E-Wastes: Bridging the Knowledge Gaps in Global Production Budgets, Composition, Recycling and Sustainability Implications
Abstract
1. Introduction
2. Composition of E-Waste
3. Diverse Materials in E-Waste: Sources and Implications
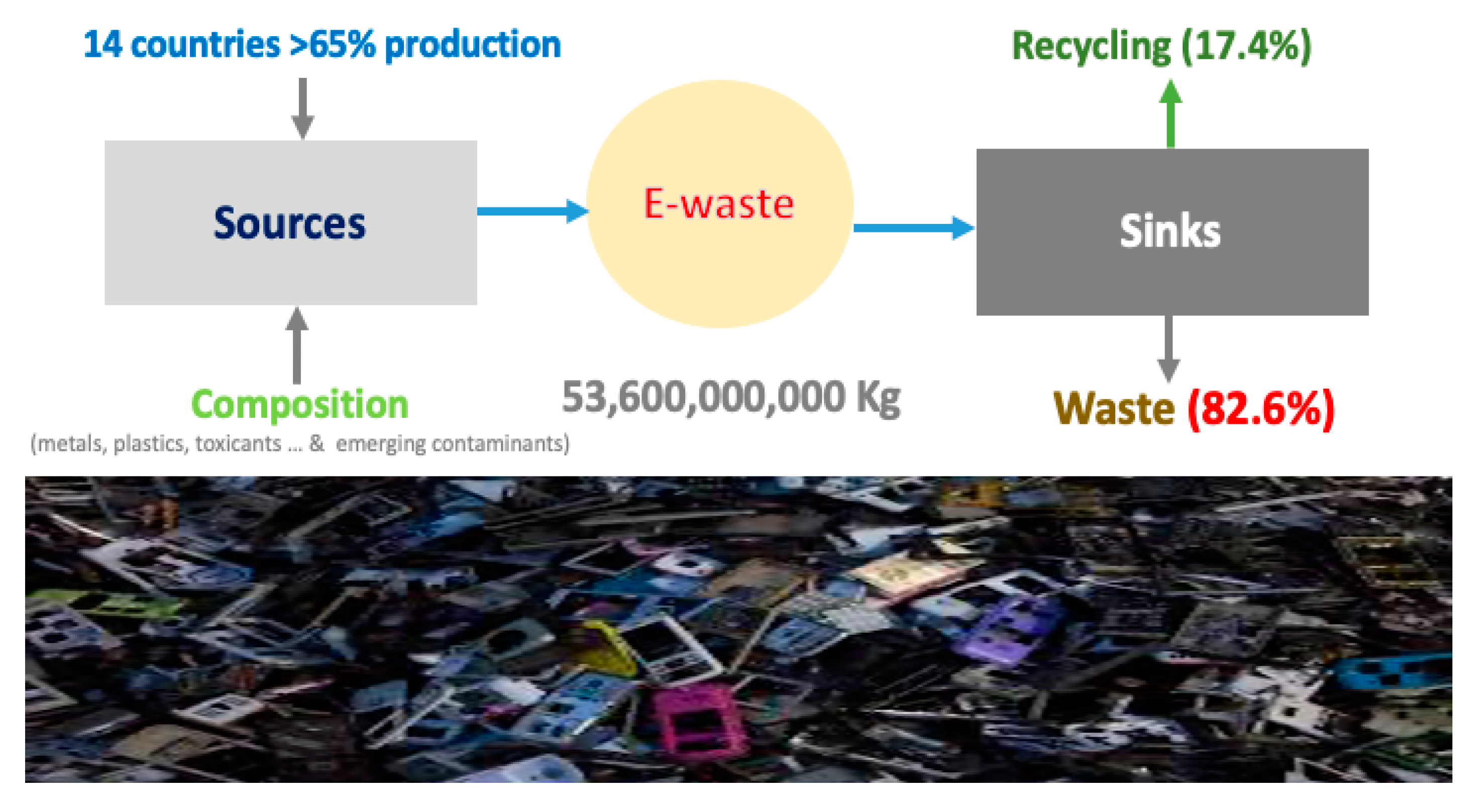
4. Global, Continental, and Regional Budgets of E-Waste
4.1. Global Overview
4.2. Continental Overview
4.3. Regional Overview
4.3.1. China
4.3.2. United States
4.3.3. Germany
4.3.4. United Kingdom
4.3.5. France
4.3.6. Italy
4.3.7. Canada
4.3.8. Japan
4.3.9. Australia
4.3.10. India
4.3.11. Russia
4.3.12. Mexico
4.3.13. Brazil
4.3.14. South Africa
5. Initiatives in Tackling the E-Waste Problem
5.1. The Basel Convention
5.2. The European Union (EU) Waste from Electrical and Electronic Equipment (WEEE) Directive
5.3. Restriction of Hazardous Substances (RoHS)
5.4. Solving the E-Waste Problem (StEP)
5.5. Global E-Waste Statistics Partnership (GESP)
5.6. Basel Action Network, Silicon Valley Toxics Coalition, and Electronics TakeBack Coalition
6. Opportunities and Challenges of Secondary Mining
7. Current Practices of E-Waste Handling
7.1. Direct Treatments
7.1.1. Disposal to Landfill
7.1.2. Incineration (Pyrolysis)
7.2. Reuse/Repair
7.3. Recycling
7.3.1. Pyrometallurgical (Smelting) Processing
7.3.2. Hydrometallurgical Processing
7.3.3. Biometallurgical Processing
7.4. Other Sustainable Technologies to Reduce E-Waste
7.4.1. Use of Airborne Nanoparticle for Effective and Efficient Recycling of E-Waste
7.4.2. Clay Minerals and Kaolin Nano and Microtraps to Remove and Recycle E-Waste Components
7.4.3. Combination of Bioreactors and Nanotechnology for Removal and Recycling E-Waste
8. Concluding Remarks
9. Future Directions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| Ag | Silver |
| Al | Aluminum |
| As | Arsenic |
| Au | Gold |
| Ba | Barium |
| Be | Beryllium |
| Br | Bromine |
| Cd | Cadmium |
| Co | Cobalt |
| Cr | Chromium |
| Cu | Copper |
| Fe | Iron |
| Hg | Mercury |
| In | Indium |
| Li | Lithium |
| Mn | Manganese |
| Ni | Nickel |
| Pb | Lead |
| Pd | Palladium |
| Pt | Platinum |
| Sb | Antimony |
| Se | Selenium |
| Sn | Tin |
| Zn | Zinc |
| BFRs | Brominated flame retardants |
| EAA | European Economic Area |
| CFCs | Chlorofluorocarbons |
| CRT | Cathode Ray Tube |
| EEE | Electrical and electronic equipment |
| HBr | Hydrogen bromide |
| HCs | Hydrocarbons |
| HCFC | Hydrochlorofluorocarbon |
| HFC | Hydrofluorocarbon |
| LCD | Liquid Crystal Display |
| OECD | Organization for Economic Co-operation and Development |
| PAHs | Polycyclic aromatic hydrocarbons |
| PBBs | Polybrominated biphenyls |
| PBDDs | Polybrominated dibenzo-dioxins |
| PBDFs | Polybrominated dibenzo-furans |
| PBDEs | Polybrominated diphenyl ethers |
| PCBs | Polychlorinated biphenyls |
| PCDDs | Polychlorinated dibenzo-dioxins |
| PCDFs | Polychlorinated dibenzofurans |
| PHAHs | Polyhalogenated aromatic hydrocarbons |
| WEEE | Waste Electrical and Electronic Equipment |
| BTEX | Benzene, toluene, ethylbenzene and toluene |
References
- Directive 2012/19/Eu of the European Parliament and of the Council. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A32012L0019 (accessed on 20 July 2020).
- Balakrishnan Ramesh, B.; Anand Kuber, P.; Chiya Ahmed, B. Electrical and electronic waste: A global environmental problem. Waste Manag. Res. 2007, 25, 307–318. [Google Scholar] [CrossRef]
- Vanessa Forti, C.P.B.; Ruediger, K.; Garam, B. The Global E-Waste Monitor 2020: Quantities, Flows, and the Circular Economy Potential. 2020. Available online: https://globalewaste.org/?fbclid=IwAR1AgKGT2S6NnFTBpuLZPlujoE_oYu2qeQErEq8sel-9XmdW2GZLJVG0Fws (accessed on 20 July 2020).
- Tue, N.M.; Takahashi, S.; Subramanian, A.; Sakai, S.; Tanabe, S. Environmental contamination and human exposure to dioxin-related compounds in e-waste recycling sites of developing countries. Environ. Sci. Process. Impacts 2013, 15, 1326. [Google Scholar] [CrossRef] [PubMed]
- Weber, R.; Kuch, B. Relevance of BFRs and thermal conditions on the formation pathways of brominated and brominated-chlorinated dibenzodioxins and dibenzofurans. Environ. Int. 2003, 29, 699–710. [Google Scholar] [CrossRef]
- Söderström, G.; Marklund, S. PBCDD and PBCDF from incineration of waste-containing brominated flame retardants. Environ. Sci. Technol. 2002, 36, 1959–1964. [Google Scholar] [CrossRef] [PubMed]
- Lundgren, K. The Global Impact of E-Waste: Addressing the Challenge. 2012. Available online: https://www.ilo.org/sector/Resources/publications/WCMS_196105/lang--en/index.htm (accessed on 20 July 2020).
- Brigden, K.; Labunska, I.; Santillo, D.; Johnston, P. Chemical Contamination at E-Waste Recycling and Disposal Sites in Accra and Korforidua, Ghana; Greenpeace: Amsterdam, The Netherlands, 2008. [Google Scholar]
- Baldé, C.; Forti, V.; Gray, V.; Kuehr, R.; Stegmann, P. The Global E-Waste Monitor–2017; ISBN Electronic Version; United Nations University (UNU): Bonn, Germany; International Telecommunication Union (ITU): Geneva, Switzerland; International Solid Waste Association (ISWA): Vienna, Austria, 2017; pp. 978–992. [Google Scholar]
- Perkins, D.N.; Drisse, M.N.B.; Nxele, T.; Sly, P.D. E-Waste: A Global Hazard. Ann. Glob. Health 2014, 80, 286–295. [Google Scholar] [CrossRef] [PubMed]
- Widmer, R.; Oswald-Krapf, H.; Sinha-Khetriwal, D.; Schnellmann, M.; Böni, H.W. Global perspectives on e-waste. Environ. Impact Assess. Rev. 2005, 25, 436–458. [Google Scholar] [CrossRef]
- Santhanam, N.; Samuel, M.; Chidambaram, R. Electronic waste–An emerging threat to the environment of urban India. J. Environ. Health Sci. Eng. 2014, 12, 36. [Google Scholar]
- Robinson, B.H. E-waste: An assessment of global production and environmental impacts. Sci. Total Environ. 2009, 408, 183–191. [Google Scholar] [CrossRef]
- Li, Y.; Xu, X.; Liu, J.; Wu, K.; Gu, C.; Shao, G.; Chen, S.; Chen, G.; Huo, X. The hazard of chromium exposure to neonates in Guiyu of China. Sci. Total Environ. 2008, 403, 99–104. [Google Scholar] [CrossRef]
- Blass, V.D.; Fuji, M.; Neira, J.; Favret, L.; Mahdavi, S.; Miller, R.; Geyer, R. End-of-Life Management of Cell Phones in the United States. Master’s Thesis, Donald Bren School of Environmental Science and Management University of California, Santa Barbara, CA, USA, 2006. [Google Scholar]
- Liu, Q.; Li, K.Q.; Zhao, H.; Li, G.; Fan, F.Y. The global challenge of electronic waste management. Environ. Sci. Pollut. Res. 2009, 16, 248–249. [Google Scholar] [CrossRef] [PubMed]
- Hageluken, C. Improving Metal Returns and Eco-Efficiency In Electronics Recycling—A Holistic Approach for Interface Optimisation between Pre-Processing and Integrated Metals Smelting and Refining. In Proceedings of the 2006 IEEE International Symposium on Electronics and the Environment, Scottsdale, AZ, USA, 8–11 May 2006. [Google Scholar]
- Cui, J.; Forssberg, E. Characterization of shredded television scrap and implications for materials recovery. Waste Manag. 2007, 27, 415–424. [Google Scholar] [CrossRef]
- Legarth, J.; Alting, L.; Danzer, B.; Tartler, D.; Brodersen, K.; Scheller, H.; Feldmann, K. A New Strategy in the Recycling of Printed Circuit Boards. Circuit World 1995, 21, 10–15. [Google Scholar] [CrossRef]
- Zhang, S.; Forssberg, E. Electronic scrap characterization for materials recycling. J. Waste Manag. Resour. Recover. 1997, 3, 157–167. [Google Scholar]
- Amoyaw-Osei, Y.; Agyekum, O.O.; Pwamang, J.A.; Mueller, E.; Fasko, R.; Schluep, M. Ghana E-Waste Country Assessment. SBC E-Waste Africa Project. Dubendorf, EMPA. 2011. Available online: http://www.basel.int/Portals/4/Basel%20Convention/docs/eWaste/E-wasteAssessmentGhana.pdf (accessed on 1 September 2020).
- Caravanos, J.; Clark, E.; Fuller, R.; Lambertson, C. Assessing Worker and Environmental Chemical Exposure Risks at an e-waste Recycling and Disposal Site in Accra, Ghana. J. Health Pollut. 2011, 1, 16–25. [Google Scholar] [CrossRef]
- Oteng-Ababio, M.; Chama, M.A.; Amankwaa, E.F. Qualitative analysis of the presence of PBDE in ashes, soils and vegetables from Agbogbloshie e-waste recycling site. Studies 2014, 5, 71–80. [Google Scholar]
- Asante, K.A.; Adu-Kumi, S.; Nakahiro, K.; Takahashi, S.; Isobe, T.; Sudaryanto, A.; Devanathan, G.; Clarke, E.; Ansa-Asare, O.D.; Dapaah-Siakwan, S.; et al. Human exposure to PCBs, PBDEs and HBCDs in Ghana: Temporal variation, sources of exposure and estimation of daily intakes by infants. Environ. Int. 2011, 37, 921–928. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Xu, X.; Yekeen, T.A.; Zhang, Y.; Chen, A.; Kim, S.S.; Dietrich, K.N.D.K.N.; Ho, S.M.; Lee, S.A.; Reponen, T.; et al. Ambient air heavy metals in PM2.5 and potential human health risk assessment in an informal electronic-waste recycling site of China. Aerosol Air Qual. Res. 2016, 16, 388–397. [Google Scholar] [CrossRef]
- Grant, K.; Goldizen, F.C.; Sly, P.D.; Brune, M.-N.; Neira, M.; Berg, M.V.D.; Norman, R.E. Health consequences of exposure to e-waste: A systematic review. Lancet Glob. Health 2013, 1, e350–e361. [Google Scholar] [CrossRef]
- Horne, R.; Gertsakis, J. A Literature Review on the Environmental and Health Impacts of Waste Electrical and Electronic Equipment; RMIT University, Prepared for the Ministry for the Environment; Government of New Zealand: Melbourne, Australia, 2006.
- Hu, H. Human health and heavy metals exposure. In Life Support: The Environment and Human Health; MIT Press: Cambridge, UK, 2002; pp. 65–82. [Google Scholar]
- Kumar, A.; Holuszko, M.; Espinosa, D.C.R. E-waste: An overview on generation, collection, legislation and recycling practices. Resour. Conserv. Recycl. 2017, 122, 32–42. [Google Scholar] [CrossRef]
- Parzefall, W. Risk assessment of dioxin contamination in human food. Food Chem. Toxicol. 2002, 40, 1185–1189. [Google Scholar] [CrossRef]
- Boffetta, P.; Jourenkova, N.; Gustavsson, P. Cancer risk from occupational and environmental exposure to polycyclic aromatic hydrocarbons. Cancer Causes Control. 1997, 8, 444–472. [Google Scholar] [CrossRef] [PubMed]
- United Nations. World Population Prospects 2019. Population Data 2019. Available online: https://population.un.org/wpp/Download/Standard/Population/ (accessed on 31 March 2020).
- ITU. The Global E-Waste Statistics Partnership: Country and Regional Sheets. 2020. Available online: https://globalewaste.org/country-sheets/ (accessed on 5 July 2020).
- Zoeteman, B.C.J.; Krikke, H.R.; Venselaar, J. Handling WEEE waste flows: On the effectiveness of producer responsibility in a globalizing world. Int. J. Adv. Manuf. Technol. 2009, 47, 415–436. [Google Scholar] [CrossRef]
- Baldé, C.P.; Wang, F.; Kuehr, R.; Huisman, J. The Global E-Waste Monitor 2014 Quantities, Flows and Resources. 2015. Available online: https://i.unu.edu/media/unu.edu/news/52624/UNU-1stGlobal-E-Waste-Monitor-2014-small.pdf. (accessed on 7 April 2020).
- Galloway, B.L. Five Superpowers Ruling the World in 2050. 2020. Available online: http://www.bbc.com/travel/story/20200322-five-superpowers-ruling-the-world-in-2050 (accessed on 1 April 2020).
- Ylä-Mella, J.; Román, E. Waste electrical and electronic equipment management in Europe: Learning from best practices in Switzerland, Norway, Sweden and Denmark. In Waste Electrical and Electronic Equipment (WEEE) Handbook; Elsevier: Amsterdam, The Netherlands, 2019; pp. 483–519. [Google Scholar]
- Yang, W. Regulating Electrical and Electronic Wastes in China. Rev. Eur. Community Int. Environ. Law. 2008, 17, 337–346. [Google Scholar] [CrossRef]
- Balde, C.P.; Kuehr, R.; Blumenthal, K.; Gill, S.F.; Kern, M.; Micheli, P.; Magpantay, E.; Huisman, J. E-Waste Statistics: Guidelines on Classifications, Reporting and Indicators; United Nations University, IAS—SCYCLE: Bonn, Germany, 2015. [Google Scholar]
- Shinkuma, T.; Huong, N.T.M. The flow of E-waste material in the Asian region and a reconsideration of international trade policies on E-waste. Environ. Impact Assess. Rev. 2009, 29, 25–31. [Google Scholar] [CrossRef]
- Chi, X.; Streicher-Porte, M.; Wang, M.Y.; Reuter, M. Informal electronic waste recycling: A sector review with special focus on China. Waste Manag. 2011, 31, 731–742. [Google Scholar] [CrossRef]
- Eurostat. Waste Electrical and Electronic Equipment (WEEE) by Waste Management Operations. 2020. Available online: https://appsso.eurostat.ec.europa.eu/nui/show.do?dataset=env_waselee&lang=en (accessed on 20 June 2020).
- Golev, A.; Schmeda-Lopez, D.R.; Smart, S.K.; Corder, G.D.; McFarland, E.W. Where next on e-waste in Australia? Waste Manag. 2016, 58, 348–358. [Google Scholar] [CrossRef]
- Borthakur, A.; Sinha, K. Generation of electronic waste in India: Current scenario, dilemmas and stakeholders. Afr. J. Environ. Sci. Technol. 2013, 7, 899–910. [Google Scholar]
- Mihai, F.C.; Gnoni, M.G.; Meidiana, C.; Ezeah, C.; Elia, V. Chapter 1—Waste Electrical and Electronic Equipment (WEEE): Flows, Quantities, and Management—A Global Scenario. In Electronic Waste Management and Treatment Technology; Prasad, M.N.V., Vithanage, M., Eds.; Butterworth-Heinemann: Oxford, UK, 2019; pp. 1–34. [Google Scholar]
- Gavilán-García, A.; Román-Moguel, G.; Almada-Calvo, F.; Aburto-Mejía, S. Electronic waste generation and technology for an appropriate management in Mexico. Bol. Soc. Quím. Méx. 2009, 3, 85–92. [Google Scholar]
- Araújo, M.G.; Magrini, A.; Mahler, C.F.; Bilitewski, B. A model for estimation of potential generation of waste electrical and electronic equipment in Brazil. Waste Manag. 2012, 32, 335–342. [Google Scholar] [CrossRef]
- Schluep, M.; Hageluken, C.; Kuehr, R.; Magalini, F. Recycling: From E-Waste to Resources; UNEP: Nairobi, Kenya; United Nations University (UNU): Tokyo, Japan, 2009. [Google Scholar]
- Sthiannopkao, S.; Wong, M.H. Handling e-waste in developed and developing countries: Initiatives, practices, and consequences. Sci. Total Environ. 2013, 463, 1147–1153. [Google Scholar] [CrossRef]
- UNEP. Basel Convention, Controlling Transboundary Movements of Hazardous Wastes and Their Disposal. 1992. Available online: http://www.basel.int/TheConvention/Overview/tabid/1271/Default.aspx/ (accessed on 27 August 2020).
- European Commission. Waste Electrical & Electronic Equipment (WEEE). Available online: https://ec.europa.eu/environment/waste/weee/ (accessed on 16 August 2020).
- Directive 2002/96/EC of the European Parliament and of the Council of 27 January 2003 on Waste Electrical and Electronic Equipment (WEEE). 2003. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX:32002L0096 (accessed on 17 August 2020).
- Directive 2012/19/EU of the European Parliament and of the Council of 4 July 2012 on Waste Electrical and Electronic Equipment (WEEE). 2012. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX:32012L0019 (accessed on 17 August 2020).
- Salhofer, S.; Steuer, B.; Ramusch, R.; Beigl, P. WEEE Management in Europe and China—A Comparison. Waste Manag. 2016, 57, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Directive 2011/65/EU of the European Parliament and of the Council of 8 June 2011 on the Restriction of the Use of Certain Hazardous Substances in Electrical and Electronic Equipment. 2011. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX:32011L0065 (accessed on 18 August 2020).
- Step: Solving the E-Waste Problem. Available online: https://www.step-initiative.org/organisation-rev.html (accessed on 18 August 2020).
- The Global E-Waste Statistics Partnership. Available online: https://www.itu.int/en/ITU-D/Environment/Pages/Priority-Areas/Global-Ewaste-Statistics-Partnership.aspx (accessed on 18 August 2020).
- Electronics Take Back Coalition. Available online: http://www.electronicstakeback.com/how-to-recycle-electronics/manufacturer-takeback-programs/ (accessed on 24 July 2020).
- Rujanavech, C.; Lessard, J.; Chandler, S.; Shannon, S.; Dahmus, J.; Guzzo, R. Liam—An Innovation Story Apple. 2016. Available online: https://www.dx2025.com/wp-content/uploads/2019/11/apple-white-paper-on-dismantling-production-line-of-liam-mobile-phone-recycling.pdf (accessed on 1 September 2020).
- Newsroom: Apple Adds Earth Day Donations to Trade-In and Recycling Program. Available online: https://www.apple.com/newsroom/2018/04/apple-adds-earth-day-donations-to-trade-in-and-recycling-program/ (accessed on 18 August 2020).
- Magalini, F.; Huisman, J. WEEE Recycling Economics—The Shortcomings of the Current Business Model. 2018. Available online: https://www.researchgate.net/publication/327282498_WEEE_Recycling_Economics_-_The_shortcomings_of_the_current_business_model/stats (accessed on 2 September 2020).
- Ghosh, B.; Ghosh, M.; Parhi, P.K.; Mukherjee, P.; Mishra, B. Waste Printed Circuit Boards recycling: An extensive assessment of current status. J. Clean. Prod. 2015, 94, 5–19. [Google Scholar] [CrossRef]
- Cucchiella, F.; D’Adamo, I.; Rosa, P.; Terzi, S. Automotive printed circuit boards recycling: An economic analysis. J. Clean. Prod. 2016, 121, 130–141. [Google Scholar] [CrossRef]
- D’Adamo, I.; Rosa, P.; Terzi, S. Challenges in Waste Electrical and Electronic Equipment Management: A Profitability Assessment in Three European Countries. Sustainability 2016, 8, 633. [Google Scholar] [CrossRef]
- Zeng, X.; Mathews, J.A.; Li, J. Urban Mining of E-Waste is Becoming More Cost-Effective than Virgin Mining. Environ. Sci. Technol. 2018, 52, 4835–4841. [Google Scholar] [CrossRef] [PubMed]
- Cucchiella, F.; D’Adamo, I.; Koh, S.L.; Rosa, P. A profitability assessment of European recycling processes treating printed circuit boards from waste electrical and electronic equipments. Renew. Sustain. Energy Rev. 2016, 64, 749–760. [Google Scholar] [CrossRef]
- Kaya, M. Recovery of metals and nonmetals from electronic waste by physical and chemical recycling processes. Waste Manag. 2016, 57, 64–90. [Google Scholar] [CrossRef]
- Hagelüken, C.; Refining, U.P.M. Opportunities & Challenges to Recover Scarce and Valuable Metals from Electronic Devices. In Proceedings of the Vortrag Anlässlich Der OECD-UNEP Conference on Resource Efficiency, Paris, France, 23–25 April 2008. [Google Scholar]
- Huisman, J.; Stevels, A.; Baldé, K.; Magalini, F.; Kuehr, R. Chapter 3—The e-waste development cycle, part II—Impact assessment of collection and treatment. In Waste Electrical and Electronic Equipment (WEEE) Handbook; Woodhead Publishing: Cambridge, UK, 2019; pp. 57–92. [Google Scholar]
- Tanskanen, P. Management and recycling of electronic waste. Acta Mater. 2013, 61, 1001–1011. [Google Scholar] [CrossRef]
- De Boer, M.A.; Lammertsma, K. Scarcity of Rare Earth Elements. Chem. Sustain. Chem. 2013, 6, 2045–2055. [Google Scholar] [CrossRef]
- Sun, Z.; Cao, H.; Xiao, Y.; Sietsma, J.; Jin, W.; Agterhuis, H.; Yang, Y. Toward Sustainability for Recovery of Critical Metals from Electronic Waste: The Hydrochemistry Processes. ACS Sustain. Chem. Eng. 2016, 5, 21–40. [Google Scholar] [CrossRef]
- Ning, C.; Lin, C.S.K.; Hui, D.C.W.; McKay, G. Waste Printed Circuit Board (PCB) Recycling Techniques. Top. Curr. Chem. 2017, 375, 331. [Google Scholar]
- Williams, E.; Kahhat, R.; Allenby, B.; Kavazanjian, E.; Kim, J.; Xu, M. Environmental, Social, and Economic Implications of Global Reuse and Recycling of Personal Computers. Environ. Sci. Technol. 2008, 42, 6446–6454. [Google Scholar] [CrossRef] [PubMed]
- Czuczwa, J.M.; Hites, R.A. Environmental fate of combustion-generated polychlorinated dioxins and furans. Environ. Sci. Technol. 1984, 18, 444–450. [Google Scholar] [CrossRef] [PubMed]
- Kiddee, P.; Naidu, R.; Wong, M.H. Metals and polybrominated diphenyl ethers leaching from electronic waste in simulated landfills. J. Hazard. Mater. 2013, 252, 243–249. [Google Scholar] [CrossRef]
- Kasassi, A.; Rakimbei, P.; Karagiannidis, A.; Zabaniotou, A.; Tsiouvaras, K.; Nastis, A.; Tzafeiropoulou, K. Soil contamination by heavy metals: Measurements from a closed unlined landfill. Bioresour. Technol. 2008, 99, 8578–8584. [Google Scholar] [CrossRef]
- Tiller, K. Heavy metals in soils and their environmental significance. In Advances in Soil Science; Springer: Berlin/Heidelberg, Germany, 1989; pp. 113–142. [Google Scholar]
- Kiddee, P.; Naidu, R.; Wong, M.H.; Hearn, L.; Müller, J.F. Field investigation of the quality of fresh and aged leachates from selected landfills receiving e-waste in an arid climate. Waste Manag. 2014, 34, 2292–2304. [Google Scholar] [CrossRef]
- Spalvins, E.; Dubey, B.K.; Townsend, T. Impact of Electronic Waste Disposal on Lead Concentrations in Landfill Leachate. Environ. Sci. Technol. 2008, 42, 7452–7458. [Google Scholar] [CrossRef]
- De Marco, I.; Caballero, B.; Chomón, M.; Laresgoiti, M.; Torres, A.; Fernández, G.; Arnaiz, S. Pyrolysis of electrical and electronic wastes. J. Anal. Appl. Pyrolysis 2008, 82, 179–183. [Google Scholar] [CrossRef]
- Jie, G.; Ying-Shun, L.; Mai-Xi, L. Product characterization of waste printed circuit board by pyrolysis. J. Anal. Appl. Pyrolysis 2008, 83, 185–189. [Google Scholar] [CrossRef]
- Barontini, F.; Cozzani, V. Formation of hydrogen bromide and organobrominated compounds in the thermal degradation of electronic boards. J. Anal. Appl. Pyrolysis 2006, 77, 41–55. [Google Scholar] [CrossRef]
- Duan, H.; Li, J.; Liu, Y.; Yamazaki, N.; Jiang, W. Characterization and Inventory of PCDD/Fs and PBDD/Fs Emissions from the Incineration of Waste Printed Circuit Board. Environ. Sci. Technol. 2011, 45, 6322–6328. [Google Scholar] [CrossRef] [PubMed]
- Gullett, B.K.; Linak, W.P.; Touati, A.; Wasson, S.J.; Gatica, S.; King, C.J. Characterization of air emissions and residual ash from open burning of electronic wastes during simulated rudimentary recycling operations. J. Mater. Cycles Waste Manag. 2007, 9, 69–79. [Google Scholar] [CrossRef]
- Ortuño, N.; Conesa, J.A.; Molto, J.; Font, R. Pollutant emissions during pyrolysis and combustion of waste printed circuit boards, before and after metal removal. Sci. Total Environ. 2014, 499, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Guo, J.; Xu, Z. Recycling of non-metallic fractions from waste printed circuit boards: A review. J. Hazard. Mater. 2009, 168, 567–590. [Google Scholar] [CrossRef] [PubMed]
- Ni, M.; Xiao, H.; Chi, Y.; Yan, J.; Buekens, A.; Jin, Y.-Q.; Lu, S. Combustion and inorganic bromine emission of waste printed circuit boards in a high temperature furnace. Waste Manag. 2012, 32, 568–574. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Sun, L.; Xiang, J.; Hu, S.; Su, S. Pyrolysis and dehalogenation of plastics from waste electrical and electronic equipment (WEEE): A review. Waste Manag. 2013, 33, 462–473. [Google Scholar] [CrossRef]
- Long, Y.; Feng, Y.-J.; Cai, S.-S.; Ding, W.-X.; Shen, D.-S. Flow analysis of heavy metals in a pilot-scale incinerator for residues from waste electrical and electronic equipment dismantling. J. Hazard. Mater. 2013, 261, 427–434. [Google Scholar] [CrossRef]
- Kurien, U.; Hu, Z.; Lee, H.; Dastoor, A.; Ariya, P.A. Radiation enhanced uptake of Hg0(g) on iron (oxyhydr)oxide nanoparticles. RSC Adv. 2017, 7, 45010–45021. [Google Scholar] [CrossRef]
- Hu, Z.; Beuret, M.; Khan, H.; Ariya, P.A. Development of a Recyclable Remediation System for Gaseous BTEX: Combination of Iron Oxides Nanoparticles Adsorbents and Electrochemistry. ACS Sustain. Chem. Eng. 2014, 2, 2739–2747. [Google Scholar] [CrossRef]
- Wieser, H.; Tröger, N. Exploring the inner loops of the circular economy: Replacement, repair, and reuse of mobile phones in Austria. J. Clean. Prod. 2018, 172, 3042–3055. [Google Scholar] [CrossRef]
- Cairns, C.N. E-Waste and the Consumer: Improving Options to Reduce, Reuse and Recycle. In Proceedings of the 2005 IEEE International Symposium on Electronics and the Environment, New Orleans, LA, USA, 19 May 2005. [Google Scholar]
- Cui, J.; Forssberg, E. Mechanical recycling of waste electric and electronic equipment: A review. J. Hazard. Mater. 2003, 99, 243–263. [Google Scholar] [CrossRef]
- Button, K. 20 Staggering E-Waste Facts. 2016. Available online: https://earth911.com/eco-tech/20-e-waste-facts/ (accessed on 5 April 2020).
- Yamane, L.H.; De Moraes, V.T.; Espinosa, D.C.R.; Tenório, J.A.S. Recycling of WEEE: Characterization of spent printed circuit boards from mobile phones and computers. Waste Manag. 2011, 31, 2553–2558. [Google Scholar] [CrossRef] [PubMed]
- Taurino, R.; Pozzi, P.; Zanasi, T. Facile characterization of polymer fractions from waste electrical and electronic equipment (WEEE) for mechanical recycling. Waste Manag. 2010, 30, 2601–2607. [Google Scholar] [CrossRef] [PubMed]
- Hubau, A.; Chagnes, A.; Minier, M.; Touzé, S.; Chapron, S.; Guezennec, A.G. Recycling-oriented methodology to sample and characterize the metal composition of waste Printed Circuit Boards. Waste Manag. 2019, 91, 62–71. [Google Scholar] [CrossRef]
- Petter, P.; Veit, H.M.; Bernardes, A.M. Evaluation of gold and silver leaching from printed circuit board of cellphones. Waste Manag. 2014, 34, 475–482. [Google Scholar] [CrossRef]
- Sahajwalla, V.; Pahlevani, F.; Maroufi, S.; Rajarao, R. Green Manufacturing: A Key to Innovation Economy. J. Sustain. Met. 2016, 2, 273–275. [Google Scholar] [CrossRef]
- Huisman, J.; Stevels, A.; Baldé, K.; Magalini, F.; Kuehr, R. Chapter 4—The e-waste development cycle, part III—Policy & legislation, business & finance, and technologies & skills. In Waste Electrical and Electronic Equipment (WEEE) Handbook, 2nd ed.; Goodship, V., Stevels, A., Huisman, J., Eds.; Woodhead Publishing: Cambridge, UK, 2019; pp. 93–141. [Google Scholar]
- Ebin, B.; Isik, M.I. Chapter 5—Pyrometallurgical Processes for the Recovery of Metals from WEEE. In WEEE Recycling; Chagnes, A., Ed.; Elsevier: Amsterdam, The Netherlands, 2016; pp. 107–137. [Google Scholar]
- Cui, J.; Zhang, L. Metallurgical recovery of metals from electronic waste: A review. J. Hazard. Mater. 2008, 158, 228–256. [Google Scholar] [CrossRef]
- Andrews, D.; Raychaudhuri, A.; Frias, C. Environmentally sound technologies for recycling secondary lead. J. Power Sources 2000, 88, 124–129. [Google Scholar] [CrossRef]
- Yazici, E.; Deveci, H. E-atıklardan Metallerin Geri Kazanımı (Recovery of Metals from E-wastes). Madencilik 2009, 48, 3–18. [Google Scholar]
- Tuncuk, A.; Stazi, V.; Akcil, A.; Yazici, E.Y.; Deveci, H. Aqueous metal recovery techniques from e-scrap: Hydrometallurgy in recycling. Miner. Eng. 2012, 25, 28–37. [Google Scholar] [CrossRef]
- Kamberović, Ž.; Korać, M.; Ivšić, D.; Nikolić, V.; Ranitović, M. Hydrometallurgical process for extraction of metals from electronic waste—Part I: Material characterization and process option selection. Metal. J. Metall. 2009, 15, 231–245. [Google Scholar] [CrossRef]
- Namias, J. The Future of Electronic Waste Recycling in the United States: Obstacles and Domestic Solutions. Master’s Thesis, Columbia University, New York, NY, USA, 2013. [Google Scholar]
- Birloaga, I.; De Michelis, I.; Ferella, F.; Buzatu, M.; Veglio, F. Study on the influence of various factors in the hydrometallurgical processing of waste printed circuit boards for copper and gold recovery. Waste Manag. 2013, 33, 935–941. [Google Scholar] [CrossRef] [PubMed]
- Gloe, K.; Mühl, P.; Knothe, M. Recovery of precious metals from electronic scrap, in particular from waste products of the thick-layer technique. Hydrometallurgy 1990, 25, 99–110. [Google Scholar] [CrossRef]
- Baba, H. An efficient recovery of gold and other noble metals from electronic and other scraps. Conserv. Recycl. 1987, 10, 247–252. [Google Scholar] [CrossRef]
- Kołodzziej, B.; Adamski, Z. A ferric chloride hydrometallurgical process for recovery of silver from electronic scrap materials. Hydrometallurgy 1984, 12, 117–127. [Google Scholar] [CrossRef]
- Kononova, O.; Patrushev, V.; Kononov, Y. Recovery of noble metals from refractory sulfide black-shale ores. Hydrometallurgy 2014, 144, 156–162. [Google Scholar] [CrossRef]
- Hilson, G.; Monhemius, A. Alternatives to cyanide in the gold mining industry: What prospects for the future? J. Clean. Prod. 2006, 14, 1158–1167. [Google Scholar] [CrossRef]
- Konyratbekova, S.S.; Baikonurova, A.; Akcil, A. Non-cyanide Leaching Processes in Gold Hydrometallurgy and Iodine-Iodide Applications: A Review. Miner. Process. Extr. Met. Rev. 2014, 36, 198–212. [Google Scholar] [CrossRef]
- Dönmez, B.; Sevim, F.; Colak, S. A Study on Recovery of Gold from Decopperized Anode Slime. Chem. Eng. Technol. 2001, 24, 91–95. [Google Scholar] [CrossRef]
- Gökelma, M.; Birich, A.; Stopic, S.; Friedrich, B. A Review on Alternative Gold Recovery Re-agents to Cyanide. J. Mater. Sci. Chem. Eng. 2016, 4, 10. [Google Scholar]
- Aylmore, M.; Muir, D. Thiosulfate leaching of gold—A review. Miner. Eng. 2001, 14, 135–174. [Google Scholar] [CrossRef]
- Sheng, P.P.; Etsell, T.H. Recovery of gold from computer circuit board scrap using aqua regia. Waste Manag. Res. 2007, 25, 380–383. [Google Scholar] [CrossRef] [PubMed]
- Liang, G.; Tang, J.; Liu, W.; Zhou, Q. Optimizing mixed culture of two acidophiles to improve copper recovery from printed circuit boards (PCBs). J. Hazard. Mater. 2013, 250, 238–245. [Google Scholar] [CrossRef] [PubMed]
- Ilyas, S.; Ruan, C.; Bhatti, H.N.; Ghauri, M.; Anwar, M. Column bioleaching of metals from electronic scrap. Hydrometallurgy 2010, 101, 135–140. [Google Scholar] [CrossRef]
- Faramarzi, M.A.; Stagars, M.; Pensini, E.; Krebs, W.; Brandl, H. Metal solubilization from metal-containing solid materials by cyanogenic Chromobacterium violaceum. J. Biotechnol. 2004, 113, 321–326. [Google Scholar] [CrossRef]
- Morin, D.; Lips, A.; Pinches, T.; Huisman, J.; Frias, C.; Norberg, A.; Forssberg, E. BioMinE—Integrated project for the development of biotechnology for metal-bearing materials in Europe. Hydrometallurgy 2006, 83, 69–76. [Google Scholar] [CrossRef]
- Mishra, D.; Kim, D.J.; Ahn, J.G.; Rhee, Y.H. Bioleaching: A microbial process of metal recovery. Met. Mater. Int. 2005, 11, 249–256. [Google Scholar] [CrossRef]
- Pradhan, J.K.; Kumar, S. Metals bioleaching from electronic waste by Chromobacterium violaceum and Pseudomonads sp. Waste Manag. Res. 2012, 30, 1151–1159. [Google Scholar] [CrossRef]
- Hong, Y.; Valix, M. Bioleaching of electronic waste using acidophilic sulfur oxidising bacteria. J. Clean. Prod. 2014, 65, 465–472. [Google Scholar] [CrossRef]
- Rozas, E.E.; Mendes, M.A.; Nascimento, C.A.; Espinosa, D.C.R.; Oliveira, R.; Oliveira, G.; Custodio, M.R. Bioleaching of electronic waste using bacteria isolated from the marine sponge Hymeniacidon heliophila (Porifera). J. Hazard. Mater. 2017, 329, 120–130. [Google Scholar] [CrossRef]
- Natarajan, G.; Ting, Y.P. Gold biorecovery from e-waste: An improved strategy through spent medium leaching with pH modification. Chemosphere 2015, 136, 232–238. [Google Scholar] [CrossRef] [PubMed]
- Baniasadi, M.; Vakilchap, F.; Bahaloo-Horeh, N.; Mousavi, S.M.; Farnaud, S. Advances in bioleaching as a sustainable method for metal recovery from e-waste: A review. J. Ind. Eng. Chem. 2019, 76, 75–90. [Google Scholar] [CrossRef]
- Valix, M. 18—Bioleaching of Electronic Waste: Milestones and Challenges. In Current Developments in Biotechnology and Bioengineering; Wong, J.W.C., Tyagi, R.D., Pandey, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 407–442. [Google Scholar]
- Chatterjee, A.; Abraham, J. Efficient management of e-wastes. Int. J. Environ. Sci. Technol. 2016, 14, 211–222. [Google Scholar] [CrossRef]
- Volesky, B. Removal and recovery of heavy metals by biosorption. In Biosorption of Heavy Metals; Volesky, B., Ed.; CRC Press: Boca Raton, FL, USA, 1973; pp. 7–39. [Google Scholar]
- De Vargas, I.; Macaskie, L.; Guibal, E. Biosorption of palladium and platinum by sulfate-reducing bacteria. J. Chem. Technol. Biotechnol. 2003, 79, 49–56. [Google Scholar] [CrossRef]
- Mack, C.; Wilhelmi, B.; Duncan, J.; Burgess, J. Biosorption of precious metals. Biotechnol. Adv. 2007, 25, 264–271. [Google Scholar] [CrossRef]
- Schiewer, S.; Volesky, B. Ionic Strength and Electrostatic Effects in Biosorption of Divalent Metal Ions and Protons. Environ. Sci. Technol. 1997, 31, 2478–2485. [Google Scholar] [CrossRef]
- Schiewer, S.; Volesky, B. Modeling Multi-Metal Ion Exchange in Biosorption. Environ. Sci. Technol. 1996, 30, 2921–2927. [Google Scholar] [CrossRef]
- Schiewer, S.; Volesky, B. Ionic Strength and Electrostatic Effects in Biosorption of Protons. Environ. Sci. Technol. 1997, 31, 1863–1871. [Google Scholar] [CrossRef]
- Choudhary, B.; Paul, D.; Borse, A.U.; Garole, D.J. Surface functionalized biomass for adsorption and recovery of gold from electronic scrap and refinery wastewater. Sep. Purif. Technol. 2018, 195, 260–270. [Google Scholar] [CrossRef]
- Sheel, A.; Pant, D. Recovery of gold from electronic waste using chemical assisted microbial biosorption (hybrid) technique. Bioresour. Technol. 2018, 247, 1189–1192. [Google Scholar] [CrossRef]
- Sinha, R.; Chauhan, G.; Singh, A.; Kumar, A.; Acharya, S. A novel eco-friendly hybrid approach for recovery and reuse of copper from electronic waste. J. Environ. Chem. Eng. 2018, 6, 1053–1061. [Google Scholar] [CrossRef]
- Harikrushnan, B.; Shreyass, G.; Hemant, G.; Pandimadevi, M. Recovery of metals from printed circuit boards (pcbs) using a combination of hydrometallurgical and biometallurgical processes. Int. J. Environ. Res. 2016, 10, 511–518. [Google Scholar]
- Ganguly, M.; Tao, Y.; Lee, B.; Ariya, P.A. Natural Kaolin: Sustainable Technology for the Instantaneous and Energy-Neutral Recycling of Anthropogenic Mercury Emissions. ChemSusChem 2019, 13, 165–172. [Google Scholar] [CrossRef] [PubMed]
- Connie, Z.Y.; Ariya, P.A. Co-adsorption of gaseous benzene, toluene, ethylbenzene, m-xylene (BTEX) and SO2 on recyclable Fe3O4 nanoparticles at 0–101% relative humidities. J. Environ. Sci. 2015, 31, 164–174. [Google Scholar]
- Hu, Z.; Kurien, U.; Murwira, K.; Ghoshdastidar, A.; Nepotchatykh, O.; Ariya, P.A. Development of a Green Technology for Mercury Recycling from Spent Compact Fluorescent Lamps Using Iron Oxides Nanoparticles and Electrochemistry. ACS Sustain. Chem. Eng. 2016, 4, 2150–2157. [Google Scholar] [CrossRef]
- Eltouny, N.; Ariya, P.A. Enhanced Reactivity toward Oxidation by Water Vapor: Interactions of Toluene and NO2 on Hydrated Magnetite Nanoparticles. J. Phys. Chem. C. 2014, 118, 23654–23663. [Google Scholar] [CrossRef]
- Eltouny, N.; Ariya, P.A. Competing reactions of selected atmospheric gases on Fe3O4 nanoparticles surfaces. Phys. Chem. Chem. Phys. 2014, 16, 23056–23066. [Google Scholar] [CrossRef]
- Eltouny, N.; Ariya, P.A. Fe3O4 Nanoparticles and Carboxymethyl Cellulose: A Green Option for the Removal of Atmospheric Benzene, Toluene, Ethylbenzene, and o-Xylene (BTEX). Ind. Eng. Chem. Res. 2012, 51, 12787–12795. [Google Scholar] [CrossRef]
- Ganguly, M.; Dib, S.; Ariya, P.A. Fast, Cost-effective and Energy Efficient Mercury Removal-Recycling Technology. Sci. Rep. 2018, 8, 1–10. [Google Scholar] [CrossRef]
- Rocha, A.A.; Wilde, C.; Hu, Z.; Nepotchatykh, O.; Nazarenko, Y.; Ariya, P.A. Development of a hybrid photo-bioreactor and nanoparticle adsorbent system for the removal of CO2, and selected organic and metal co-pollutants. J. Environ. Sci. 2017, 57, 41–53. [Google Scholar] [CrossRef]




| EEE/E-Waste | Content (%w/w) | Content (ppm) | Ref | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Fe | Cu | Al | Pb | Ni | Sn | Plastics | Glass | Ag | Au | Pd | ||
| TV boards | 28 | 10 | 10 | 1.0 | 0.3 | 1.4 | 28 | 6 | 280 | 17 | 10 | [17] |
| PC boards | 7 | 20 | 5 | 1.5 | 1 | 2.9 | 23 | 18 | 1000 | 250 | 110 | [17] |
| Cell phone | 5 | 13 | 1 | 0.3 | 0.1 | 0.5 | 57 | 2 | 1340 | 350 | 210 | [17] |
| Portable audio | 23 | 21 | 1 | 0.14 | 0.03 | 0.1 | 47 | - | 150 | 10 | 4 | [17] |
| DVD | 62 | 5 | 2 | 0.3 | 0.05 | 0.2 | 24 | - | 115 | 15 | 4 | [17] |
| Calculator | 3 | 3 | 5 | 0.1 | 0.5 | 0.2 | 61 | 13 | 260 | 50 | 5 | [17] |
| TV scrap | - | 3.4 | 1.2 | 0.2 | 0.038 | - | - | - | 20 | <10 | <6 | [18] |
| PC scrap | - | 14.3 | 2.8 | 2.2 | 1.1 | - | - | - | 639 | 566 | - | [19] |
| PCBs scrap | - | 10 | 7 | 1.2 | 0.85 | - | - | - | 280 | 110 | - | [20] |
| Components | Typical Sources | Effects on Humans | Ref. |
|---|---|---|---|
| Mercury | Thermostats, sensors, monitors, printed circuit boards, cathode ray tubes, fluorescent lamps, etc. | Causes chronic brain damage. | [26,27] |
| Lead | Printed circuit boards, Cathode ray tubes, light bulbs, and batteries. | Harms nervous system, blood system, and kidneys; affects brain development of children. | [26,27] |
| Cadmium | Switches, springs, connectors, printed circuit boards, semiconductor chips, photocopy machines, cathode ray tubes, mobile phones, etc. | Respiratory irritation, chronic lung disease, toxicity to kidneys, etc. | [26,28] |
| Chromium | Anticorrosion coatings, data tapes, etc. | Strong allergic reactions such as asthmatic bronchitis, can cause DNA damage. | [26,27] |
| Barium | Cathode ray tubes and florescent lamps | Causes brain swelling, muscle weakness, damage to heart, liver, and spleen | [26,27] |
| Beryllium | Power supply boxes, computers, X-ray machines, ceramic parts of electronics, etc. | May cause lung cancer and skin diseases | [26,27] |
| Arsenic | Doping agents in transistors and printed wiring boards | Skin ailment as well as decrease nerve conduction velocity, and lung cancer. | [27] |
| Brominated flame retardants (BFRs) Polybrominated diphenyl ethers (PBDEs) | Fire retardants for electronic equipment | Affects growth hormones, sexual development, immune systems, and brain development in animals | [26,29] |
| Polychlorinated biphenyls (PCBs) | Dielectric fluids, lubricants, coolants in generators, capacitors, and transformers, fluorescent lights, ceiling fan, dishwashers, and electric motors | Affects immune hormone, nervous, and enzyme systems. Probable carcinogens for humans. | [26,27] |
| Polychlorinated dibenzo-dioxins (PCDDs) Polychlorinated dibenzofurans (PCDFs) | Released as byproducts in open combustion. | Induces chloracne, increases cholesterol levels, decreases testosterone levels, increases the chances of diabetes. | [26,30] |
| Polycyclic aromatic hydrocarbons (PAHs) | Released as combustion byproducts | increases risk of skin, lung, and bladder cancer | [26,31] |
| Material | Amount in E-Waste (in kt) | Potential Value (in Million USD) |
|---|---|---|
| Iron/steel | 20,466 | 24,645 |
| Copper | 1808 | 10,960 |
| Aluminum | 3046 | 6062 |
| Gold | 0.2 | 9481 |
| Silver | 1.2 | 579 |
| Palladium | 0.1 | 3532 |
| Antimony | 76 | 644 |
| Cobalt | 13 | 1036 |
| Total | 25,410 | 56,939 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ghimire, H.; Ariya, P.A. E-Wastes: Bridging the Knowledge Gaps in Global Production Budgets, Composition, Recycling and Sustainability Implications. Sustain. Chem. 2020, 1, 154-182. https://doi.org/10.3390/suschem1020012
Ghimire H, Ariya PA. E-Wastes: Bridging the Knowledge Gaps in Global Production Budgets, Composition, Recycling and Sustainability Implications. Sustainable Chemistry. 2020; 1(2):154-182. https://doi.org/10.3390/suschem1020012
Chicago/Turabian StyleGhimire, Hem, and Parisa A. Ariya. 2020. "E-Wastes: Bridging the Knowledge Gaps in Global Production Budgets, Composition, Recycling and Sustainability Implications" Sustainable Chemistry 1, no. 2: 154-182. https://doi.org/10.3390/suschem1020012
APA StyleGhimire, H., & Ariya, P. A. (2020). E-Wastes: Bridging the Knowledge Gaps in Global Production Budgets, Composition, Recycling and Sustainability Implications. Sustainable Chemistry, 1(2), 154-182. https://doi.org/10.3390/suschem1020012






