Olive Growing Farming System and Damage by Cicadas
Abstract
1. Introduction
2. Materials and Methods
2.1. Description of the Study Area
2.1.1. Olive Groves with Ecological Farming
2.1.2. Olive Grove with Conventional Farming
2.2. Sampling on the Vegetation Cover (VC1; VC2)
2.3. Sampling in Olive Tree Canopy (VC1; VC2; CONV)
2.4. Statistical Analysis
3. Results
3.1. Study of Herbaceous Cover (ECO-VC1 & ECO-VC2)
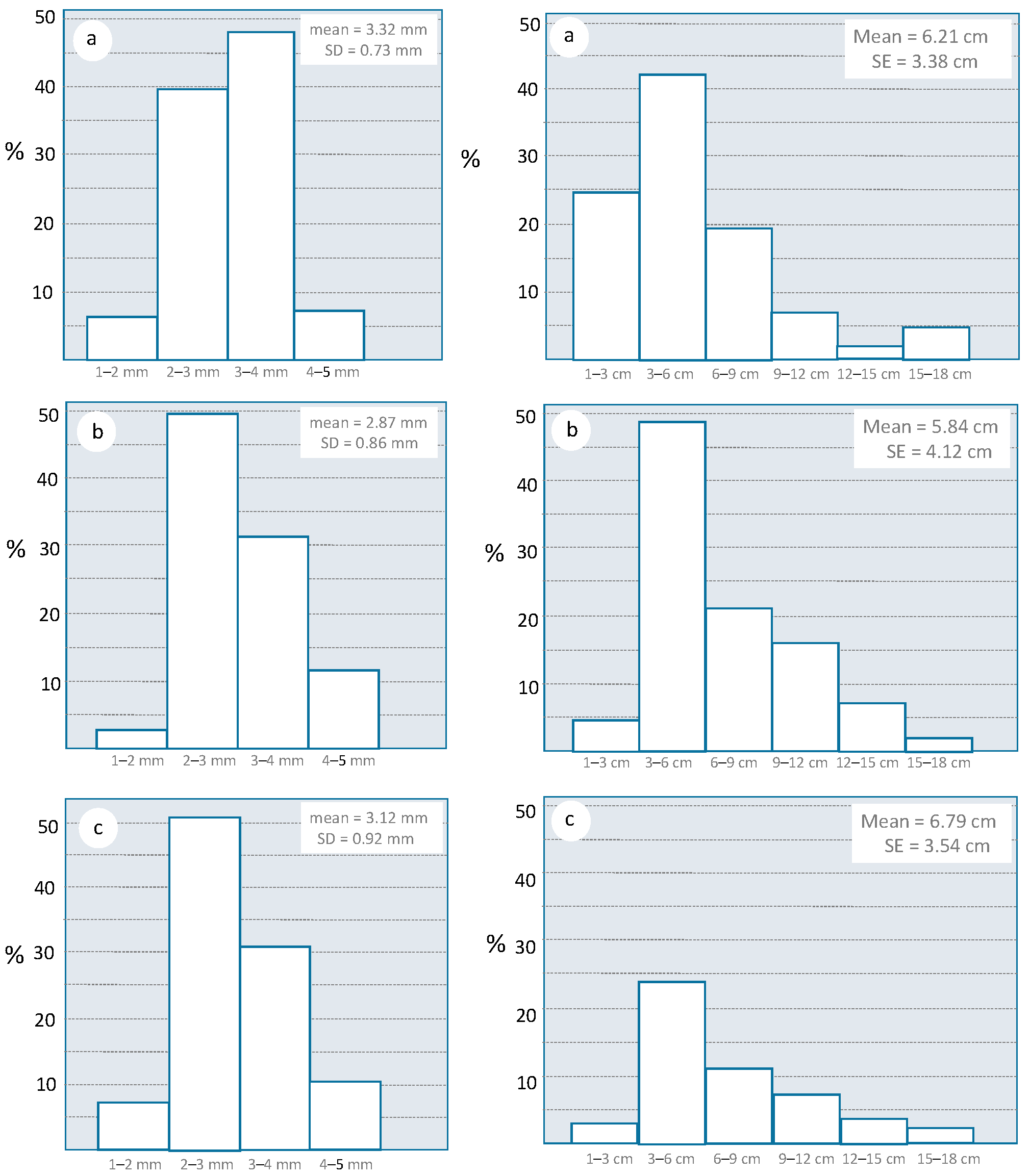
3.2. Description of Oviposition Injuries by Cicadas
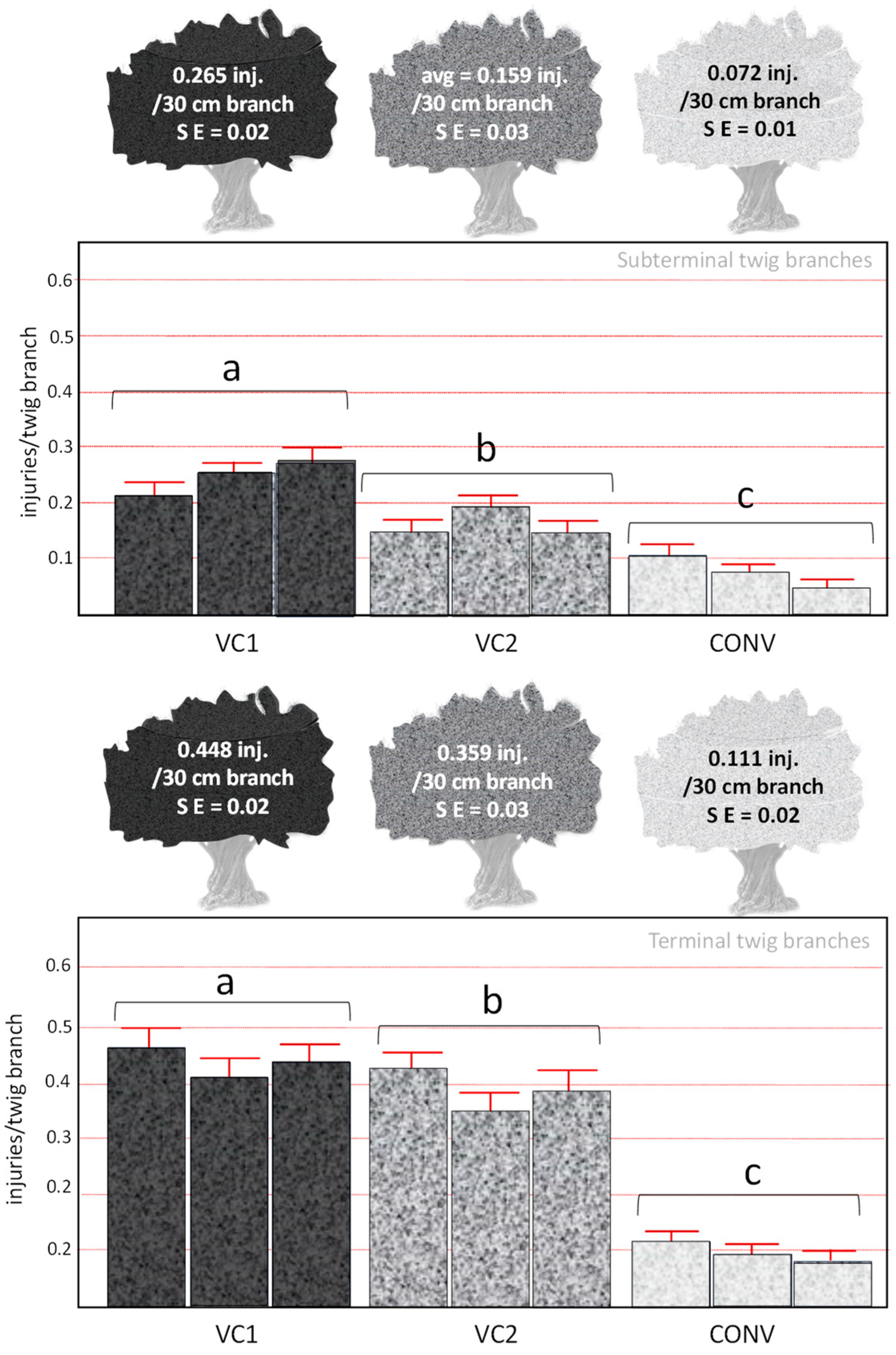
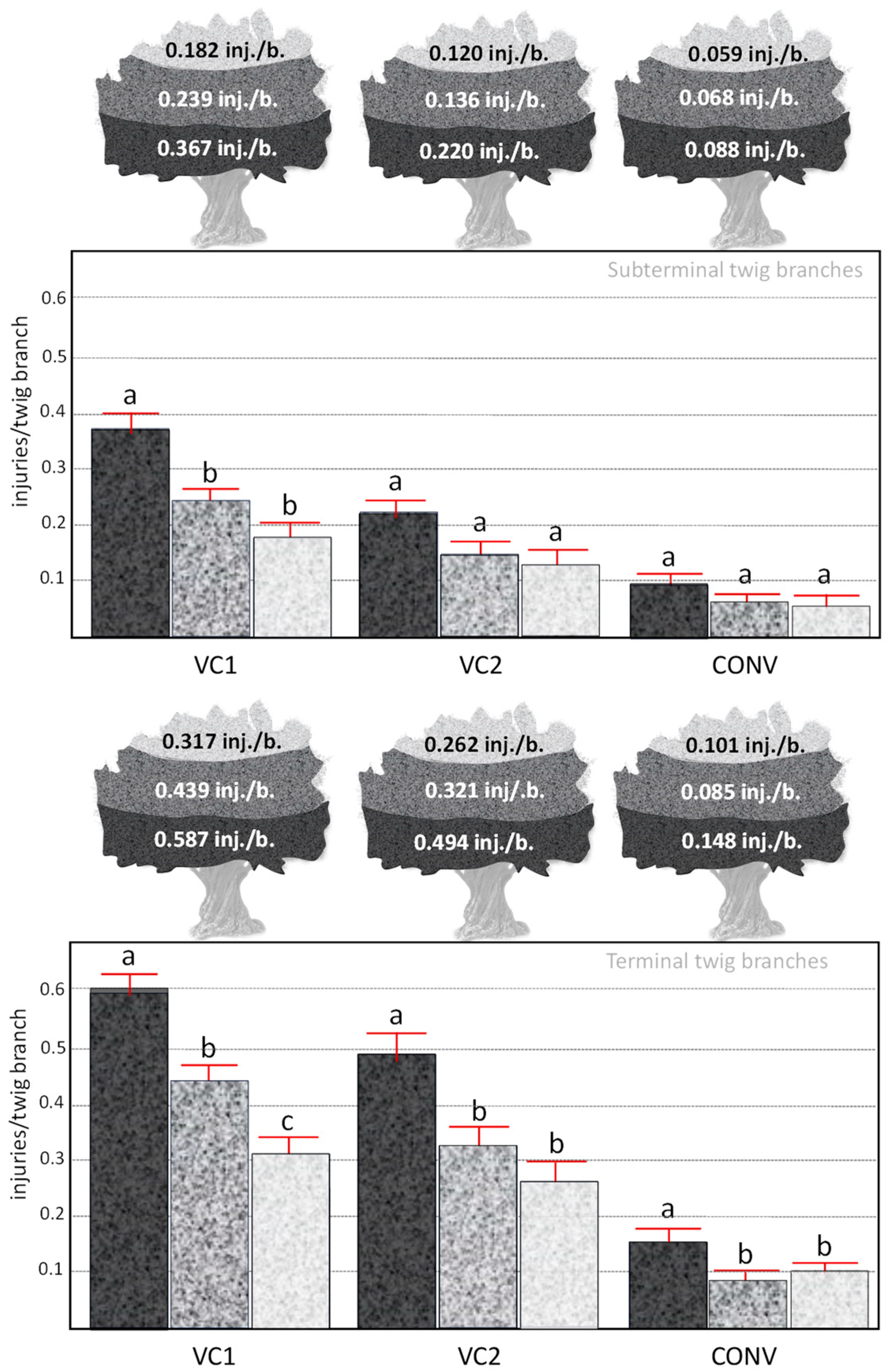
3.3. Influence of the Olive Farming System on the Density of Oviposition Injuries
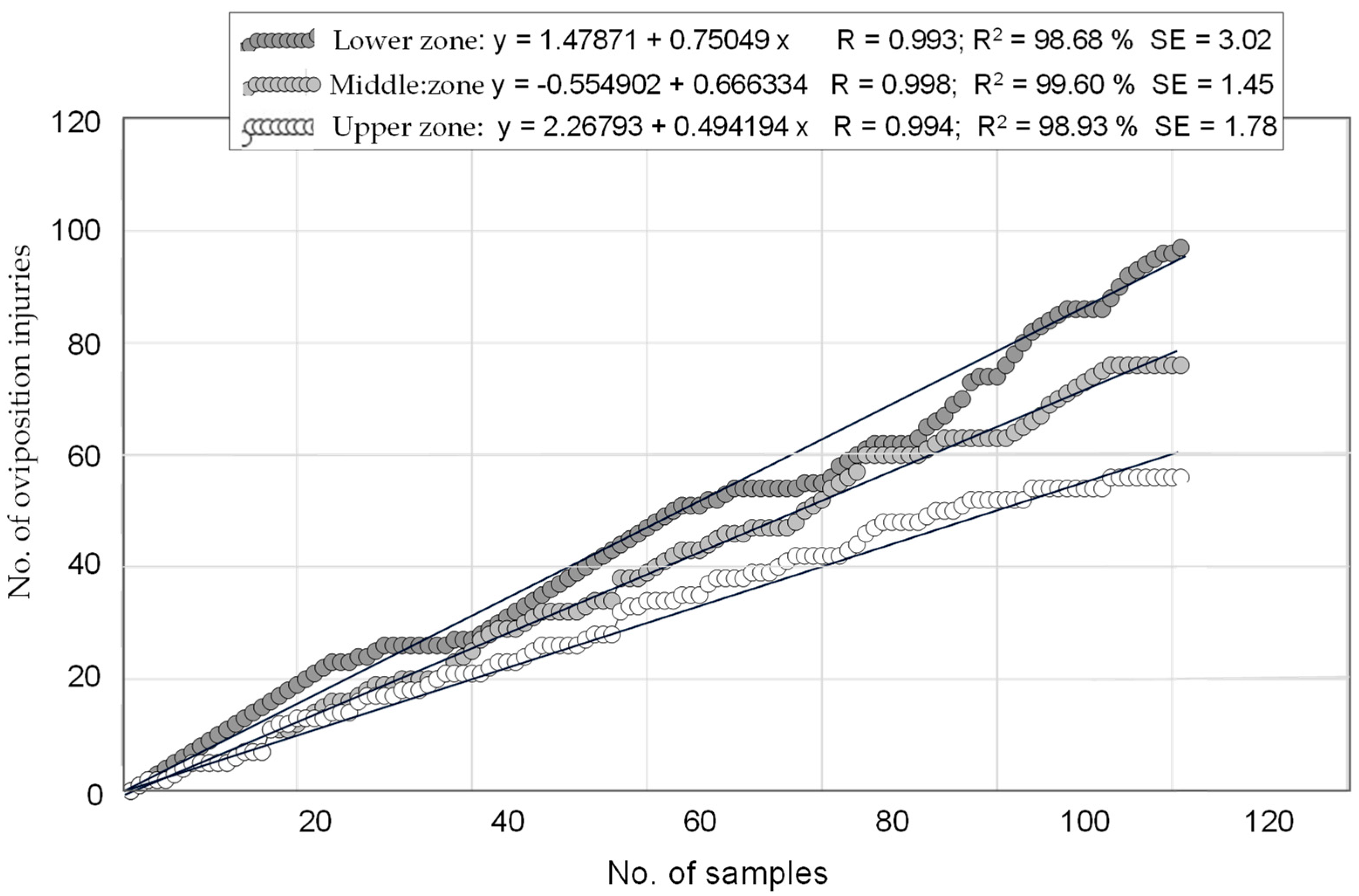
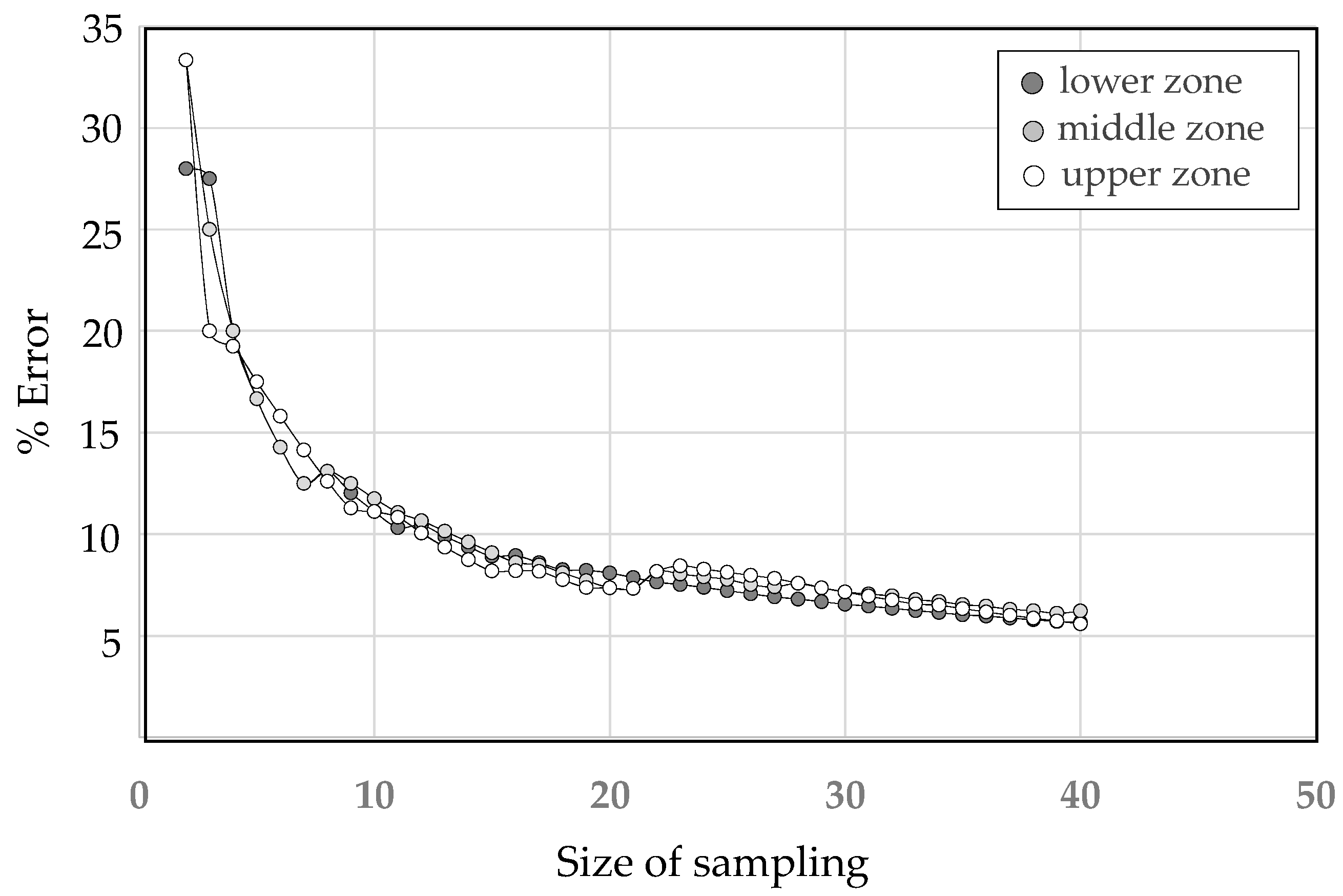
3.4. Determination of an Extensive Sampling for Cataloging Olive Groves Based on Injuries Density
4. Discussion
- Conventional chemical control, using products with a notable knockdown effect on adults during the reproductive period [67]. Obviously, this would not be a viable solution in organic crops, so for these, applications of kaolin-based formulation along with diatomaceous earth and essential oil have demonstrated acceptable control of oviposition injuries [68].
- Complementary cultural methods, based on the suppression during the summer of plant specimens that develop in the peripheral area of olive trees, close to irrigation drippers, which would have a very negative impact on the survival rate of nymphs.
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Karban, R. Addition of periodical cicada nymphs to an oak forest: Effects on cicada density, acorn production, and rootlet density. J. Kans. Entom. Soc. 1985, 58, 269–276. [Google Scholar]
- Logan, D.; Connolly, P. Cicadas from kiwifruit orchards in New Zealand and identification of their final instar exuviae (Cicadidae: Homoptera). N. Z. Entomol. 2005, 28, 37–48. [Google Scholar] [CrossRef]
- Spooner-Hart, R.; Tesoriero, L.; Hall, B.H. Field Guide to Olive Pests, Diseases and Disorders in Australia; Rural industries Research and Development Corporation: Brisbane, Australia, 2007; pp. 1–80. [Google Scholar]
- Moulds, M.S. Cicadas. In Encyclopedia of Insects, 2nd ed.; Resh, V.H., Cardé, R.T., Eds.; Academic Press: San Diego, CA, USA, 2009; pp. 163–164. [Google Scholar]
- Boulard, M. Les cigales du Portugal, contribution à leur étude [Hom. Cicadidae]. Ann. Société Entomol. Fr. 1982, 18, 181–198. [Google Scholar] [CrossRef]
- Quartau, J.A. A numerical taxonomic analysis of interspecific morphological differences in two closely related species of Cicada (Homoptera, Cicadidae) in Portugal. Great Basin Nat. Mem. 1988, 12, 171–181. [Google Scholar]
- Ribeiro, M. Diferenciação em Duas Espécies Gémeas do Género Cicada L. (Insecta, Homoptera, Cicadoidea): Cicada orni L. e Cicada barbara (Stål). Ph.D. Thesis, Faculty of Sciences, University of Lisbon, Lisbon, Portugal, 1998. [Google Scholar]
- Seabra, S.G.; Pinto-Juma, G.; Quartau, J.A. Calling songs of sympatric and allopatric populations of Cicada barbara and C. orni (Hemiptera: Cicadidae) on the Iberian Peninsula. Eur. J. Entomol. 2006, 103, 843–852. [Google Scholar] [CrossRef]
- Louça, J.; Symons, J.; Rodrigues, D.; Morais, A. Pattern-oriented analysis of communication flow: The case study of Cicada barbara lusitanica. In Proceedings of the 21st European Conference on Modelling and Simulation-ECMS, Prague, Czech Republic, 4–6 June 2007; Zelinka, I., Zuzana, O., Orsoni, A., Eds.; ECMS Proceedings; ECSM: Coon Rapids, MN, USA, 2007; pp. 229–234. [Google Scholar]
- Sueur, J.; Puissant, S.; Simoes, P.C.; Seabra, S.; Boulard, M.; Quartau, J.A. Cicadas from Portugal: Revised 1004 list of species with eco-ethological data (Hemiptera: Cicadidae). Insect. Syst. Evol. 2004, 35, 177–187. [Google Scholar] [CrossRef]
- Popov, A.V. The structure of the tymbals and the characteristics of the sound signals in singing cicadas (Homoptera, Cicadidae) in the southern regions of the USSR. Entomol. Rev. 1975, 54, 7–35. [Google Scholar]
- Quartau, J.A.; Fonseca, P.J. An annotated check-list of the species of cicadas known to occur in Portugal (Homoptera: Cicadoidea). In Proceedings of the 6th Auchenorrhyncha Meeting, Turin, Italy, 7–11 September 1987; Vidano, C., Arzone, A., Eds.; Consiglio Nazionale delle Ricerche: Ottawa, Canada, 1988; pp. 367–375. [Google Scholar]
- Pinto-Juma, G.A.; Quartau, J.A.; Bruford, M.W. Population structure of Cicada barbara Stål (Hemiptera, Cicadoidea) from the Iberian Peninsula and Morocco based on mitochondrial DNA analysis. Bull. Entomol. Res. 2008, 98, 15–25. [Google Scholar] [CrossRef] [PubMed]
- Quartau, J.A. Cigarras-esses insectos quase desconhecidos. Correio Natur. 1995, 19, 33–38. [Google Scholar]
- Sueur, J.; Aubin, T. Is microhabitat segregation between two cicada species (Tibicina haematodes and Cicada orni) due to calling song propagation constraints? Naturwissenschaften 2003, 90, 322–326. [Google Scholar] [CrossRef] [PubMed]
- Patterson, I.J.; Massei, G.; Genov, P. The density of cicadas Cicada orni in Mediterranean coastal habitats. Ital. J. Zool. 1997, 64, 141–146. [Google Scholar] [CrossRef]
- Silvestri, F. Compendio di Entomologia Applicata. Parte Especiale, I; Stab. Tip. Bellavista: Portici, Italy; Roma, Italy, 1939. [Google Scholar]
- Jardak, T.; Ksantini, M. La estructuración de la protección fitosanitaria del olivo en Túnez: Elementos básicos y necesidades económicas y ecológicas. Olivae 1996, 61, 24–33. [Google Scholar]
- Ruiz Castro, A. The Insect Fauna of Olive in Spain. A Study of the Classification and Biology of the Species of Greatest Economic Importance; Consejo Superior de Investigaciones Científicas (CSIC), Instituto Español de Entomología: Madrid, Spain, 1951. [Google Scholar]
- Arambourg, Y. Traité d’Entomologie Oléicole; Conseil Oleicole International (COI): Madrid, Spain, 1986. [Google Scholar]
- Lay, J.M.B.; Fraga, D.E.; Font, R.C.; Ferran, P.P.; Rodríguez, M.F.; Fontelles, F.; Funosas, D.; Fusellas, M.; Puig-Gironès, R.; Roca, C.T.; et al. Diversitat, distribució i fenologia de les cigales (Hemiptera: Cicadidae) a Catalunya (NE Península Iberica). Butl. Inst. Catalana Hist. Nat. 2021, 85, 59–72. [Google Scholar]
- González, M.I.; Alvarado, M.; Duran, J.M.; Serrano, A.; De la Rosa, A. Estudios sobre Cicada sp. (Homoptera: Cicadidae) en olivo. Bol. Sanid. Veg. Plagas 1998, 24, 803–816. [Google Scholar]
- Ruiz Torres, M. Análisis del estado de las plagas del olivar andaluz en 2008 y Reflexiones sobre algunas dificultades de Control. Incidencia de las principales plagas del olivar. Vida Rural 2008, 44–48. Available online: https://www.mapa.gob.es/ministerio/pags/biblioteca/revistas/pdf_Vrural/Vrural_2008_276_44_48.pdf (accessed on 11 August 2024).
- Cornara, D.; Marra, M.; Tedone, B.; Cavalieri, V.; Porcelli, F.; Fereres, A.; Purcell, A.; Saponari, M. No evidence for cicadas’ implication in Xylella fastidiosa epidemiology. Entomol. Gen. 2020, 40, 125–132. [Google Scholar] [CrossRef]
- White, E.G.; Sedcole, J.R. A study of the abundance and patchiness of cicada nymphs (Homoptera: Tibicinidae) in a New Zealand subalpine shrub-grassland. N. Z. J. Zool. 1993, 20, 38–51. [Google Scholar] [CrossRef]
- Yang, L.H. Periodical cicadas as resource pulses in North American forests. Science 2004, 306, 1565–1567. [Google Scholar] [CrossRef] [PubMed]
- Raupp, M.J. Egg-Laying and the Dark Side of Cicadas, Magicicada spp. Web Editor: Chris Sargent; Web Designer: Kris Keochinda. 2021. Available online: https://bugoftheweek.com/blog/2021/6/14/egg-laying-and-the-dark-side-of-cicadas-cicadas-magicicada-spp (accessed on 11 August 2024).
- Casado, G.G.; Pulido, L.F. Manejo de la Cubierta Vegetal en el Olivar Ecológico en Andalucía: Siembra de Leguminosas Entre Calles; Dirección General de Agricultura Ecológica; Consejería de Agricultura y Pesca, Junta de Andalucía: Seville, Spain, 2007. [Google Scholar]
- Gómez, J.A.; Sobrinho, T.A.; Giráldez, J.V.; Fereres, E. Soil management effects on runoff, erosion and soil properties in an olive grove of Southern Spain. Soil Till. Res. 2009, 102, 5–13. [Google Scholar] [CrossRef]
- Rodríguez-Entrena, M.; Arriaza, M. Adoption of conservation agriculture in olive groves: Evidences from southern Spain. Land Use Policy 2013, 34, 294–300. [Google Scholar] [CrossRef]
- González-Ruiz, R.; Gómez-Guzmán, J.A.; Martínez-Rojas, M.; García-Fuentes, A.; Cordovilla, M.D.P.; Sainz-Pérez M: Rodríguez-Lizana, A. The influence of mixed green covers, a new trend in organic olive growing, on the efficiency of predatory insects. Agriculture 2023, 13, 785. [Google Scholar] [CrossRef]
- García-Martín, J.F.; Cuevas, M.; Feng, C.H.; Álvarez-Mateos, P.; Torres, M.; Sánchez, S. Energetic valorisation of olive biomass: Olive-tree pruning, olive stones and pomaces. Processes 2020, 8, 511. [Google Scholar] [CrossRef]
- Azam, F. Comparative effects of organic and inorganic nitrogen sources applied to a flooded soil on rice yield and availability of N. Plant Soil 1990, 125, 255–262. [Google Scholar] [CrossRef]
- Singh, H. Nitrogen mineralization, microbial biomass and crop yield affected by the placement of wheat residues and fertilizers in a semi-arid tropical soil with minimum tillage. J. Appl. Ecol. 1995, 32, 588–595. [Google Scholar] [CrossRef]
- Rodríguez-Lizana, A.; Pereira, M.J.; Ribeiro, M.C.; Soares, A.; Márquez-García, F.; Ramos, A.; Gil-Ribes, J. Assessing Local Uncertainty of Soil Protection in an Olive Grove Area with Pruning Residues Cover: A Geostatistical Cosimulation Approach. Land Degrad. Dev. 2017, 28, 2086–2097. [Google Scholar] [CrossRef]
- García-Fuentes, A.; Lendínez, M.L.; Salazar, C. Pérdida de diversidad vegetal en los olivares del Alto Valle del Guadalquivir: Alternativas agroecológicas. In La Cultura del Olivo, Ecología, Economía, Sociedad; Anta, J.L., Palacios, J., Guerrero, F., Eds.; Universidad de Jaén: Jaén, Spain, 2005; pp. 300–430. [Google Scholar]
- Calatrava, J.; Franco, J.A. Using pruning residues as mulch: Analysis of its adoption and process of diffusion in southern Spain olive orchards. J. Environ. Manag. 2011, 92, 620–629. [Google Scholar] [CrossRef] [PubMed]
- Repullo, M.A.; Carbonell, R.; Hidalgo, J.; Rodríguez-Lizana, A.; Ordóñez, R. Using olive pruning residues to cover soil and improve fertility. Soil Tillage Res. 2012, 124, 36–46. [Google Scholar] [CrossRef]
- Taguas, E.V.; Gómez, J.A. Vulnerability of olive orchards under the current CAP (Common Agricultural Policy) regulations on soil erosion: A study case in Southern Spain. Land Use Policy 2015, 42, 683–694. [Google Scholar] [CrossRef]
- Medina, J.; Monreal, C.; Barea, J.M.; Arriaga, C.; Borie, F.; Cornejo, P. Crop residue stabilization and application to agricultural and degraded soils: A review. Waste Manag. 2015, 42, 41–54. [Google Scholar] [CrossRef] [PubMed]
- Cheung, W.K.; Marshall, A.T. Water and ion regulation in cicadas in relation to xylem feeding. J. Insect Physiol. 1973, 19, 1801–1816. [Google Scholar] [CrossRef]
- White, J.; Strehl, C.E. Xylem feeding by periodical cicada nymphs on tree roots. Ecol. Entomol. 1978, 3, 323–327. [Google Scholar] [CrossRef]
- Carpio, A.J.; Solana, M.; Tortosa, F.S.; Castro, J. Effect of cover crops in olive groves on Cicadomorpha communities. Span. J. Agric. Res. 2020, 18, e0303. [Google Scholar] [CrossRef]
- Redak, R.A.; Purcell, A.H.; Lopes, J.S.; Blua, M.J.; Mizell, R.F.; Andersen, P.C. The biology of xylem fluid feeding insect vectors of Xylella fastidiosa and their relation to disease epidemiology. Annu. Rev. Entomol. 2004, 49, 243–270. [Google Scholar] [CrossRef] [PubMed]
- Cueto Romero, M.; Blanca López, G.; Salazar Mendías, C.; Cabezudo-Artero, B. Diversity and ecological characteristics of the vascular flora in the Western Mediterranean (Eastern Andalusia, Spain). Universidad de Málaga, Servicio de Publicaciones: Malaga, Spain, 2014. [Google Scholar]
- Raunkiaer, C. The life-forms of plants and their bearing on geography. In The Life Forms of Plants and Statistical Plant Geography; Oxford University Press: London, UK, 1934; pp. 2–104. [Google Scholar]
- González-Ruiz, R. Estudio Bioecológico de Phloeotribus scarabaeoides Bernard (Coleoptera: Scolytidae) en la Provincia de Granada. Ph.D. Thesis, University of Granada, Granada, Spain, 1989. [Google Scholar]
- González-Ruiz, R.; Campos, M. A preliminary study of the effect of attacks by Phloeotribus scarabaeoides (Bern.)(Coleoptera: Scolytidae) on the productivity of the olive tree (Olea europaea L.). Mitteilungen Der Schweiz. Entomol. Ges. 1994, 67, 67–75. [Google Scholar]
- Orians, C.M.; Jones, C.G. Plants as resource mosaics: A functional model for predicting patterns of within-plant resource heterogeneity to consumers based on vascular architecture and local environmental variability. Oikos 2001, 94, 493–504. [Google Scholar] [CrossRef]
- Potter, K.; Davidowitz, G.; Woods, H.A. Insect eggs protected from high temperatures by limited homeothermy of plant leaves. J. Exp. Biol. 2009, 212, 3448–3454. [Google Scholar] [CrossRef]
- Pincebourde, S.; Sinoquet, H.; Combes, D.; Casas, J. Regional climate modulates the canopy mosaic of favourable and risky microclimates for insects. J. Anim. Ecol. 2007, 76, 424–438. [Google Scholar] [CrossRef] [PubMed]
- Moriyama, M.; Yasuyama, K.; Numata, H. The formation of a hatching line in the serosal cuticle confers multifaceted adaptive functions on the eggshell of a cicada. Zool. Lett. 2021, 7, 8. [Google Scholar] [CrossRef] [PubMed]
- Lloyd, M.; Dybas, H.S. The periodical cicada problem II. Evolution. Evolution 1966, 20, 466–505. [Google Scholar] [CrossRef] [PubMed]
- Novotny, V.; Wilson, M.R. Why are there no small species among xylem-sucking insects? Evol. Ecol. 1997, 11, 419–437. [Google Scholar] [CrossRef]
- Karban, R. Prolonged development in cicadas. In The Evolution of Insect Life Cycles; Springer: New York, NY, USA, 1986; pp. 222–235. [Google Scholar]
- Karban, R. Why cicadas (Hemiptera: Cicadidae) develop so slowly. Biol. J. of the Lin. Soc. 2022, 135, 291–298. [Google Scholar] [CrossRef]
- Callaham, M.A., Jr.; Whiles, M.R.; Meyer, C.K.; Brock, B.L.; Charlton, R.E. Feeding ecology and emergence production of annual cicadas (Homoptera: Cicadidae) in tallgrass prairie. Oecologia 2000, 123, 535–542. [Google Scholar] [CrossRef] [PubMed]
- Ito, Y.; Nagamine, M. Why a cicada, Mogannia minuta Matsumura, became a pest of sugarcane: An hypothesis based on the theory of escape. Ecol. Entomol. 1981, 6, 273–283. [Google Scholar] [CrossRef]
- Bozsik, A.; González-Ruiz, R. First data on the sibling species of the common green lacewings in Spain (Neuroptera: Chrysopidae): (The taxonomic status of the most important cryptic species of Chrysoperla carnea complex in Spain). In Proceedings of the 4th International Plant Protection Symposium at Debrecen University and 11th Trans-Tisza Plant Protection Forum, Debrecen, Hungary, 18–19 October 2006; pp. 3–11. [Google Scholar]
- González-Ruiz, R.; Al-Asaad, S.; Bozsik, A. Influencia de las masas forestales en la diversidad y abundancia de los crisópidos (Neur.:” Chrysopidae”) del olivar. Cuad. Soc. Española Cienc. For. 2008, 26, 33–38. [Google Scholar]
- Pappas, M.L.; Broufas, G.D.; Koveos, D.S. Effects of various prey species on development, survival and reproduction of the predatory lacewing Dichochrysa prasina (Neu.: Chrysopidae). Biol. Control 2007, 43, 163–170. [Google Scholar] [CrossRef]
- Manojlovic, B.; Zabel, A.; Stankovic, S. Additional diet of the parasitoids (Hymenoptera: Braconidae) and the parasitizing of the Elm Bark Beetle (Coleoptera: Scolytidae). Anz. Schädlingskunde 2001, 74, 66–71. [Google Scholar] [CrossRef]
- Bozsik, A. Nahrungsanalytische untersuchungen an einigen mitteleuropäischen chrysopiden-imagines (Neuroptera: Chrysopidae). Beiträge Zur Entomol. Contrib. Entomol. 2000, 50, 237–246. [Google Scholar] [CrossRef]
- Canard, M. Natural food and feeding habits of lacewings. In Lacewings in the Crop Environment; McEwen, P.K., New, T.R., Whittington, A.E., Eds.; Cambridge University Press: Cambridge, UK, 2001; pp. 116–129. [Google Scholar]
- Tóth, M.; Bozsik, A.; Szentkirályi, F.; Letardi, A. Phenylacetaldehyde: A chemical attractant for common green lacewings (Chrysoperla carnea sl, Neuroptera: Chrysopidae). Eur. J. Entomol. 2006, 103, 267–271. [Google Scholar] [CrossRef]
- Baur, R.; Wijnands, F.; Malavolta, C.; Alaphilippe, B.G.; Baur, R. Integrated Production—Objectives, Principles and Technical Guidelines. IOBC/WPRS Bulletin, Special Issue. 2018. pp. 1–8. Available online: https://iobc-wprs.org/wp-content/uploads/2022/06/IOBC-WPRS_IP_objectives_and_principles_4th_edition_2018_EN.pdf (accessed on 23 September 2024).
- Hogmire, H.W.; Baugher, T.A.; Crim, V.L.; Walter, S.I. Effects and control of periodical cicada (Homoptera: Cicadidae) oviposition injury on nonbearing apple trees. J. Econ. Entomol. 1990, 83, 2401–2404. [Google Scholar] [CrossRef]
- Valizadeh, H.; Abbasipour, H.; Farazmand, H. Evaluation of Kaolin Application on Oviposition Control of the Vine Cicada, Psalmocharias alhageos in Vineyards. Entomol. Gen. 2012, 34, 279–286. [Google Scholar] [CrossRef]
- Frank, D.L. Evaluation of organically acceptable methods to control periodical cicada (Hemiptera: Cicadidae) oviposition injury on nonbearing apple trees. J. Entomol. Sci. 2020, 55, 210–218. [Google Scholar] [CrossRef]


| (Biotype) 1 | Months | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sp | [Life Form] 2 | N | %VC1 | %VC2 | p | I | II | III | IV | V | VI | VII | VIII | IX | X | XI | XII |
| Filago | (Th.) [e.] | 606 | 15.2 | 84.8 | <0.001 | ||||||||||||
| pyramidata L. | |||||||||||||||||
| Malva | (H./Th.) [fasc.] | 410 | 65.8 | 34.2 | <0.05 | ||||||||||||
| parviflora L. | |||||||||||||||||
| Bromus | (Th.) [caesp.] | 300 | 75.3 | 24.7 | >0.05 | ||||||||||||
| rubens L. | |||||||||||||||||
| Astragalus | (Th.) [e. fasc.] | 149 | 95.3 | 4.7 | <0.01 | ||||||||||||
| hamosus L. | |||||||||||||||||
| Trisetaria | (Th.) [caesp.] | 104 | 50 | 50 | >0.05 | ||||||||||||
| panicea (Lam.) | |||||||||||||||||
| Filago | (Th.) [e.] | 103 | 89.3 | 10.7 | <0.001 | ||||||||||||
| fuscescens Pomel | |||||||||||||||||
| Herniaria | (Th.) [creep.] | 91 | 80.2 | 19.8 | >0.05 | ||||||||||||
| cinerea D. C. | |||||||||||||||||
| Crucianella | (Th.) [e.] | 47 | 0 | 100 | >0.05 | ||||||||||||
| patula L. | |||||||||||||||||
| Diplotaxis | (Th.) [e.] | 35 | 0 | 100 | <0.01 | ||||||||||||
| virgata (Cav.) DC. | |||||||||||||||||
| Hordeum murinum | (Th.) [e.] | 34 | 85.3 | 14.7 | >0.05 | ||||||||||||
| leporinum Arcang. | |||||||||||||||||
| Trigonella | (Th.) [e.] | 26 | 23.1 | 76.9 | <0.01 | ||||||||||||
| monspeliaca L. | |||||||||||||||||
| Phalaris | (Th.) [caesp.] | 23 | 47.8 | 52.2 | >0.05 | ||||||||||||
| minor Retz. | |||||||||||||||||
| Heliotropium | (H.) [ros.] | 23 | 55.4 | 44.6 | >0.05 | ||||||||||||
| europaeum L. | |||||||||||||||||
| Plantago | (H./Th.) [ros.] | 22 | 100 | 0 | >0.05 | ||||||||||||
| lagopus L. | |||||||||||||||||
| Erodium | (Th.) [creep.] | 20 | 0 | 100 | >0.05 | ||||||||||||
| malacoides (L.) L’Hér. | |||||||||||||||||
| Ononis | (Th.) [e.] | 19 | 15.0 | 85.0 | >0.05 | ||||||||||||
| biflora Desf. | |||||||||||||||||
| Melilotus | (Th.) [e.] | 11 | 18.2 | 81.8 | >0.05 | ||||||||||||
| elegans Salzm. ex Ser. | |||||||||||||||||
| Leontodon longirostris | (H.) [ros.] | 10 | 0 | 100 | >0.05 | ||||||||||||
| (F. & P. D. Sell) Talav. | |||||||||||||||||
| Stellaria | (Th.) [e.] | 1 | 100 | 0 | >0.05 | ||||||||||||
| pallida (Dumort.) Piré | |||||||||||||||||
| Anagallis | (Th.) [e.] | 1 | 100 | 0 | >0.05 | ||||||||||||
| foemina Mill. | |||||||||||||||||
| N | Lower | Middle | Upper | N | Lower | Middle | Upper |
|---|---|---|---|---|---|---|---|
| 1 | - | - | - | 21 | 7.86 | 7.33 | 7.33 |
| 2 | 28.00 | 33.33 | 33.33 | 22 | 7.64 | 8.16 | 8.16 |
| 3 | 27.50 | 25.00 | 20.00 | 23 | 7.52 | 8.03 | 8.44 |
| 4 | 20.00 | 20.00 | 19.25 | 24 | 7.37 | 7.90 | 8.28 |
| 5 | 16.67 | 16.67 | 17.50 | 25 | 7.21 | 7.77 | 8.12 |
| 6 | 14.29 | 14.29 | 15.81 | 26 | 7.06 | 7.51 | 7.97 |
| 7 | 12.50 | 12.50 | 14.14 | 27 | 6.91 | 7.40 | 7.82 |
| 8 | 13.09 | 13.09 | 12.60 | 28 | 6.80 | 7.59 | 7.59 |
| 9 | 12.03 | 12.50 | 11.29 | 29 | 6.67 | 7.36 | 7.36 |
| 10 | 11.11 | 11.75 | 11.11 | 30 | 6.55 | 7.15 | 7.15 |
| 11 | 10.32 | 11.07 | 10.83 | 31 | 6.45 | 7.05 | 6.94 |
| 12 | 10.44 | 10.66 | 10.05 | 32 | 6.34 | 6.95 | 6.75 |
| 13 | 9.88 | 10.14 | 9.35 | 33 | 6.23 | 6.77 | 6.56 |
| 14 | 9.37 | 9.61 | 8.73 | 34 | 6.13 | 6.69 | 6.50 |
| 15 | 8.91 | 9.09 | 8.18 | 35 | 6.03 | 6.52 | 6.33 |
| 16 | 8.94 | 8.61 | 8.20 | 36 | 5.96 | 6.45 | 6.17 |
| 17 | 8.58 | 8.49 | 8.16 | 37 | 5.87 | 6.30 | 6.01 |
| 18 | 8.25 | 8.08 | 7.75 | 38 | 5.78 | 6.23 | 5.86 |
| 19 | 8.22 | 7.71 | 7.37 | 39 | 5.69 | 6.10 | 5.72 |
| 20 | 8.09 | 7.36 | 7.36 | 40 | 5.64 | 6.21 | 5.58 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
González-Ruiz, R.; Cuevas-López, V.; Sainz-Pérez, M.; Cuesta Cocera, J.F.; García-Fuentes, A. Olive Growing Farming System and Damage by Cicadas. World 2024, 5, 832-847. https://doi.org/10.3390/world5040043
González-Ruiz R, Cuevas-López V, Sainz-Pérez M, Cuesta Cocera JF, García-Fuentes A. Olive Growing Farming System and Damage by Cicadas. World. 2024; 5(4):832-847. https://doi.org/10.3390/world5040043
Chicago/Turabian StyleGonzález-Ruiz, Ramón, Valentina Cuevas-López, María Sainz-Pérez, Juan F. Cuesta Cocera, and Antonio García-Fuentes. 2024. "Olive Growing Farming System and Damage by Cicadas" World 5, no. 4: 832-847. https://doi.org/10.3390/world5040043
APA StyleGonzález-Ruiz, R., Cuevas-López, V., Sainz-Pérez, M., Cuesta Cocera, J. F., & García-Fuentes, A. (2024). Olive Growing Farming System and Damage by Cicadas. World, 5(4), 832-847. https://doi.org/10.3390/world5040043






