Biofuels have long been firmly established in the energy landscape in order to meet a considerable portion of the world’s energy demand and to contribute to the reduction in CO
2 emissions. In fact, according to the World energy outlook 2024 report [
1], the world liquid biofuel demand in 2023 reached 2.3 million barrels per day (mb/day, expressed in energy-equivalent volumes of million barrels of gasoline and diesel). It is expected that in 2050, this demand will rise to 4.1 mb/day in the Stated Policies Scenario (STEPS), namely in the current context, including the trends in the energy sector, based on the current market data, technology costs, and predominant worldwide policies about energy and climate. Considering the Announced Pledges Scenario (APS), i.e., the fulfilment of each nation’s energy and climate targets, including net-zero goals, in 2050, the world liquid biofuel demand shall increase to 7 mb/day.
Therefore, it will be necessary to modify current biofuel production to address future demands or find feasible and sustainable approaches to ensure the scalability of promising liquid biofuels.
Among these, 2,5-dimethylfuran (DMF) has currently garnered interest as a biofuel candidate due to its physicochemical properties.
DMF possesses a high energy density (31.5 MJ/L) [
2], very similar to that of gasoline and diesel, with a higher research octane number (RON = 119) [
3] and a higher boiling point (365 K–367 K) but lower oxygen content (O/C = 0.17) [
4], with respect to that of gasoline and ethanol. Furthermore, DMF is immiscible in water but miscible in any proportion with gasoline and diesel. Thus, these physicochemical properties make it an interesting biofuel candidate usable in both spark and compression engines. Recent studies [
2,
5] have shown that DMF blended with gasoline and diesel can be used without any specific customization, allowing improvements in the emissions profile, with a low production of particulate matter and without compromising the performance and durability of engines.
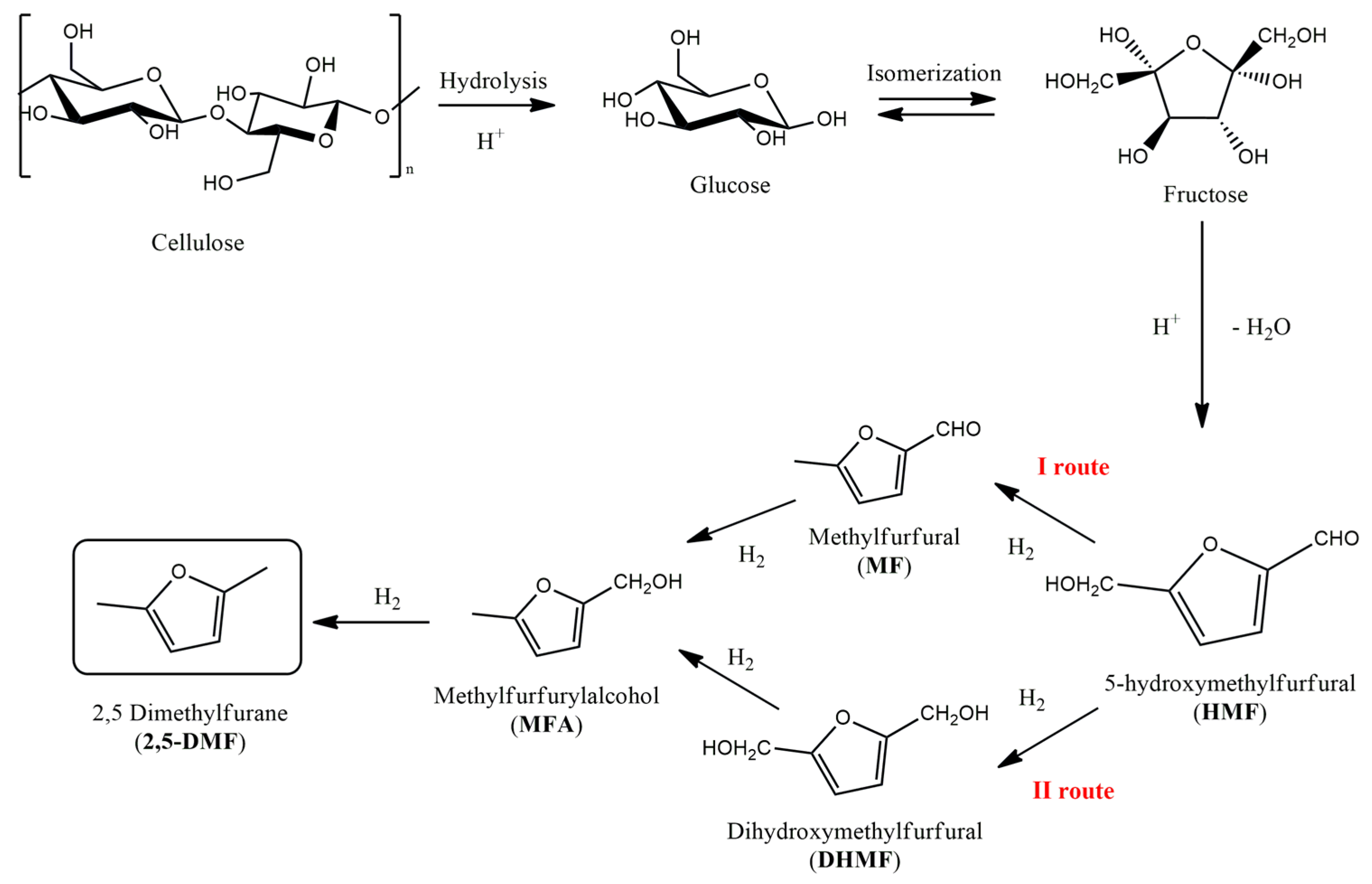
In light of its great potential, the production of DMF from lignocellulosic biomass has become a rather challenging but increasingly explored field of research [
6,
7]. Nevertheless, DMF is not yet used commercially. Before explaining the reasons for the lack of industrial production and considering a possible approach to changing this system, a brief description of the chemical sequence for DMF production is provided. DMF is easily produced by using cellulose, which is very abundant, as a starting material. After various steps, including hydrolysis, the isomerization of sugars and dehydration, intermediate 5-hydroxymethylfurfural (HMF) is produced. HMF represents a platform molecule, since DMF is produced through two possible interchangeable routes involved in hydrodeoxygenation reactions, as reported in
Figure 1.
Despite the extensive literature on DMF synthesis, recent, thorough analyses of the technical and economic features of its large-scale production are still missing. In 2011, Kazi et al. [
8] conducted a detailed study on all the steps affecting the production costs of DMF. The analysis was based on the exploitation of a two-step integrated process, patented by Dumesic and a coworker [
9,
10], for DMF production using fructose as a starting material.
In the first step, HMF is produced by the acid (homogeneous HCl)-catalyzed conversion of fructose in a bi-phasic water–butanol reactor in the presence of NaCl to promote the extraction of HMF, from aqueous to organic phase. In the second step, HMF is converted to DMF using a Cu-Ru/C catalyst and molecular hydrogen. In this context, the economic analysis reported by Kazi et al. considered that an industrial plant, appropriately sized to allow for a DMF capacity of 96 metric tons/day using 300 metric tons/day of fructose, required an installed equipment cost of around USD 121.9 million and USD 36.4 million the initial costs of the catalyst (with a further biennial replacement cost of USD 0.2585 million). On this basis, a minimum selling price (MSP) of USD 2.02/L was estimated. This price was obviously much higher compared with the price of gasoline (USD 0.77 /L) in 2010 and with the price of corn-derived ethanol (USD 0.6 /L) or cellulosic-derived ethanol (USD 1.6/L).
The major contribution of DMF to the MSP is the use of fructose as a starting material, which affects up to 47% of the final price. The recovery of raw materials (fructose and butanol), DMF purification, catalyst costs, reactor costs and other costs account for 21%, 11%, 6%, 2%, and 13% of the MSP, respectively.
This analysis reflects the situation at that time; however, even considering the progress that has been achieved since then, two main obstacles to the mass-scale production of DMF still exist: (i) the availability of large quantities of low-cost HMF obtained from the cellulosic fraction of lignocellulosic biomass, and (ii) the lack of noble metal-free catalytic systems that can be used to carry out hydrodeoxygenation steps using 5-hydroxymethylfurfural (HMF) as well as by using alternative hydrogen sources, that are effective in terms of allowing for high selectivity and high yields to be obtained. To improve the sustainability and the efficiency of the process, it would be feasible to integrate the main steps into a cascade process that would allow us to use a cheap substrate like cellulose as a starting material for the production of HMF and for its conversion to DMF. Although this may cause lead to a high-complexity process and large quantities of by-products, it can still be justified for research to be focused in this direction. Otherwise, the direct conversion of cellulose to HMF should be taken into account. Indeed, the development of multifunctional heterogeneous catalytic materials containing both Bronsted and Lewis acidic active sites, which can enable the cascade processes involved in isomerization and dehydration reactions, would be highly desirable. With regards to the conversion of HMF to DMF, in which hydrodeoxygenation reactions are involved, the use of catalytic noble metal-free materials would be convenient. According to EuChems [
11], a great deal of importance is placed on the need to carefully consider strategical elements due to their rarity, geopolitically affected supply, and overexploitation; otherwise, the already limited recycling infrastructures may become entirely unavailable. Thus, the development of non-critical-element-based catalysts is mandatory.
Another way to ensure the sustainability of the process is to use 2-butanol as an organic phase in a bi-phasic system. Indeed, this could not only improve the solubility of cellulose but lead to high yields of HMF, preventing its further degradation into humines. Another advantage is the capability of 2-butanol to act as a hydrogen donor in the catalysed transfer hydrogenation, which leads to DMF production. In this way, it is possible to avoid the use of H2 gas, with a resulting reduction in the costs of constructing and maintaining industrial plants. Moreover, the use of hydrogen requires a close proximity to hydrogen-producing plants, and a potential consequence is a reduction in DMF renewability features if fossil-derived hydrogen is used.
The use of a bi-phasic system allows us to use a cheap and green solvent like water, but the proper utilization of this important natural resource should be kept into account. In fact, the abovementioned industrial process produces a large amount of wastewater during HMF production (910.2 metric ton/day), which is also due to the use of NaCl for the salting out of HMF from an aqueous phase to an organic phase. The use of NaCl increases the cost of the process due to its energy-intensive removal, which is required during the purification steps. For this reason, a further improvement could be the use of a newly developed solvent like a deep eutectic solvent [
12], in order to circumvent the water utilization-related issues.
Finally, by taking advantage of the research results on DMF synthesis and the possibility of combining it with existing biorefineries, the market for DMF could be expected to grow over the next 10 years to partially meet the continuously growing need for biomass-derived fuels and chemicals.