Does Polluted Air Increase COVID-19 Severity? A Critical Review of the Evidence and Proposals to Clarify a Potentially Dramatic Interaction
Abstract
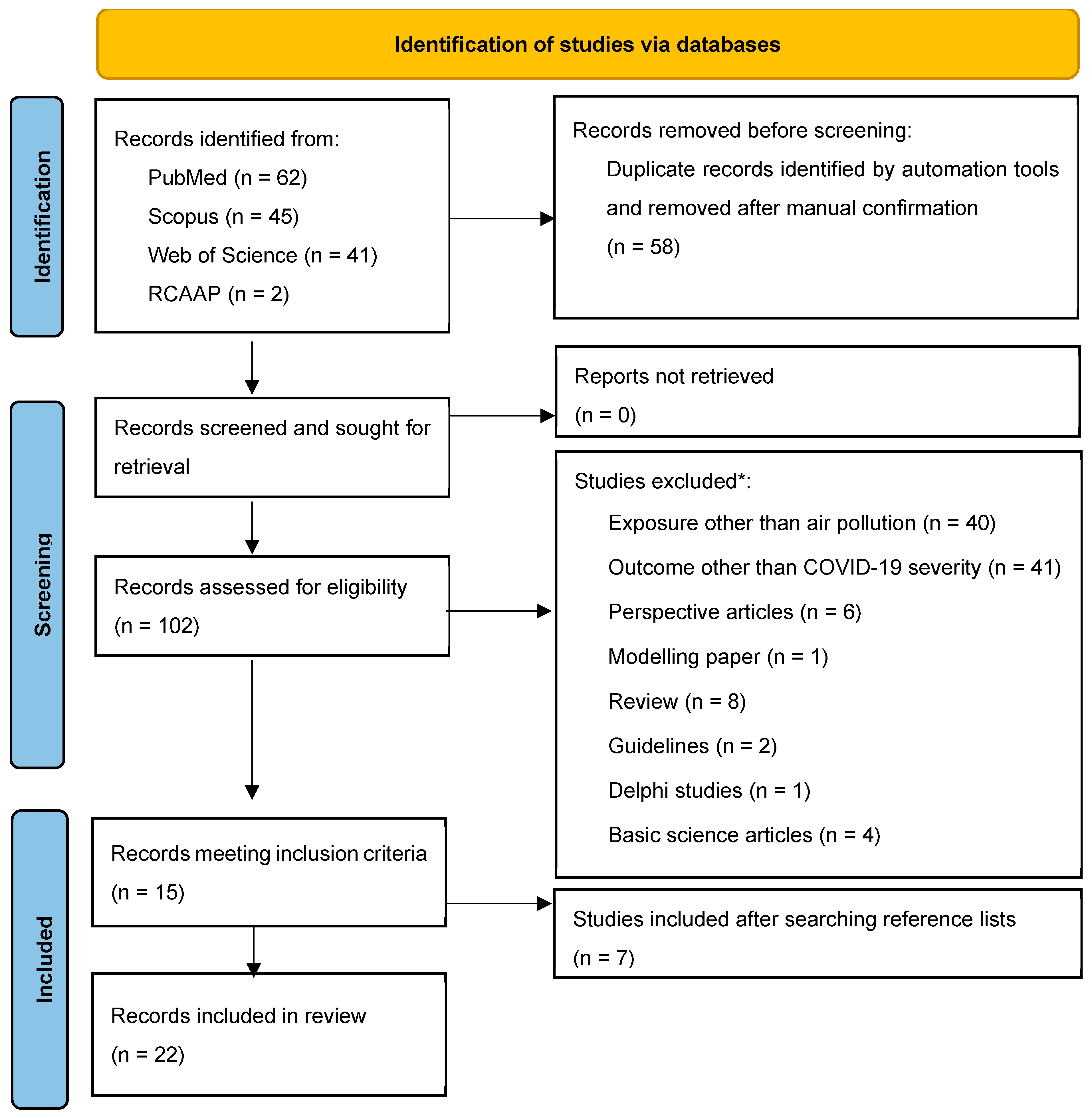
1. Introduction
2. Research Questions
- -
- Among individuals infected with SARS-CoV-2, does exposure to higher levels of long-term outdoor air pollution worsen the course of infection and disease?
- -
- Among individuals infected with SARS-CoV-2, does exposure to higher levels of short-term outdoor air pollution worsen the course of infection and disease?
2.1. Measurement Issues: Diagnostic Tests, Infection Severity, Air Pollution Levels
2.2. Confounding Effects and Possible Strategies to Untangle Them
2.3. What Have We Learnt from Studies Other than Ecological Studies?
| Authors | Publication Year Study Design | Setting | Sample Size | Population | Environmental Exposure (Pollutants, Geographical Resolution and Periods) | Measured Outcome | Adjustment | Findings | Newcastle-Ottawa Scale |
|---|---|---|---|---|---|---|---|---|---|
| Beloconi et al. [33] | 2023 Cohort | Switzerland | n = 28,540 | Hospitalized patients | PM2.5 and NO2 1 sq km 5-year averages | ICU admission Death | Individual comorbidities, Aggregated socio-economics | PM2.5 associated with higher mortality (OR 1.16 [95% CI 1.04–1.28] but not with ICU admission NO2 associated with both (OR 1.17 [95% CI 1.05, 1.30] and 1.15 [1.03, 1.27] Results held only for the first pandemic wave | 8 * |
| Bowe et al. [34] | 2021 Cohort | USA | n = 169,102 | US Armed Forces Veterans COVID-19 cases | PM2.5 1 sq km 2018 averages | Hospitalizations | Individual demographics, race, Aggregated socio-economics | PM2.5 associated with a 10% (95% CI: 8–12%) higher risk of hospitalization | 8 * |
| Bozack et al. [28] | 2022 Cohort | New York City | n = 6542 | Hospitalized patients | PM2.5, black carbon and NO2 1 km radius 2019 averages | Death ICU Intubation | Individual demographics, race, individual insurance | PM2.5 associated with death and ICU admission (RR 1.11 [95% CI 1.02–1.21] and 1.13 [95% CI 1.00–1.28] Black carbon and NO2 were not | 8 * |
| Chen C et al. [35] | 2022 Cohort | Ontario | n = 151,105 | Ontario’s Case and Contact Management System COVID-19 cases | PM2.5, NO2, and O3 average postal code–specific annual concentrations 5-year averages | Death ICU admission Hospitalizations | Individual demographics (age, sex, and race), healthcare access, Aggregated socio-economics | PM2.5 associated with hospital and ICU admission (OR 1.06 [95% CI 1.01–1.12] and death (1.09 [95% CI 0.98–1.21]) O3 associated with all three outcomes (1.15 [95% CI 1.06–1.23], 1.30 [95% CI 1.12–1.50] and 1.18 [95% CI 1.02–1.36] NO2 associated with hospital admission (OR 1.09 [95% CI 0.97–1.21]) | 8 * |
| Elliott J et al. [29] | 2021 Cohort | UK | n = 473,550 (459 COVID deaths) | UK Biobank COVID-19 cases | PM2.5, PM10, and NOx Unspecified spatial resolution 2010 averages | COVID-19 mortality | Individual demographics (age, sex, and ethnicity), comorbidities, and socio-economics | No associations found between pollutants and mortality | 6 * |
| Chen Z et al. [36] | 2022 Cohort | California, USA | n = 75,010 | Kaiser Permanente (insurance company) COVID-19 cases | NOx Residential address 1-month and 1-year (previous to COVID diagnosis) averages | ICU admissions Intensive respiratory support (IRS) Death | Individual demographics (Age, sex, ethnicity), comorbidities, insurance type, Aggregated socio-economics | Exposure to non-freeway near roadway NOx associated with increased risk of IRS and ICU admission [OR (95% CI): 1.07 (1.01, 1.13) and 1.11 (1.04, 1.19), respectively]; increased risk of mortality (HR = 1.10, 95% CI = 1.03, 1.18) | 6 * |
| Hoskovec L et al. [37] | 2022 Cohort | Denver, USA | n= 55,273 | Denver Public Health COVID-19 cases | PM2.5 Address-based inverse distance weighing 2019 averages | ICU admission Death | Individual demographics (age, sex, pregnancy, and ethnicity) Aggregated socio-economic data | Exposure to PM2.5 was associated with an increased risk of being hospitalized (OR 1.24 [95% CI 1.08–1.43]) and admitted to the ICU when combined with high levels of ozone (1.83 [95% CI 1.01–3.33]) and temperature (1.48 [95% CI 1.12–2.00]) | 6 * |
| Hyman S et al. [38] | 2023 Cohort | Manchester, UK | n = 313,657 | Greater Manchester COVID-19 cases | PM2.5, PM10, O3, NO2, SO2, and benzene 1 sq km grid 2019 averages | Hospitalization Death | Individual age, sex, ethnicity, BMI, smoking status, history of comorbidities Area-level socio-economic status | Significant associations with hospital admissions and PM2.5, PM10, NO2 (OR 1.27 [95% CI 1.25–1.30], 1.15 [95% CI 1.13–1.17], and 1.12 [95% CI 1.10–1.14]; death and PM2.5 and PM10 (OR 1.39 [95% CI 1.31–1.48] and 1.23 [95% CI 1.17–1.30]) | 8 * |
| Jerrett M et al. [39] | 2023 Cohort | Southern California, USA | n = 21,415 | Insurance network hospitalized patients | PM2.5, O3, NO2, ultra-fine particulate matter (PM0.1), PM chemical species, and PM sources 1 sq km 5-year averages | Death | Individual age, sex, ethnicity, comorbidities, insurance (Medicaid) Community-level socio-economics | PM2.5 associated with death among hospitalized patients HR = 1.12 [95% CI 1.06, 1.17] | 6 * |
| Lavigne E et al. [24] | 2023 Case-crossover | Alberta and Ontario, Canada | n = 78,255 | Emergency Department visits of COVID-19 cases | PM2.5, O3, NO2 10 sq km Daily averages over a 3-day period | Emergency Department visits | Aggregated sex and age No socio-economic data | Exposure to PM2.5 and NO2 were associated with ED visits for COVID-19 (OR 1.010; 95% CI 1.004 to 1.015 and OR 1.021; 95% CI 1.015 to 1.028) | 4 * |
| Lopez-Feldman et al. [40] | 2021 Cohort | Mexico | No info on sample size | Mexico City confirmed COVID-19 cases | PM2.5 Mexico City municipalities short- (daily) and long-term (2000–2018) | Death | Individual age and sex Aggregated socio-economics | Long-term PM2.5 exposure related to death (p = 0.048) | 7 * |
| Mendy et al. [41] | 2021 Cohort | Ohio | n = 14,783 | COVID-19 cases diagnosed at the University of Cincinnati healthcare system | PM2.5 0.01° × 0.01° grid 2009–2018 averages | COVID-19 hospitalization | Individual sex, age, and comorbidities Aggregated socio-economics | Long-term PM2.5 exposure is associated with increased hospitalization in COVID-19 OR: 1.18, 95% CI: 1.11–1.26 | 7 * |
| Pegoraro et al. [30] | 2021 Cohort | Italy | n = 6483 | COVID-19 cases diagnosed at GP’s in Italy IQVIA Database | PM10 Italian regions 30-day period preceding the Index Date | Pneumonia cases | Individual age, sex, and comorbidities No socio-economic data | PM10 exposure associated with higher likelihood of pneumonia (OR 1.93 [95% CI 1.55–2.39]) | 6 * |
| Ponzano et al. [26] | 2022 Case–control | Italy | n = 49 | Multiple sclerosis patients diagnosed with COVID-19 | PM2.5, PM10, and NO2 resolution not specified 2018–2020 | Pneumonia cases | Individual age, sex, comorbidities, disease type, and treatment No socio-economic data | Higher long-term exposure to PM2.5, PM10, and NO2 increased the odds of COVID-19 pneumonia (OR 2.26 [95% CI 1.29;3.96], 2.12 [95% CI 1.22;3.68], and 2.12 [95% CI 1.22;3.69]) | 8 * |
| Rigolon et al. [42] | 2023 Cohort | Denver, USA | n = 18,042 | COVID-19 cases diagnosed at Uni Colorado health system | PM2.5 Census block groups 2016 | Hospitalization | Individual demographics, race, comorbidities; Aggregated socio-economics | Incidence Rate Ratio for hospitalization 1.19 [95% CI 1.151–1.230] | 7 * |
| Di Ciaula et al. [32] | 2022 Cohort | Apulia, Italy | n = 147 | Hospitalized patients | NO2 and PM2.5 Unspecified spatial resolution 2 weeks before admission | Mortality | Individual demographics, comorbidities No socio-enconomic data | NO2 OR for mortality 1.045 [95% CI 1.003–1.088]; PM2.5 not significant | 7 * |
| Kogevinas et al. [43] | 2021 Cohort | Catalonia | n = 481 | COVID-19 and non-COVID-19 individuals recruited from previous healthy cohorts | PM2.5 100 sq m grids based on hybrid models 2018–2019 averages | Hospitalization | Individual demographics, BMI, comorbidities Aggregated socio-economics | Relative Risk Ratio for hospitalization 1.83 (1.01, 3.31) for NO2; 2.12 (1.13, 3.96) | 7 * |
| English et al. [44] | 2022 Cohort | California, USA | n = 3.1 million (n = 49,691 deaths) | COVID-19 cases obtained from the California Department of Public Health | PM2.5 1 sq km 2000–2018 averages | Death | Individual demographics Aggregated race/ethnicity and socio-economics | Individuals living in the highest quintile of exposure had mortality risks 51% higher than those in the lowest quintile | 7 * |
| Bronte et al. [45] | 2023 Cohort | Spain | n = 1548 | Hospitalized patients | PM10, PM2.5, O3, NO2, NO, and NOx Spatial resolution not specified 2019 averages | Death C Reactive Protein levels PaO2/FiO2 | Individual demographics and comorbidities Aggregated socio-economics | PM10, NO2, NO, and NOx increased risk of death (5.33%, 3.59%, 10.79%, and 2.24% p < 0.05) | 7 * |
| Zhang et al. [27] | 2023 Cohort | Danmark | n = 3.7 million (n = 138,742 infections) | Nationwide cohort | NO2, PM2.5, PM10, black carbon, and O3 1 sq km complex land model 1979–2019 averages | Hospitalization Death | Individual age and sex, individual and aggregated socio-economics No comorbidities | PM2.5 and NO2 associated with hospitalizations (HR 1.09 (95% CI 1.01–1.17) and HR 1.19 (95% CI 1.12–1.27) and death (HR 1.23 (95% CI 1.04–1.44) and HR 1.18 (95% CI 1.03–1.34) | 9 * |
| Kim H et al. [25] | 2022 Case-crossover | Cook County, USA | n = 7462 | COVID-19 death reports | PM2.5 and O3 Inverse-distance weighing interpolation 21 days before death | Death | Individual age, sex, race, and comorbidities No socio-economic data | Short-term increases in PM2.5 and O3 associated with increased risk of death (69.3% [95% confidence interval (CI): 34.6, 113.8] and 29.0% (95% CI: 9.9, 51.5), respectively) | 7 * |
| Izadi et al. [31] | 2022 Cohort | 23 countries | n = 14,044 | Global Rheumatic Alliance Registry | Average monthly PM2.5 Country and US state | COVID-19 mortality | Individual demographics, comorbidities; characteristics of rheumatic disease; No socio-enconomic adjustment | PM2.5 increased odds of death (OR 1·10 per 10 μg/m3 [95% CI 1·01–1·17]) | 7 * |
2.4. Beyond Ecological and Large Registry-Based Cohort Studies
3. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Ambient (Outdoor) Air Pollution. 2022. Available online: https://www.who.int/news-room/fact-sheets/detail/ambient-(outdoor)-air-quality-and-health (accessed on 27 April 2024).
- Global Burden of Disease Collaboration Network. In Global Burden of Disease Study 2017 (GBD 2017) Results; Institute for Health Metrics and Evaluation: Seattle, WA, USA, 2018.
- Burnett, R.; Chen, H.; Szyszkowicz, M.; Fann, N.; Hubbell, B.; Pope, C.A., 3rd; Apte, J.S.; Brauer, M.; Cohen, A.; Weichenthal, S.; et al. Global Estimates of Mortality associated with long-term exposure to outdoor fine particulate matter. Proc. Natl. Acad. Sci. USA 2018, 115, 9592–9597. [Google Scholar] [CrossRef]
- Horn, S.A.; Dasgupta, P.K. The Air Quality Index (AQI) in historical and analytical perspective a tutorial review. Talanta 2024, 267, 125260. [Google Scholar] [CrossRef]
- Dominski, F.H.; Lorenzetti Branco, J.H.; Buonanno, G.; Stabile, L.; Gameiro da Silva, M.; Andrade, A. Effects of air pollution on health: A mapping review of systematic reviews and meta-analyses. Environ. Res. 2021, 201, 111487. [Google Scholar] [CrossRef] [PubMed]
- Barnett-Itzhaki, Z.; Levi, A. Effects of chronic exposure to ambient air pollutants on COVID-19 morbidity and mortality—A lesson from OECD countries. Environ. Res. 2021, 195, 110723. [Google Scholar] [CrossRef]
- Wang, B.; Eum, K.-D.; Kazemiparkouhi, F.; Li, C.; Manjourides, J.; Pavlu, V.; Suh, H. The impact of long-term PM2.5 exposure on specific causes of death: Exposure-response curves and effect modification among 53 million U.S. Medicare beneficiaries. Environ. Health 2020, 19, 20. [Google Scholar]
- Pozzer, A.; Dominici, F.; Haines, A.; Witt, C.; Münzel, T.; Lelieveld, J. Long-term exposure to fine particulate matter air pollution: An ecological study of its effect on COVID-19 cases and fatalities in Germany. Environ. Res. 2021, 204, 111948. [Google Scholar] [CrossRef]
- Hernandez-Carballo, I.; Bakola, M.; Stuckler, D. The impact of air pollution on COVID-19 incidence, severity, and mortality: A systematic review of studies in Europe and North America. Environ. Res. 2022, 215 Pt 1, 114155. [Google Scholar] [CrossRef] [PubMed]
- Wakefield, J. Ecologic studies revisited. Annu. Rev. Public. Health 2008, 29, 75–90. [Google Scholar] [CrossRef] [PubMed]
- Villeneuve, P.; Goldberg, M. Ecological studies of COVID-19 and air pollution: How useful are they? Environ. Epidemiol. 2022, 6, e195. [Google Scholar] [CrossRef]
- Mertens, T.E.; Hayes, R.J.; Smith, P.G. Epidemiological methods to study the interaction between HIV infection and other sexually transmitted diseases. AIDS 1990, 4, 57–65. [Google Scholar] [CrossRef]
- Kabir, A.; Ahmed, R.; Iqbal, S.M.A.; Chowdhury, R.; Paulmurugan, R.; Demirci, U.; Asghar, W. Diagnosis for COVID-19: Current status and future prospects. Expert. Rev. Mol. Diagn. 2021, 21, 269–288. [Google Scholar] [CrossRef] [PubMed]
- Bobrovitz, N.; Ware, H.; Ma, X.; Li, Z.; Hosseini, R.; Cao, C.; Selemon, A.; Whelan, M.; Premji, Z.; Issa, H.; et al. Protective effectiveness of previous SARS-CoV-2 infection and hybrid immunity against the omicron variant and severe disease: A systematic review and meta-regression. Lancet Infect. Dis. 2023, 23, 556–567. [Google Scholar] [CrossRef]
- Karagulian, F.; Gerboles, M.; Barbiere, M.; Kotsev, A.; Lagler, F.; Borowiak, A. Review of Sensors for Air Quality Monitoring; JRC Technical Reports; European Commission: Brussels, Belgium, 2019. [Google Scholar]
- Korhonen, A.; Lehtomäki, H.; Rumrich, I.; Karvosenoja, N.; Paunu, V.-V.; Kupiainen, K.; Sofiev, M.; Palamarchuk, Y.; Kukkonen, J.; Kangas, L.; et al. Influence of spatial resolution on population PM2.5 exposure and health impacts. Air Qual. Atmos. Health 2019, 12, 705–718. [Google Scholar] [CrossRef]
- Cousens, S.N.; Mertens, T.E.; Kirkwood, B.R.; Smith, P.G.; Feachem, R.G. Case-Control Studies of Common Childhood Diseases; Macmillan: London, UK, 1995; pp. 14–15. [Google Scholar]
- Shavers, V.L. Measurement of socioeconomic status in health disparities research. J. Natl. Med. Assoc. 2007, 99, 1013–1023. [Google Scholar] [PubMed]
- Magesh, S.; John, D.; Li, W.T.; Li, Y.; Mattingly-App, A.; Jain, S.; Chang, E.Y.; Ongkeko, W.M. Disparities in COVID-19 outcomes by race, ethnicity, and socioeconomic status: A systematic review and meta-analysis. JAMA Netw. Open 2021, 4, e2134147. [Google Scholar] [CrossRef]
- Blakely, T.; Hunt, D.; Woodward, A. Confounding by socioeconomic position remains after adjusting for neighborhood deprivation: An example using smoking and mortality. J. Epidemiol. Community Health 2004, 58, 1030–1031. [Google Scholar] [CrossRef]
- Mertens, T.E. Estimating the effects of misclassification. Lancet 1993, 42, 418–421. [Google Scholar] [CrossRef]
- Sheppard, L.; Burnett, R.T.; Szpiro, A.A.; Kim, S.-Y.; Jerrett, M.; Pope, C.A.; Brunekreef, B. Confounding and exposure measurement error in air pollution epidemiology. Air Qual. Atmos. Health 2012, 5, 203–216. [Google Scholar] [CrossRef]
- Ratshikhopha, E.; Muvhali, M.; Naicker, N.; Tlotleng, N.; Jassat, W.; Singh, T. Disease severity and comorbidities among healthcare worker COVID-19 admissions in South Africa: A retrospective analysis. Int. J. Environ. Res. Public. Health 2022, 19, 5519. [Google Scholar] [CrossRef] [PubMed]
- Lavigne, E.; Ryti, N.; Gasparrini, A.; Sera, F.; Weichenthal, S.; Chen, H.; To, T.; Evans, G.J.; Sun, L.; Dheri, A.; et al. Short-term exposure to ambient air pollution and individual emergency department visits for COVID-19: A case-crossover study in Canada. Thorax 2023, 78, 459–466. [Google Scholar] [CrossRef]
- Kim, H.; Samet, J.M.; Bell, M.L. Association between Short-Term Exposure to Air Pollution and COVID-19 Mortality: A Population-Based Case-Crossover Study Using Individual-Level Mortality Registry Confirmed by Medical Examiners. Environ. Health Perspect. 2022, 130, 117006. [Google Scholar] [CrossRef]
- Ponzano, M.; Schiavetti, I.; Bergamaschi, R.; Pisoni, E.; Bellavia, A.; MuSC-19 Study Group. The impact of PM2.5, PM10, and NO2 on COVID-19 severity in patients with multiple sclerosis: A case-control study. Mult. Scler. Relat. Disord. 2022, 68, 104243. [Google Scholar] [CrossRef]
- Zhang, J.; Lim, Y.-H.; So, R.; Jørgensen, J.T.; Mortensen, L.H.; Napolitano, G.M.; Cole-Hunter, T.; Loft, S.; Bhatt, S.; Hoek, G.; et al. Long-term exposure to air pollution and risk of SARS-CoV-2 infection and COVID-19 hospitalization or death: Danish nationwide cohort study. Eur. Respir. J. 2023, 62, 2300280. [Google Scholar] [CrossRef]
- Bozack, A.; Pierre, S.; DeFelice, N.; Colicino, E.; Jack, D.; Chillrud, S.N.; Rundle, A.; Astua, A.; Quinn, J.W.; McGuinn, L.; et al. Long-Term Air Pollution Exposure and COVID-19 Mortality: A Patient-Level Analysis from New York City. Am. J. Respir. Crit. Care Med. 2022, 205, 651–662. [Google Scholar] [CrossRef]
- Elliott, J.; Bodinier, B.; Whitaker, M.; Delpierre, C.; Vermeulen, R.; Tzoulaki, I.; Elliott, P.; Chadeau-Hyam, M. COVID-19 mortality in the UK Biobank cohort: Revisiting and evaluating risk factors. Eur. J. Epidemiol. 2021, 36, 299–309. [Google Scholar] [CrossRef]
- Pegoraro, V.; Heiman, F.; Levante, A.; Urbinati, D.; Peduto, I. An Italian individual-level data study investigating the association between air pollution exposure and COVID-19 severity in primary-care settings. BMC Public Health 2021, 21, 902. [Google Scholar]
- Izadi, Z.; A Gianfrancesco, M.; Schmajuk, G.; Jacobsohn, L.; Katz, P.; Rush, S.; Ja, C.; Taylor, T.; Shidara, K.; I Danila, M.; et al. COVID-19 Global Rheumatology Alliance Registry. Environmental and societal factors associated with COVID-19-related death in people with rheumatic disease: An observational study. Lancet Rheumatol. 2022, 4, e603–e613. [Google Scholar] [CrossRef] [PubMed]
- Di Ciaula, A.; Bonfrate, L.; Portincasa, P.; IMC-19 Group; Appice, C.; Belfiore, A.; Binetti, M.; Cafagna, G.; Campanale, G.; Carrieri, A.; et al. Nitrogen dioxide pollution increases vulnerability to COVID-19 through altered immune function. Environ. Sci. Pollut. Res. Int. 2022, 29, 44404–44412. [Google Scholar] [CrossRef] [PubMed]
- Beloconi, A.; Vounatsou, P. Long-term air pollution exposure and COVID-19 case-severity: An analysis of individual-level data from Switzerland. Environ. Res. 2023, 216 Pt 1, 114481. [Google Scholar] [CrossRef] [PubMed]
- Bowe, B.; Xie, Y.; Gibson, A.K.; Cai, M.; van Donkelaar, A.; Martin, R.V.; Burnett, R.; Al-Aly, Z. Ambient fine particulate matter air pollution and the risk of hospitalization among COVID-19 positive individuals: Cohort study. Environ. Int. 2021, 154, 106564. [Google Scholar] [CrossRef]
- Chen, C.; Wang, J.; Kwong, J.; Kim, J.; van Donkelaar, A.; Martin, R.V.; Hystad, P.; Su, Y.; Lavigne, E.; Kirby-McGregor, M.; et al. Association between long-term exposure to ambient air pollution and COVID-19 severity: A prospective cohort study. CMAJ 2022, 194, E693–E700. [Google Scholar] [CrossRef]
- Chen, Z.; Huang, B.Z.; Sidell, M.A.; Chow, T.; Eckel, S.P.; Pavlovic, N.; Martinez, M.P.; Lurmann, F.; Thomas, D.C.; Gilliland, F.D.; et al. Near-roadway air pollution associated with COVID-19 severity and mortality—Multiethnic cohort study in Southern California. Environ. Int. 2021, 157, 106862. [Google Scholar] [CrossRef] [PubMed]
- Hoskovec, L.; Martenies, S.; Burket, T.L.; Magzamen, S.; Wilson, A. Association between air pollution and COVID-19 disease severity via Bayesian multinomial logistic regression with partially missing outcomes. Environmetrics 2022, 33, e2751. [Google Scholar] [CrossRef] [PubMed]
- Hyman, S.; Zhang, J.; Andersen, Z.J.; Cruickshank, S.; Møller, P.; Daras, K.; Williams, R.; Topping, D.; Lim, Y.H. Long-term exposure to air pollution and COVID-19 severity: A cohort study in Greater Manchester, United Kingdom. Environ. Pollut. 2023, 327, 121594. [Google Scholar] [CrossRef]
- Jerrett, M.; Nau, C.L.; Young, D.R.; Butler, R.K.; Batteate, C.M.; Su, J.; Burnett, R.T.; Kleeman, M.J. Air pollution and meteorology as risk factors for COVID-19 death in a cohort from Southern California. Environ. Int. 2023, 171, 107675. [Google Scholar] [CrossRef]
- Lopez-Feldman, A.; Heres, D.; Marquez-Padilla, F. Air pollution exposure and COVID-19: A look at mortality in Mexico City using individual-level data. Sci. Total Environ. 2021, 756, 143929. [Google Scholar] [CrossRef] [PubMed]
- Mendy, A.; Wu, X.; Keller, J.L.; Fassler, C.S.; Apewokin, S.; Mersha, T.B.; Xie, C.; Pinney, S.M. Air pollution and the pandemic: Long-term PM2.5 exposure and disease severity in COVID-19 patients. Respirology 2021, 26, 1181–1187. [Google Scholar] [CrossRef]
- Rigolon, A.; Németh, J.; Anderson-Gregson, B.; Miller, A.R.; deSouza, P.; Montague, B.; Hussain, C.; Erlandson, K.M.; Rowan, S.E. The neighborhood built environment and COVID-19 hospitalizations. PLoS ONE 2023, 18, e0286119. [Google Scholar] [CrossRef]
- Kogevinas, M.; Karachaliou, M.; Espinosa, A.; Aguilar, R.; Castaño-Vinyals, G.; Garcia-Aymerich, J.; Carreras, A.; Cortés, B.; Pleguezuelos, V.; Papantoniou, K.; et al. Long-Term Exposure to Air Pollution and COVID-19 Vaccine Antibody Response in a General Population Cohort (COVICAT Study, Catalonia). Environ. Health Perspect. 2023, 131, 47001. [Google Scholar] [CrossRef]
- English, P.B.; Von Behren, J.; Balmes, J.R.; Boscardin, J.; Carpenter, C.; Goldberg, D.E.; Horiuchi, S.; Richardson, M.; Solomon, G.; Valle, J.; et al. Association between long-term exposure to particulate air pollution with SARS-CoV-2 infections and COVID-19 deaths in California, USA. Environ. Adv. 2022, 9, 100270. [Google Scholar] [CrossRef]
- Bronte, O.; García-García, F.; Lee, D.J.; Urrutia, I.; Uranga, A.; Nieves, M.; Martínez-Minaya, J.; Quintana, J.M.; Arostegui, I.; Zalacain, R.; et al. Impact of outdoor air pollution on severity and mortality in COVID-19 pneumonia. Sci. Total Environ. 2023, 894, 164877. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Hsueh, L. Do wildfires exacerbate COVID-19 infections and deaths in vulnerable communities? Evidence from California. J. Environ. Manage. 2023, 328, 116918. [Google Scholar] [CrossRef] [PubMed]
- Behndig, A.F.; Mudway, I.S.; Brown, J.L.; Stenfors, N.; Helleday, R.; Duggan, S.T.; Wilson, S.J.; Boman, C.; Cassee, F.R.; Frew, A.J.; et al. Airway antioxidant and inflammatory responses to diesel exhaust exposure in healthy humans. Eur. Respir. J. 2006, 27, 359–365. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Liu, S. The Effects and Pathogenesis of PM2.5 and Its Components on Chronic Obstructive Pulmonary Disease. Int. J. Chronic Obstr. Pulm. Dis. 2023, 18, 493–506. [Google Scholar] [CrossRef]
- Lu, C.; Wang, F.; Liu, Q.; Deng, M.; Yang, X.; Ma, P. Effect of NO2 exposure on airway inflammation and oxidative stress in asthmatic mice. J. Hazard. Mater. 2023, 457, 131787. [Google Scholar] [CrossRef]
- Yang, L.; Li, C.; Tang, X. The Impact of PM2.5 on the Host Defense of Respiratory System. Front. Cell Dev. Biol. 2020, 8, 91. [Google Scholar] [CrossRef]
- Kim, J.H.; Kim, J.; Kim, W.J.; Choi, Y.H.; Yang, S.R.; Hong, S.H. Diesel Particulate Matter 2.5 Induces Epithelial-to-Mesenchymal Transition and Upregulation of SARS-CoV-2 Receptor during Human Pluripotent Stem Cell-Derived Alveolar Organoid Development. Int. J. Env. Res. Public Health 2020, 17, 8410. [Google Scholar] [CrossRef]
- Pope, C.A., 3rd; Bhatnagar, A.; McCracken, J.P.; Abplanalp, W.; Conklin, D.; O’Toole, T. Exposure to Fine Particulate Air Pollution Is Associated With Endothelial Injury and Systemic Inflammation. Circ. Res. 2016, 119, 1204–1214. [Google Scholar] [CrossRef]
- Hajat, A.; Allison, M.; Diez-Roux, A.V.; Jenny, N.S.; Jorgensen, N.W.; Szpiro, A.A.; Vedal, S.; Kaufman, J.D. Long-term exposure to air pollution and markers of inflammation, coagulation, and enodthelial activation: A repeat-measures analysis in the Multi-Ethnic Study of Atherosclerosis (MESA). Epidemiology 2015, 26, 310–320. [Google Scholar] [CrossRef]
- Polidoro, R.; Hagan, R.; Santiago, R.; Schmidt, N. Overview: Systemic inflammatory response derived from lung injury caused by SARS-CoV-2 infection explains severe outcomes in covid-19. Front. Immunol. 2020, 11, 1626. [Google Scholar] [CrossRef]
- Grimes, D.A.; Schulz, K.F. An overview of clinical research: The lay of the land. Lancet 2002, 359, 57–61. [Google Scholar] [CrossRef]
- Xia, Y.; Tong, H. Cumulative effects of air pollution on public health. Stat. Med. 2006, 25, 3548–3559. [Google Scholar] [CrossRef]
- Feng, B.; Lian, J.; Yu, F.; Zhang, D.; Chen, W.; Wang, Q.; Shen, Y.; Xie, G.; Wang, R.; Teng, Y.; et al. Impact of short-term ambient air pollution exposure on the risk of severe COVID-19. J. Environ. Sci. 2024, 135, 610–618. [Google Scholar]
- Linares, C.; Belda, F.; López-Bueno, J.A.; Luna, M.Y.; Sánchez-Martínez, G.; Hervella, B.; Culqui, D.; Díaz, J. Short-term associations of air pollution and meteorological variables on the incidence and severity of COVID-19 in Madrid (Spain): A time series study. Environ. Sci. Eur. 2021, 33, 107. [Google Scholar] [CrossRef] [PubMed]
- Jaakkola, J.J. Case-crossover design in air pollution epidemiology. Eur. Respir. J. Suppl. 2003, 40, 81s–85s. [Google Scholar] [CrossRef] [PubMed]
| Air Pollutant | Anthropogenic Sources | Health Effects a |
|---|---|---|
| Particulate matter (PM) | Motor vehicles Engines Industrial processes Construction sites Unpaved roads Cigarette smoke Biomass burning Agriculture | Cardiovascular disease Cerebrovascular disease Dementia Chronic obstructive lung disease Eye irritation and eye disease Cancer Adverse birth outcomes |
| Nitrogen oxides (NOx) | Fuel-burning motor vehicles Power plants Residential fuel-burning | Reduced lung function Asthma Exacerbation of chronic respiratory and cardiovascular disease Cancer |
| Ozone (O3) | Nitrogen oxides Volatile organic compounds | Lung irritation and damage Aggravated chronic respiratory disease Immune system impairment |
| Sulfur dioxide (SO2) | Petroleum derivates and coal burning Motor vehicles Refineries Power plants Paper mills | Headaches and anxiety Cardiovascular disease |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Almeida, A.; Neves, D.; Serra, S.S.; Mertens, T.E. Does Polluted Air Increase COVID-19 Severity? A Critical Review of the Evidence and Proposals to Clarify a Potentially Dramatic Interaction. World 2025, 6, 133. https://doi.org/10.3390/world6040133
Almeida A, Neves D, Serra SS, Mertens TE. Does Polluted Air Increase COVID-19 Severity? A Critical Review of the Evidence and Proposals to Clarify a Potentially Dramatic Interaction. World. 2025; 6(4):133. https://doi.org/10.3390/world6040133
Chicago/Turabian StyleAlmeida, André, Diana Neves, Sofia Silvério Serra, and Thierry E. Mertens. 2025. "Does Polluted Air Increase COVID-19 Severity? A Critical Review of the Evidence and Proposals to Clarify a Potentially Dramatic Interaction" World 6, no. 4: 133. https://doi.org/10.3390/world6040133
APA StyleAlmeida, A., Neves, D., Serra, S. S., & Mertens, T. E. (2025). Does Polluted Air Increase COVID-19 Severity? A Critical Review of the Evidence and Proposals to Clarify a Potentially Dramatic Interaction. World, 6(4), 133. https://doi.org/10.3390/world6040133







