Lysine-Based Silicone Surfactants
Abstract
1. Introduction
2. Results
2.1. Making Surfactants
2.1.1. Cysteine
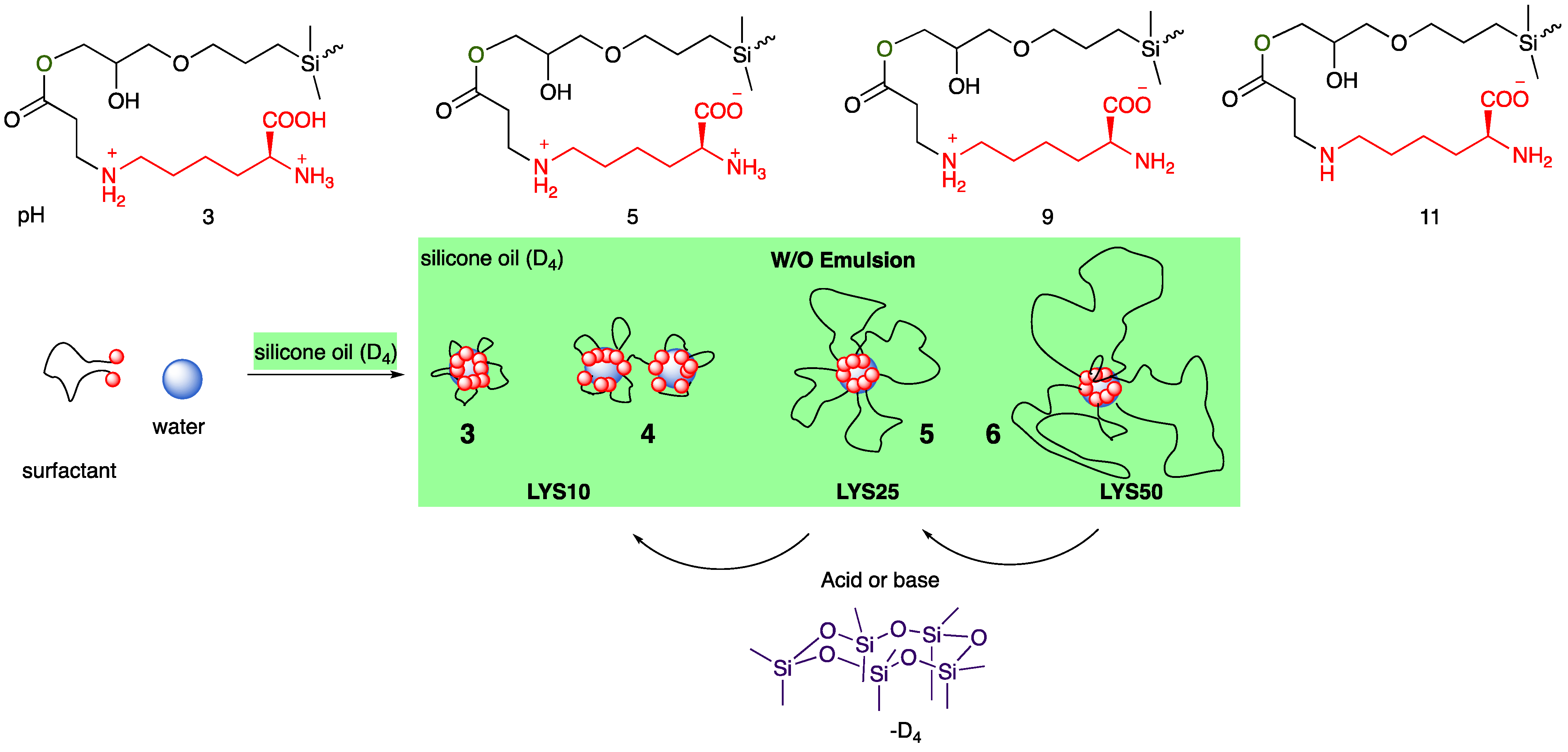
2.1.2. Lysine and Arginine
2.2. Solvency
2.3. Surfactancy
2.3.1. Emulsion Types
2.3.2. Cysteine
2.3.3. Lysine and Arginine
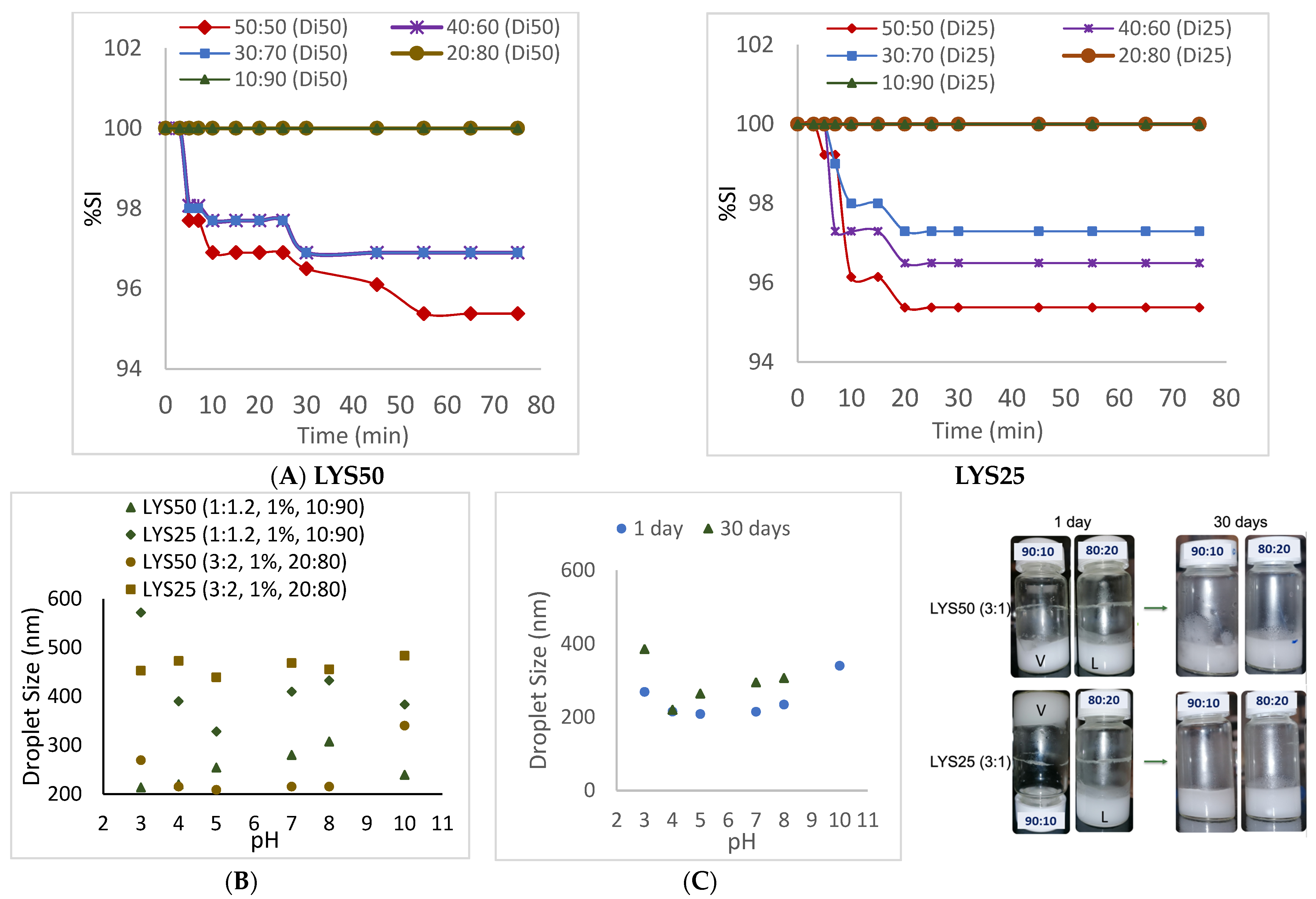
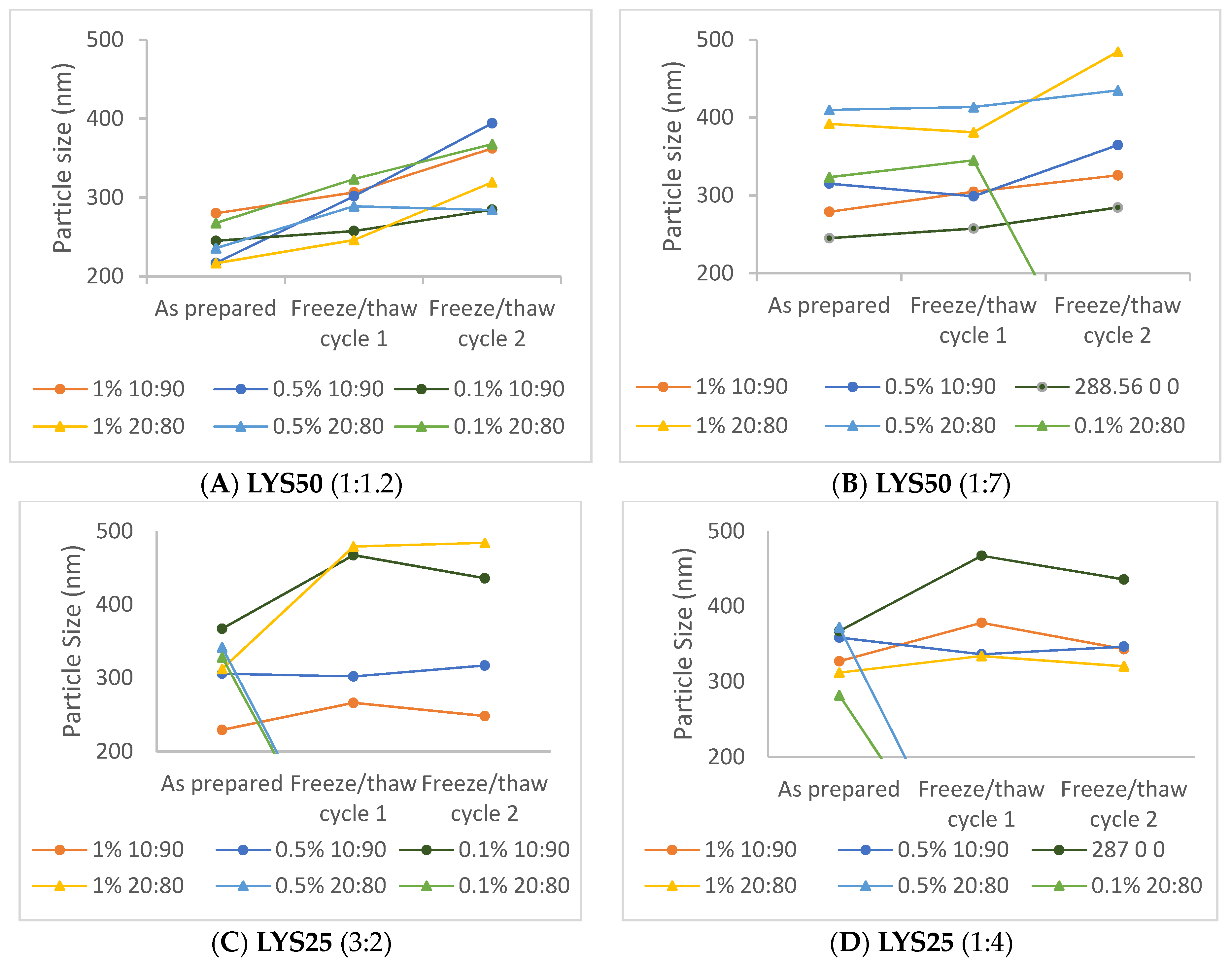
2.4. Emulsion Response to Stress: Droplet Size
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Characterization
4.3. Synthesis of Emulsifiers
4.3.1. Cysteine-Derived Silicones
4.3.2. Arginine-And LYSINE-Derived Silicones
4.4. Emulsion Preparation
4.5. Emulsion Characterization
4.5.1. Visual Stability
4.5.2. Particle Size Analysis
4.6. Stressing the Emulsions
4.6.1. pH
4.6.2. Temperature
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Anastas, P.T.; Warner, J.C. Green Chemistry Theory and Practice; Oxford University Press: Oxford, UK, 2000. [Google Scholar]
- Stevens, P.J.G. Organosilicone surfactants as adjuvants for agrochemicals. Pestic. Sci. 1993, 38, 103–122. [Google Scholar] [CrossRef]
- Snow, S.A.; Pernisz, U.C.; Braun, R.J. “Tying up loose ends”—Silicone surfactants as stabilizing agents for flexible polyurethane foam. Silicon Chem. 2006, 3, 1–10. [Google Scholar] [CrossRef]
- Hill, R.M. Silicone Surfactants; Dekker: New York, NY, USA, 1999. [Google Scholar]
- Bonnington, L.S. Analysis of Organosilicone Surfactants and Their Degradation Products. Ph.D. Thesis, The University of Waikato, Hamilton, New Zealand, 2000. [Google Scholar]
- Radulovic, J.; Sefiane, K.; Shanahan, M.E.R. Ageing of trisiloxane solutions. Chem. Eng. Sci. 2010, 65, 5251–5255. [Google Scholar] [CrossRef]
- Brook, M.A. Silicones. In Silicon in Organic, Organometallic and Polymer Chemistry, Chapters 9 and 12; Wiley: New York, NY, USA, 2000; pp. 256–308, 381–458. [Google Scholar]
- Svitova, T.; Hoffmann, H.; Hill, R.M. Trisiloxane surfactants: Surface interfacial tension dynamics and spreading on hydrophobic surfaces. Langmuir 1996, 12, 1712–1721. [Google Scholar] [CrossRef]
- Stoebe, T.; Lin, Z.X.; Hill, R.M.; Ward, M.D.; Davis, H.T. Surfactant-enhanced spreading. Langmuir 1996, 12, 337–344. [Google Scholar] [CrossRef]
- Hill, R.M. Superspreading. Curr. Opin. Colloid Interface Sci. 1998, 3, 247–254. [Google Scholar] [CrossRef]
- Chen, J.; Mullin, C.A. Characterization of Trisiloxane Surfactants from Agrochemical Adjuvants and Pollinator-Related Matrices Using Liquid Chromatography Coupled to Mass Spectrometry. J. Agric. Food Chem. 2015, 63, 5120–5125. [Google Scholar] [CrossRef]
- Lusterio, A.; Brook, M.A. Naturally Derived Silicone Surfactants Based on Saccharides and Cysteamine. Molecules 2021, 26, 4802. [Google Scholar] [CrossRef]
- Morán, M.C.; Pinazo, A.; Pérez, L.; Clapés, P.; Angelet, M.; García, M.T.; Vinardell, M.P.; Infante, M.R. “Green” amino acid-based surfactants. Green Chem. 2004, 6, 233–240. [Google Scholar] [CrossRef]
- Provatas, A.; Matisons, J.G.; Smart, R.S.C. α-Amino Acid−β-Hydroxysiloxanes on E-Glass Fibers. Langmuir 1998, 14, 1656–1663. [Google Scholar] [CrossRef]
- Genest, A.; Portinha, D.; Pouget, E.; Lamnawar, K.; Ganachaud, F.; Fleury, E. Zwitterionic Silicone Materials Derived from Aza-Michael Reaction of Amino-Functional PDMS with Acrylic Acid. Macromol. Rapid Commun. 2021, 42, 2000372. [Google Scholar] [CrossRef]
- Xu, C.; He, R.; Xie, B.; Ismail, M.; Yao, C.; Luan, J.; Li, X. Silicone hydrogels grafted with natural amino acids for ophthalmological application. J. Biomater. Sci. Polym. Ed. 2016, 27, 1354–1368. [Google Scholar] [CrossRef] [PubMed]
- LaRonde, F.J.; Brook, M.A.; Hu, G. Amino acid and peptide chemistry on silicones. Silicon Chem. 2002, 1, 215–222. [Google Scholar] [CrossRef]
- Lucas, P.; Fleury, E.; Estur, J.-F.; Lapinte, V.; Robin, J.-J. Peroxide-Grafted PDMS: Hydrosilylation Reaction and Thiol-Ene Chemistry as an Alternative Pathway. Macromol. Chem. Phys. 2009, 210, 1933–1941. [Google Scholar] [CrossRef]
- Zheng, S.; Zlatin, M.; Selvaganapathy, P.R.; Brook, M.A. Multiple modulus silicone elastomers using 3D extrusion printing of low viscosity inks. Addit. Manuf. 2018, 24, 86–92. [Google Scholar] [CrossRef]
- Hoyle, C.E.; Bowman, C.N. Thiol-Ene Click Chemistry. Angew. Chem. Int. Ed. 2010, 49, 1540–1573. [Google Scholar] [CrossRef]
- Lu, G.; Yepremyan, A.; Godfrey, S.; Mohr, C.; Herrlein, M.; Brook, M.A. Aza-Michael silicone cure is accelerated by β-hydroxyalkyl esters. J. Polym. Sci. 2021, 59, 1935–1941. [Google Scholar] [CrossRef]
- O’Lenick, A.J., Jr.; Parkinson, J.K. Three-Dimensional HLB: This revolutionary development helps formulators choose surfactants for stable oil, water and silicone emulsions. Cosmet. Toilet. 1996, 111, 37–44. [Google Scholar]
- O’Lenick, A.J., Jr. Silicone emulsions and surfactants. J. Surfactants Deterg. 2000, 3, 387–393. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, C. Supramolecular amphiphiles. Chem. Soc. Rev. 2011, 40, 94–101. [Google Scholar] [CrossRef]
- Mansuri, E.; Zepeda-Velazquez, L.; Schmidt, R.; Brook, M.A.; DeWolf, C.E. Surface Behavior of Boronic Acid-Terminated Silicones. Langmuir 2015, 31, 9331–9339. [Google Scholar] [CrossRef] [PubMed]
- Pelton, R.; Osterroth, A.; Brook, M.A. Silicone stabilized poly(methyl methacrylate) nonaqueous latex: 2. Flocculation by degradation of the steric layer. J. Colloid Interface Sci. 1991, 147, 523–530. [Google Scholar] [CrossRef]
- Annan, W.S.; Fairhead, M.; Pereira, P.; Walle, C.F.v.d. Emulsifying performance of modular β-sandwich proteins: The hydrophobic moment and conformational stability. Protein Eng. Design Sel. 2006, 19, 537–545. [Google Scholar] [CrossRef] [PubMed]
- McClements, D.J. Critical Review of Techniques and Methodologies for Characterization of Emulsion Stability. Crit. Rev. Food Sci. Nutr. 2007, 47, 611–649. [Google Scholar] [CrossRef]

| Sample | Solubility (a) | Solubility in Organic Solvents | HLB | 3D-HLB | Predicted Emulsion Type by 3D HLB | |
|---|---|---|---|---|---|---|
| D4 | H2O | |||||
| CYS10 | N | N | – | – | – | – |
| CYS-P2 | N | N | EtOH P (a) MeOH P | 4.61 | 4.61, 15.38 | W/O |
| ARG10 | ||||||
| (1:2) (b) | N | N | EtOH S MeOH S | 8.8 | 8.8, 11.1 | W/O |
| (3:1) | N | N | EtOH S MeOH S CHCl3 S TOL (c) S | W/O | ||
| LYS10 | ||||||
| (1:2) | N | EtOH S MeOH S | 8.4 | 8.4, 11.6 | W/O | |
| (3:1) | N | N | EtOH S MeOH P | W/O | ||
| LYS25 | ||||||
| (1:2) | P | N | TOL P | 4.6 | 4.6, 15.3 | W/O |
| (3:1) | S | N | CHCl3: MeOH (1:1) P TOL P | W/O | ||
| LYS50 | ||||||
| (1:2) | P | N | TOL S | 2.7 | 2.7, 17.8 | W/O |
| (3:1) | S | N | CHCl3: MeOH (1:1) P TOL S | W/O | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Camacho Ramirez, A.; Melendez-Zamudio, M.; Guerra Contreras, A.; Brook, M.A. Lysine-Based Silicone Surfactants. Sustain. Chem. 2023, 4, 197-208. https://doi.org/10.3390/suschem4020015
Camacho Ramirez A, Melendez-Zamudio M, Guerra Contreras A, Brook MA. Lysine-Based Silicone Surfactants. Sustainable Chemistry. 2023; 4(2):197-208. https://doi.org/10.3390/suschem4020015
Chicago/Turabian StyleCamacho Ramirez, Abygail, Miguel Melendez-Zamudio, Antonio Guerra Contreras, and Michael A. Brook. 2023. "Lysine-Based Silicone Surfactants" Sustainable Chemistry 4, no. 2: 197-208. https://doi.org/10.3390/suschem4020015
APA StyleCamacho Ramirez, A., Melendez-Zamudio, M., Guerra Contreras, A., & Brook, M. A. (2023). Lysine-Based Silicone Surfactants. Sustainable Chemistry, 4(2), 197-208. https://doi.org/10.3390/suschem4020015








