Anaerobic Co-Digestion of Brewers’ Spent Grain from Craft Beer and Cattle Manure for Biogas Production
Abstract
1. Introduction
2. Materials and Methods
2.1. Biomass Sampling Procedure
2.2. Preparation of Bioreactors
2.3. Inoculum Selection
2.4. Biogas Generation Measurement
2.5. Statistical Analysis
2.6. Kinetic Modeling and Data Evaluation
3. Results and Discussion
3.1. Material Characterization
3.2. Experimental AcoD
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BSG | Brewers’ spent grain |
| AcoD | Anaerobic co-digestion |
| AD | Anaerobic digestion |
| TGA | Thermogravimetric analysis |
| VS | Volatile Solids |
| BMP | Biochemical Methane Potential |
References
- Issah, A.A.; Kabera, T.; Kemausuor, F. Biomass utilization for sustainable energy. Biomass Bioenergy 2020, 133, 105449. [Google Scholar]
- Sarker, A.; Ahmmed, R.; Ahsan, S.M.; Rana, J.; Ghosh, M.K.; Nandi, R. Sustainable food production from waste biomass. Sustain. Food Technol. 2024, 2, 48. [Google Scholar] [CrossRef]
- Kohli, K.; Prajapati, R.; Shah, R.; Das, M.; Sharma, B.K. Biorefining of algal biomass. Sustain. Food Technol. 2024, 2, 70. [Google Scholar] [CrossRef]
- Wellinger, A.; Murphy, J.D.; Baxter, D. The Biogas Handbook: Science, Production and Applications; Elsevier: Amsterdam, The Netherlands, 2013. [Google Scholar]
- Randazzo, A.; Folino, A.; Tassi, F.; Tatàno, F.; de Rosa, S.; Gambioli, A. Volatile organic compounds from green waste anaerobic degradation at lab-scale: Evolution and comparison with landfill gas. Detritus 2022, 19, 63–74. [Google Scholar] [CrossRef]
- López-Aguilar, H.A.; Kennedy Puentes, G.; Lozoya Márquez, L.A.; Chávez Acosta, O.; Monreal Romero, H.A.; López Meléndez, C.; Pérez-Hernández, A. Evaluation of Energy Potential in a Landfill Through the Integration of a Biogas–Solar Photovoltaic System. Urban. Sci. 2025, 9, 17. [Google Scholar] [CrossRef]
- Aguilera, E. Gestión de residuos sólidos urbanos en Nicaragua. Rev. Cient. FAREM-Estelí 2017, 24, 60. [Google Scholar]
- Zhang, Z.; Zhang, G.; Li, W. Hydrogen production from organic waste. Int. J. Hydrogen Energy 2016, 41, 9153. [Google Scholar] [CrossRef]
- Rasmeni, Z.Z.; Madyira, D.M.; Matheri, A.N. Anaerobic digestion modeling. Energy Rep. 2022, 8, 1141. [Google Scholar] [CrossRef]
- Szaja, A.; Montusiewicz, A.; Lebiocka, M. Co-digestion of food waste and sewage sludge. Waste Manag. 2021, 135, 448. [Google Scholar] [CrossRef]
- Tasnim, F.; Iqbal, S.A.; Chowdhury, A.R. Anaerobic treatment performance. Renew. Energy 2017, 109, 434. [Google Scholar] [CrossRef]
- Mirmohamadsadeghi, S.; Karimi, K.; Tabatabaei, M. Pretreatment methods for biogas production. Bioresour. Technol. Rep. 2019, 7, 100202. [Google Scholar]
- Vázquez-Alfaro, M.; Aguilar-Ávila, J.; Palacios-Rangel, M.I. Caracterización del potencial energético de residuos agroindustriales. Nova Sci. 2021, 13, 27. [Google Scholar]
- Galan, B.I.V.; Corrales, S.C.; Avila, M.E.G.; Sidón, G.M. Evaluación de co-sustratos para digestión anaerobia. Acta Univ. 2023, 33, 1. [Google Scholar]
- Malakhova, D.V.; Egorova, M.A.; Prokudina, L.I.; Netrusov, A.I.; Tsavkelova, E.A. Microbial degradation in biogas processes. World J. Microbiol. Biotechnol. 2015, 31, 2015. [Google Scholar]
- Mallen, E.; Najdanovic-Visak, V. Bioconversion of food waste. Bioresour. Technol. Rep. 2018, 1, 16. [Google Scholar]
- Drosou, F.; Kekes, T.; Boukouvalas, C.; Oikonomopoulou, V.; Krokida, M. Thermal processing for sustainable food systems. Sustain. Food Technol. 2024, 2, 1476. [Google Scholar] [CrossRef]
- Bonato, S.V.; de Jesus Pacheco, D.A.; ten Caten, C.S.; Caro, D. Environmental impact of agroindustrial chains. J. Clean. Prod. 2022, 336, 130275. [Google Scholar] [CrossRef]
- Sganzerla, W.G.; Tena, M.; Sillero, L. Valorization of brewery waste. BioEnergy Res. 2023, 16, 2560. [Google Scholar]
- Sganzerla, W.G.; Costa, J.M.; Tena-Villares, M.; Buller, L.S.; Mussatto, S.I.; Forster-Carneiro, T. Dry anaerobic digestion of brewer’s spent grains toward a more sustainable brewery: Operational performance, kinetic analysis, and bioenergy potential. Fermentation 2022, 9, 2. [Google Scholar] [CrossRef]
- Sganzerla, W.; Buller, L.; Mussatto, S. Biogas production from spent grains. J. Clean. Prod. 2021, 297, 126600. [Google Scholar] [CrossRef]
- Gonçalves, I.; Fonseca, A.; Morão, A.; Pinheiro, H.; Duarte, A.; Ferra, M. Use of lignocellulosic biomass in energy systems. Renew. Energy 2015, 74, 489. [Google Scholar] [CrossRef]
- Bougrier, C.; Dognin, D.; Laroche, C.; Gonzalez, V.; Benali-Raclot, D.; Rivero, J. Sludge disintegration efficiency. Bioresour. Technol. 2018, 247, 1193. [Google Scholar]
- Szaja, A.; Montusiewicz, A.; Lebiocka, M. Anaerobic digestion of food waste and sewage sludge. PeerJ 2020, 8, e10590. [Google Scholar] [CrossRef]
- Edunjobi, T.D.; Agbede, O.O.; Aworanti, O.A.; Adebayo, A.O.; Agarry, S.E.; Ogunkunle, O.; Laseinde, O.T. Anaerobic digestion of food waste with cattle dung. Biomass Convers. Biorefin. 2024, 14, 29561. [Google Scholar]
- Nganyira, P.D.; Mahushi, D.J.; Balengayabo, J.G.; Shao, G.N.; Emmanuel, J.K. Quality of biogas generated through co-digestion of wastewater sludge and food waste. Energy Rep. 2023, 10, 2330–2336. [Google Scholar] [CrossRef]
- Polastri, P.; Moreira, W.M.; dos Santos, D.F. Pretreatment of agroindustrial residues for biomethane production. J. Environ. Chem. Eng. 2024, 12, 111929. [Google Scholar] [CrossRef]
- Santos, A.D.; Silva, J.R.; Castro, L.M.; Quinta-Ferreira, R.M. Co-digestion strategies for waste valorization. Energy Rep. 2022, 8, 153–158. [Google Scholar] [CrossRef]
- Elagroudy, S.; Radwan, A.G.; Banadda, N.; Mostafa, N.G.; Owusu, P.A.; Janajreh, I. Prospects of waste-to-energy systems in developing countries. Renew. Energy 2020, 155, 1009–1020. [Google Scholar] [CrossRef]
- Pererva, Y.; Miller, C.D.; Sims, R.C. Algal biomass and wastewater treatment. Water 2020, 12, 1831. [Google Scholar] [CrossRef]
- Achinas, S.; Euverink, G.J.W. Biogas production processes and technologies. Energies 2019, 12, 217. [Google Scholar] [CrossRef]
- Nguyen, D.D.; Jeon, B.H.; Jeung, J.H. Methane production from industrial wastewater. Bioresour. Technol. 2019, 280, 269. [Google Scholar]
- Almomani, F.; Bhosale, R.R. Bioenergy from anaerobic treatment of organic waste. Chemosphere 2020, 255, 126805. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Yang, K.; Zheng, L. Synergistic effects of co-digestion on biogas production. Waste Biomass Valor. 2020, 11, 4837. [Google Scholar]
- Roberts, S.; Mathaka, N.; Zeleke, M.A. Biogas potential of municipal solid waste in sub-Saharan Africa. J. Inst. Eng. Ser. A 2023, 104, 335. [Google Scholar] [CrossRef]
- Hakimi, M.; Manogaran, M.D.; Shamsuddin, R.; Johari, S.A.M.; Hassan, M.A.M.; Soehartanto, T. Biogas production from agro-waste under tropical conditions. Heliyon 2023, 9, e17096. [Google Scholar] [CrossRef]
- López-Aguilar, H.; Barrón, A.; Franco, M.; Paz, A.; Pérez-Hernández, A. Methane production modelling from cheese whey and livestock excreta anaerobic co-digestion. Nova Sci. 2021, 13, 27. [Google Scholar]
- López-Aguilar, H.; Kennedy-Puentes, G.; Gómez, J.; Huerta-Reynoso, E.; Peralta-Pérez, M.D.R.; Zavala-Díaz de la Serna, F.; Pérez-Hernández, A. Practical and Theoretical Modeling of Anaerobic Digestion of Sargassum spp. in the Mexican Caribbean. Po. J. Environ. Stud. 2021, 30, 4. [Google Scholar] [CrossRef]
- López-Aguilar, H.A.; Huerta-Reynoso, E.A.; Gómez, J.A.; Pérez-Hernández, A. Mathematical models for the kinetics of methane production via the anaerobic co-digestion of biomass waste. Rev. Cent. Investig. Univ. La Salle 2025, 16, 3650. [Google Scholar] [CrossRef]
- Alkhrissat, T. Modeling biogas kinetics in co-digestion systems. Case Stud. Chem. Environ. Eng. 2024, 9, 100589. [Google Scholar] [CrossRef]
- Zwietering, M.H.; Jongenburger, I.; Rombouts, F.M.; Van’t Riet, K. Modeling of microbial growth. App. Environ. Microbiol. 1990, 56, 1875. [Google Scholar] [CrossRef]
- Li, P.; Li, W.; Sun, M.; Xu, X.; Zhang, B.; Sun, Y. Biochemical methane potential of organic residues. Energies 2018, 12, 26. [Google Scholar] [CrossRef]
- Altaş, L. Inhibitory effects of heavy metals on methane production. J. Hazard. Mater. 2009, 162, 1551–1556. [Google Scholar] [CrossRef]
- ASTM 1131-08; Standard Test Method for Compositional Analysis by Thermogravimetry. ASTM: West Conshohocken, PA, USA, 2014.
- Holliger, C.; Alves, M.; Andrade, D.; Angelidaki, I.; Astals, S.; Baier, U.; Wierinck, I. Towards a standardization of biomethane potential tests. Water Sci. Technol. 2016, 74, 2515. [Google Scholar] [CrossRef]
- Ware, A.; Power, N. Modelling methane production kinetics of complex poultry slaughterhouse wastes using sigmoidal growth functions. Renew. Energy 2017, 104, 50. [Google Scholar] [CrossRef]
- Akaike, H. A new look at the statistical model identification. IEEE Trans. Autom. Control 1974, 19, 716. [Google Scholar] [CrossRef]
- McCarthy, A.L.; O’Callaghan, Y.C.; Piggott, C.O. Nutritional potential of spent grain. Proc. Nutr. Soc. 2013, 72, 117. [Google Scholar] [CrossRef] [PubMed]
- Moset, V.; Al-Zohairi, N.; Møller, H.B. Silage of sugar beet pulp for biogas production. Biomass Bioenergy 2015, 83, 474. [Google Scholar]
- Zong, P.; Jiang, Y.; Tian, Y. Comparative evaluation of methane production potential from food waste. Energy Convers. Manag. 2020, 216, 112777. [Google Scholar] [CrossRef]
- Rani, P.; Bansal, M.; Pathak, V.V. Biogas generation from co-digestion of cattle manure and food waste. Curr. Res. Green. Sustain. Chem. 2022, 5, 100283. [Google Scholar] [CrossRef]
- Hilgert, J.E.; Herrmann, C.; Petersen, S.O.; Dragoni, F.; Amon, T.; Belik, V.; Amon, B. Assessment of the biochemical methane potential of in-house and outdoor stored pig and dairy cow manure by evaluating chemical composition and storage conditions. Waste Manag. 2023, 168, 14–24. [Google Scholar] [CrossRef]
- Kapłan, M.; Klimek, K.; Syrotyuk, S.; Konieczny, R.; Jura, B.; Smoliński, A.; Szymenderski, J.; Budnik, K.; Anders, D.; Dybek, B.; et al. Raw biogas desulphurization using the adsorption-absorption technique for a pilot production of agricultural biogas from pig slurry in Poland. Energies 2021, 14, 5929. [Google Scholar] [CrossRef]
- Kafle, G.K.; Chen, L. Comparison on batch anaerobic digestion of five different livestock manures and prediction of biochemical methane potential. Waste Manag. 2016, 48, 492. [Google Scholar] [CrossRef]
- Pan, S.Y.; Tsai, C.Y.; Liu, C.W. Evolution and future of zero waste cities. iScience 2021, 24, 102704. [Google Scholar] [CrossRef]
- Sganzerla, W.; Costa, J.; Tena-Villares, M. Valorization of brewery spent grain through anaerobic digestion. Fermentation 2022, 9, 2. [Google Scholar] [CrossRef]
- Ampese, L.C.; Sganzerla, W.G.; Di Domenico Ziero, H.; Costa, J.M.; Martins, G.; Forster-Carneiro, T. Valorization of Apple Pomace for Biogas Production: A Leading Anaerobic Biorefinery Approach for a Circular Bioeconomy. Biomass Convers. Biorefin. 2022, 14, 14843–14857. [Google Scholar] [CrossRef]
- Buller, L.S.; Sganzerla, W.G.; Lima, M.N.; Muenchow, K.E.; Timko, M.T.; Forster-Carneiro, T. Ultrasonic Pretreatment of Brewers’ Spent Grains for Anaerobic Digestion: Biogas Production for a Sustainable Industrial Development. J. Clean. Prod. 2022, 355, 131802. [Google Scholar] [CrossRef]
- Rico, C.; Montes, J.A.; Lobo, A. Dry Batch Anaerobic Digestion of Food Waste in a Box-Type Reactor System: Inoculum Preparation and Reactor Performance. J. Clean. Prod. 2020, 251, 119751. [Google Scholar] [CrossRef]
| Experiment | BSG (kg) | Inoculum (kg) | Cattle Manure (kg) | Inoculum-to-Substrate Ratio SV |
|---|---|---|---|---|
| R1 | 0.50 | 1.0 | 0.5 | 1:1 |
| R2 | 0.34 | 1.7 | 0.34 | 2.5:1 |
| Model | Equation | Number of Parameters |
|---|---|---|
| Logistic model (L) | 3 | |
| Modified Logistic (ML) | 3 | |
| Modified Gompertz (MG) | 3 | |
| Weibull (W) | 4 |
| Biomass | % Volatile Solids (Fresh Matter) | % Ash | % Total Solids (Fresh Matter) |
|---|---|---|---|
| Inoculum | 26 ± 3.6 | 4 ± 1 | 29.71 ± 1.57 |
| BSG | 50.66 ± 3.21 | 2 ± 1.2 | 54.96 ± 1.36 |
| Cattle manure | 52.14 ± 3.75 | 21 ± 0.8 | 89 ± 3.8 |
| Experiment | Lag Phase Day | Maximum Methane Production mL CH4 gVS−1 | Day of Maximum Methane Production Rate Day |
|---|---|---|---|
| R1 | 10 | 27.22 | 25 |
| R2 | 20 | 66.78 | 33 |
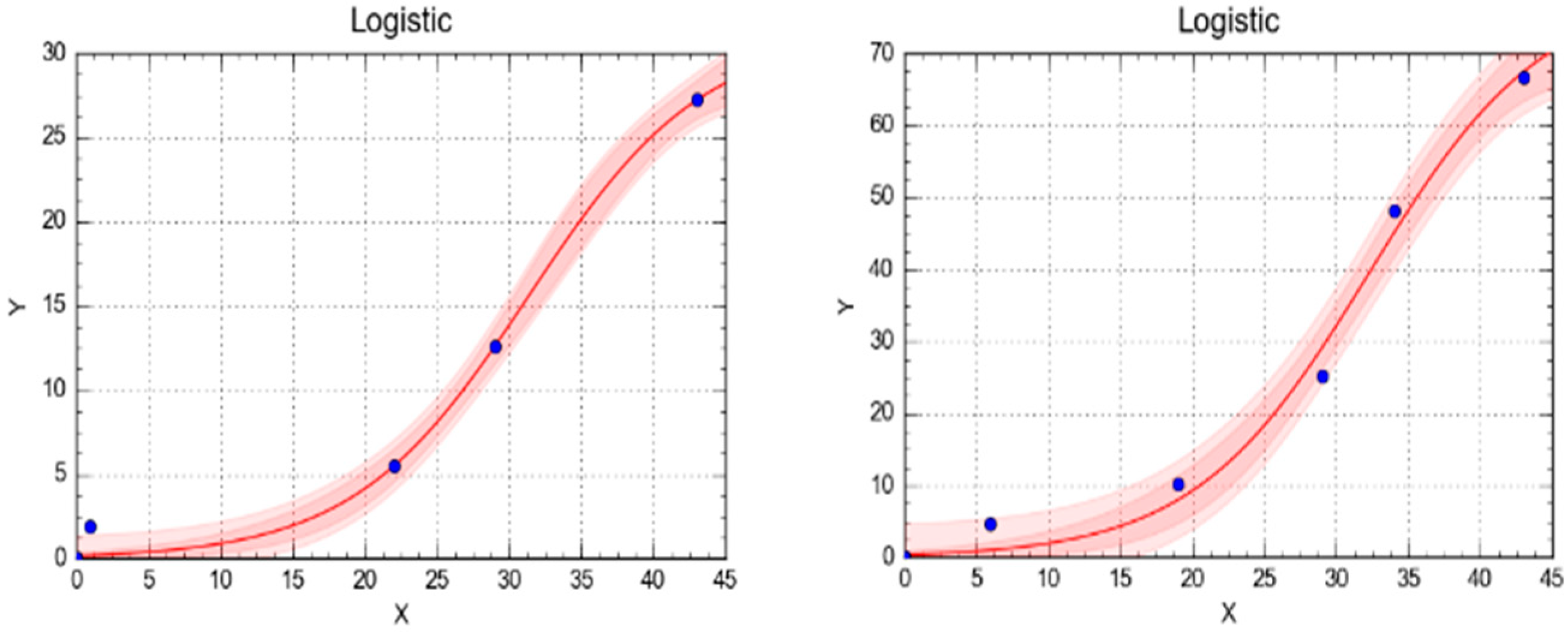
| Model | Parameters | R1 | R2 |
|---|---|---|---|
| Logistic (L) | a (mL) | 31.121 | 70.033 |
| Difference | 6.121 | 2.703 | |
| R2 | 0.9957 | 0.992 | |
| b | 177.109 | 202.645 | |
| AICC | −17.635 | 21.492 | |
| S | 0.5257 | 1.937 | |
| RSS | 3.316 | 45.039 | |
| Modified Logistic (ML) | a (mL) | 15.1 | 79.033 |
| Difference | 9.9 | 11.703 | |
| λ (h) | 43 | 3.251 | |
| R2 | 0.686 | 0.992 | |
| b | 43.799 | 3.251 | |
| AICC | 46.945 | 21.492 | |
| S | 4.525 | 1.937 | |
| RSS | 245.768 | 45.039 | |
| Modified Gompertz (MG) | a (mL) | 15.1 | 10.33 |
| Difference | 9.9 | 57 | |
| λ (h) | 11.449 | 208,152 | |
| R2 | 0.686 | 0.000000 | |
| b | 11.449 | −42,713.54 | |
| AICC | 46.945 | 94.718 | |
| S | 4.525 | 22.246 | |
| RSS | 245.768 | 5938.88 | |
| Weibull (W) | a (mL) | 30.55 | 73.365 |
| Difference | 5.55 | 6.035 | |
| R2 | 0.9956 | 0.9906 | |
| b | 30.389 | 72.788 | |
| AICC | −13.981 | 27.842 | |
| S | 0.557 | 2.248 | |
| RSS | 3.423 | 55.633 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
López-Aguilar, H.A.; Pérez-Hernández, A.; Monreal-Romero, H.A.; Meléndez, C.L.; Peralta-Pérez, M.d.R.; Serna, F.J.Z.-D.d.l. Anaerobic Co-Digestion of Brewers’ Spent Grain from Craft Beer and Cattle Manure for Biogas Production. World 2025, 6, 118. https://doi.org/10.3390/world6030118
López-Aguilar HA, Pérez-Hernández A, Monreal-Romero HA, Meléndez CL, Peralta-Pérez MdR, Serna FJZ-Ddl. Anaerobic Co-Digestion of Brewers’ Spent Grain from Craft Beer and Cattle Manure for Biogas Production. World. 2025; 6(3):118. https://doi.org/10.3390/world6030118
Chicago/Turabian StyleLópez-Aguilar, Héctor Alfredo, Antonino Pérez-Hernández, Humberto Alejandro Monreal-Romero, Claudia López Meléndez, María del Rosario Peralta-Pérez, and Francisco Javier Zavala-Díaz de la Serna. 2025. "Anaerobic Co-Digestion of Brewers’ Spent Grain from Craft Beer and Cattle Manure for Biogas Production" World 6, no. 3: 118. https://doi.org/10.3390/world6030118
APA StyleLópez-Aguilar, H. A., Pérez-Hernández, A., Monreal-Romero, H. A., Meléndez, C. L., Peralta-Pérez, M. d. R., & Serna, F. J. Z.-D. d. l. (2025). Anaerobic Co-Digestion of Brewers’ Spent Grain from Craft Beer and Cattle Manure for Biogas Production. World, 6(3), 118. https://doi.org/10.3390/world6030118









