Validation of Combined Indicator Using Joint Index Vector and Pain Score for Risk Weight Calculation of Incident Bone Fragility Fracture in Patients with Rheumatoid Arthritis
Abstract
1. Introduction
2. Materials and Methods
2.1. Inclusion Criteria of RA Patients and Parameters Chosen with RA-Specific
2.2. Introducing the JIV Measurement
2.3. Non-RA Patients Recruiting, General Parameters Picking Up, and Comparison Between the Groups
2.4. Determining the Cutoff Index of the Risk Factors and Evaluating the Candidate Risk Factors Separated by the Cutoff Index
2.5. Comparing Hazard Ratios Among the Non-RA and Strongest Hazard Ratio Groups in the Combined Criteria
2.6. Statistical Procedures
2.7. Informed Consent Attainment
2.8. Ethical Consideration
3. Results
3.1. Demographic and Clinical Characteristics and the General and RA-Specific Candidate Risk Factors in the RA and Non-RA Group—Comparison Between the RA and Non-RA Group
3.2. Evaluation of the Candidate Risk Factors in the RA and the Non-RA Group
3.3. Determining the COI of the Risk Factors and Hazard Ratios According to the COI
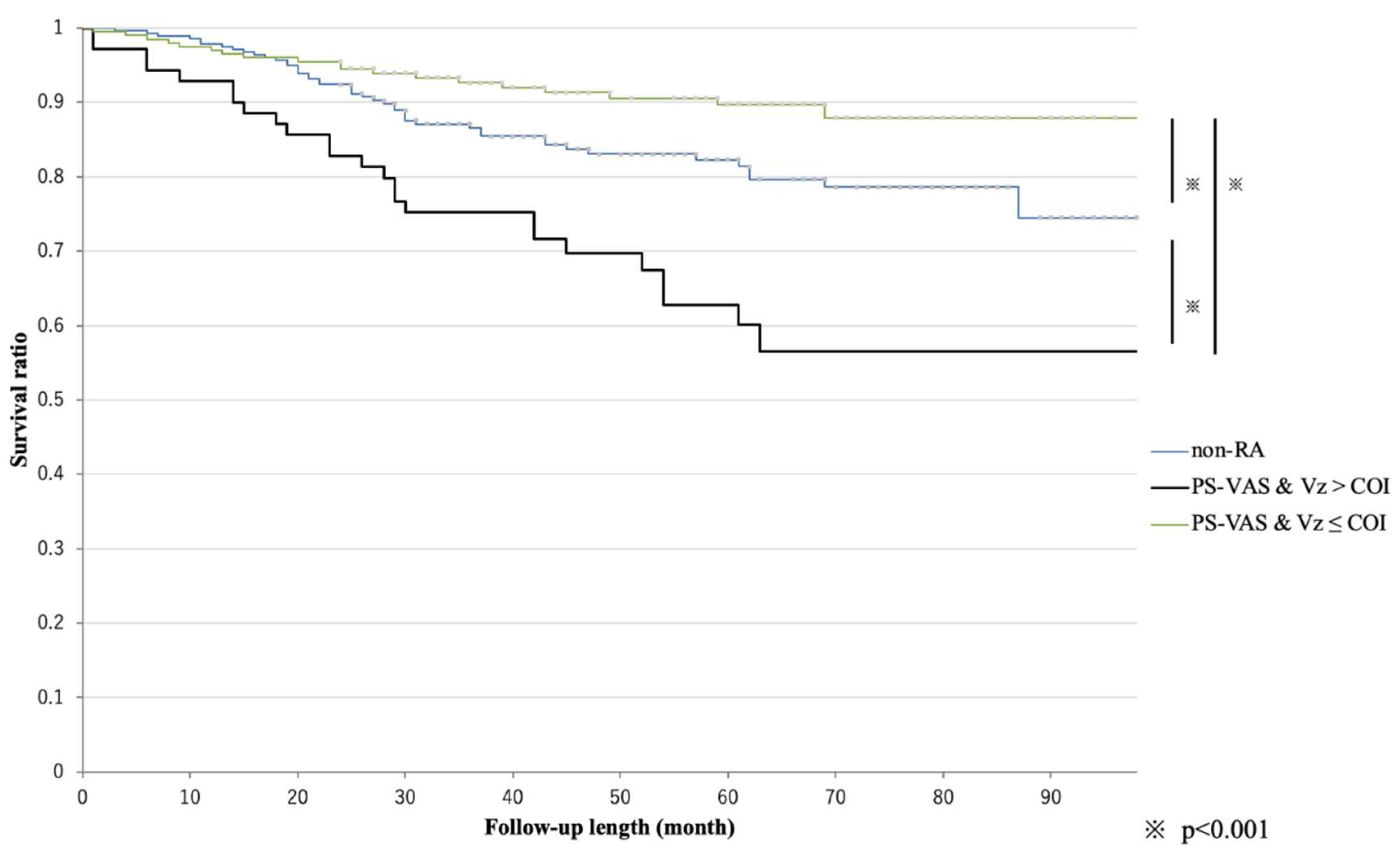
3.4. Kaplan–Meier Survival Analysis After Re-Set of the Factor Combined with Significant RA-Specific Factors
3.5. Comparing Hazard Ratios Among the Non-RA and Strongest Hazard Ratio Groups in the Combined Criteria
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Declaration of Generative AI Statement
References
- Raterman, H.G.; Lems, W.F. Pharmacological Management of Osteoporosis in Rheumatoid Arthritis Patients: A Review of the Literature and Practical Guide. Drugs Aging 2019, 36, 1061–1072. [Google Scholar] [CrossRef]
- Kanis, J.A.; Johansson, H.; Harvey, N.C.; McCloskey, E.V. A brief history of FRAX. Arch. Osteoporos. 2018, 13, 118. [Google Scholar] [CrossRef]
- Ozen, G.; Pedro, S.; Wolfe, F.; Michaud, K. Medications associated with fracture risk in patients with rheumatoid arthritis. Ann. Rheum. Dis. 2019, 78, 1041–1047. [Google Scholar] [CrossRef] [PubMed]
- Adami, G.; Saag, K.G. Osteoporosis Pathophysiology, Epidemiology, and Screening in Rheumatoid Arthritis. Curr. Rheumatol. Rep. 2019, 21, 34. [Google Scholar] [CrossRef]
- Phuan-udom, R.; Lektrakul, N.; Katchamart, W. The association between 10-year fracture risk by FRAX and osteoporotic fractures with disease activity in patients with rheumatoid arthritis. Clin. Rheumatol. 2018, 37, 2603–2610. [Google Scholar] [CrossRef]
- Kim, S.Y.; Schneeweiss, S.; Liu, J.; Daniel, G.W.; Chang, C.-L.; Garneau, K.; Solomon, D.H. Risk of osteoporotic fracture in a large population-based cohort of patients with rheumatoid arthritis. Arthritis Res. Ther. 2010, 12, R154. [Google Scholar] [CrossRef] [PubMed]
- Van Vollenhoven, R. Treat-to-target in rheumatoid arthritis—are we there yet? Nat. Rev. Rheumatol. 2019, 15, 180–186. [Google Scholar] [CrossRef] [PubMed]
- Yoshii, I.; Sawada, N.; Chijiwa, T.; Kokei, S. Impact of sustaining SDAI remission for preventing incident of bone fragility fracture in patient with rheumatoid arthritis. Ann. Rheum. Dis. 2022, 81, 296–299. [Google Scholar] [CrossRef]
- Yoshii, I.; Chijiwa, T.; Sawada, N. Rheumatoid arthritis in tight disease control is no longer risk of bone mineral density loss. Osteoporos. Sarcopenia 2020, 6, 75–81. [Google Scholar] [CrossRef]
- Yoshii, I.; Sawada, N.; Chijiwa, T. Pain score as a predictor of subsequent fragility fracture in postmenopausal patients with rheumatoid arthritis: A retrospective case-control study. Osteoporos. Sarcopenia 2023, 9, 150–156. [Google Scholar] [CrossRef]
- Kay, J.; Upchurch, K.S. ACR/EULAR 2010 rheumatoid arthritis classification criteria. Rheumatology 2012, 51, vi5–vi9. [Google Scholar] [CrossRef]
- Smolen, J.S.; Aletaha, D.; Bijlsma, J.W.; Breedveld, F.C.; Boumpas, D.; Burmester, G.; Combe, B.; Cutolo, M.; de Wit, M.; Dougados, M.; et al. Treating rheumatoid arthritis to target: Recommendations of an international task force. Ann. Rheum. Dis. 2010, 69, 631–637. [Google Scholar] [CrossRef]
- Nishiyama, S.; Sawada, T.; Nishino, J.; Tohma, S. Joint index vector: A novel assessment measure for stratified medicine in patients with rheumatoid arthritis. J. Big Data 2018, 5, 37. [Google Scholar] [CrossRef]
- Cummings, S.R.; Melton, L.J. Epidemiology and outcomes of osteoporotic fractures. Lancet 2002, 359, 1761–1767. [Google Scholar] [CrossRef] [PubMed]
- Michaud, K.; Wolfe, F. Comorbidities in rheumatoid arthritis. Best Pract. Res. Clin. Rheumatol. 2007, 21, 885–906. [Google Scholar] [CrossRef] [PubMed]
- Fonseca, H.; Moreira-Gonçalves, D.; Coriolano, H.J.; Duarte, J.A. Bone quality: The determinants of bone strength and fragility. Sports Med. 2014, 44, 37–53. [Google Scholar] [CrossRef]
- Saito, M.; Marumo, K. Effects of Collagen Crosslinking on Bone Material Properties in Health and Disease. Calcif. Tissue Int. 2015, 97, 242–261. [Google Scholar] [CrossRef]
- Compston, J. Glucocorticoid-induced osteoporosis: An update. Endocrine 2018, 61, 7–16. [Google Scholar] [CrossRef]
- Jin, S.; Hsieh, E.; Peng, L.; Yu, C.; Wang, Y.; Wu, C.; Wang, Q.; Li, M.; Zeng, X. Incidence of fractures among patients with rheumatoid arthritis: A systematic review and meta-analysis. Osteoporos. Int. 2018, 29, 1263–1275. [Google Scholar] [CrossRef] [PubMed]
- Kerschan-Schindl, K. Prevention and rehabilitation of osteoporosis. Wien. Med. Wochenschr. 2016, 166, 22–27. [Google Scholar] [CrossRef]
- Rosen, C.J. The Epidemiology and Pathogenesis of Osteoporosis; MDText.com, Inc.: South Dartmouth, MA, USA, 2020. [Google Scholar]
- Dimai, H.P.; Fahrleitner-Pammer, A. Osteoporosis and Fragility Fractures: Currently available pharmacological options and future directions. Best Pr. Res. Clin. Rheumatol. 2022, 36, 101780. [Google Scholar] [CrossRef]
- Foessl, I.; Dimai, H.P.; Obermayer-Pietsch, B. Long-term and sequential treatment for osteoporosis. Nat. Rev. Endocrinol. 2023, 19, 520–533. [Google Scholar] [CrossRef] [PubMed]
- Derksen, V.; Huizinga, T.W.J.; van der Woude, D. The role of autoantibodies in the pathophysiology of rheumatoid arthritis. Semin. Immunopathol. 2017, 39, 437–446. [Google Scholar] [CrossRef] [PubMed]
- Toes, R.; Pisetsky, D.S. Pathogenic effector functions of ACPA: Where do we stand? Ann. Rheum. Dis. 2019, 78, 716–721. [Google Scholar] [CrossRef] [PubMed]
- Krishnamurthy, A.; Ytterberg, A.J.; Sun, M.; Sakuraba, K.; Steen, J.; Joshua, V.; Tarasova, N.K.; Malmström, V.; Wähämaa, H.; Réthi, B.; et al. Citrullination Controls Dendritic Cell Transdifferentiation into Osteoclasts. J. Immunol. 2019, 202, 3143–3150. [Google Scholar] [CrossRef]
- Orsolini, G.; Caimmi, C.; Viapiana, O.; Idolazzi, L.; Fracassi, E.; Gatti, D.; Adami, G.; Rossini, M. Titer-dependent effect of anti-citrullinated protein antibodies on systemic bone mass in rheumatoid arthritis patients. Calcif. Tissue Int. 2017, 101, 17–23. [Google Scholar] [CrossRef]
- Amkreutz, J.A.M.P.; de Moel, E.C.; Theander, L.; Willim, M.; Heimans, L.; Nilsson, J.Å.; Karlsson, M.K.; Huizinga, T.W.J.; Åkesson, K.E.; Jacobsson, L.T.H.; et al. Association between bone mineral density and autoantibodies in patients with rheumatoid arthritis. Arthritis Rheumatol. 2021, 73, 921–930. [Google Scholar] [CrossRef]
- Yoshii, I.; Chijiwa, T.; Sawada, N. The influence of anti-citrullinated polypeptide antibodies on bone mineral density decrease and incident major osteoporotic fractures in patients with rheumatoid arthritis: A retrospective case-control study. Osteology 2023, 3, 47–60. [Google Scholar] [CrossRef]
- Studenic, P.; Smolen, J.S.; Aletaha, D. Near misses of ACR/EULAR criteria for remission: Effects of patient global assessment in Boolean and index-based definitions. Ann. Rheum. Dis. 2012, 71, 1702–1705. [Google Scholar] [CrossRef]

| RA Group (n = 278) | Non-RA Group (n = 278) | p-Value | |
|---|---|---|---|
| women, % | 86.0 | 86.3 | 0.985 |
| Age, years | 74.1 (10.7) | 75.0 (10.8) | 0.119 |
| BMI, kg/m2 | 22.6 (4.2) | 23.5 (4.1) | 0.187 |
| follow-up period, months | 85.1 (26.2) | 85.0 (25.8) | 0.974 |
| incident BFF, % | 15.8 | 16.5 | 0.833 |
| 10-year MOF, % | 27.0 (18.4) | 16.1 (10.7) | <0.001 |
| Lifestyle-related diseases, % | 80.9 | 75.9 | 0.131 |
| type2 DM, % | 23.4 | 28.4 | 0.170 |
| COPD, % | 10.4 | 7.6 | 0.238 |
| hypertension, % | 57.2 | 54.3 | 0.526 |
| hyperlipidemia, % | 34.2 | 34.2 | 0.976 |
| chronic heart failure, % | 20.9 | 19.4 | 0.690 |
| CKD ≥ Grade3a, % | 49.5 | 49.6 | 0.955 |
| insomnia, % | 23.4 | 23.3 | 0.939 |
| Fall-ability, % | 63.3 | 69.4 | 0.115 |
| MADS, % | 15.8 | 21.9 | 0.064 |
| osteoarthritis, % | 55.4 | 60.1 | 0.288 |
| Disuse, % | 9.0 | 4.7 | 0.051 |
| Contractures, % | 13.6 | 10.1 | 0.190 |
| Parkinsonism, % | 2.5 | 2.2 | 0.798 |
| cognitive impairment, % | 9.4 | 8.3 | 0.649 |
| T-score | −1.73 (1.17) | −1.71 (0.97) | 0.805 |
| pr-BFF, % | 50.4 | 55.8 | 0.220 |
| anti-osteoporotic drug †, % | 61.2 | 58.6 | 0.581 |
| GCS administration until baseline, % | 55.8 | 13.3 | 0.034 |
| GCS mean dosage ‡, mg/day | 2.7 (3.4) | 5.9 (7.7) | <0.001 |
| GCS total dose ever ‡, mg | 2460 (6481) | 859 (4972) | <0.001 |
| disease duration of RA, years | 7.6 (8.8) | ||
| ACPA titer at BL, U/mL | 176.6 (478.9) | ||
| RF titer at BL, IU/mL | 93.2 (214.0) | ||
| SDAI score at BL | 5.67 (7.91) | ||
| HAQ-DI at BL | 0.548 (0.656) | ||
| SHS at BL | 60.3 (73.6) | ||
| mean SDAI score after BL | 4.39 (4.38) | ||
| mean SDAI remission rate after BL, % | 39.5 (32.5) |
| Univariate Model | Multivariate Model | |||
|---|---|---|---|---|
| Candidate Risk Factors | Hazard Ratio | p-Value | Hazard Ratio | p-Value |
| (95% CI) | (95% CI) | |||
| General | ||||
| female | 6.42 | 0.07 | ||
| (0.88–46.70) | ||||
| older age | 1.01 | 0.33 | ||
| (0.99–1.04) | ||||
| lower T-score | 0.83 | 0.12 | ||
| (0.66–1.05) | ||||
| prevalent bone fragility fractures | 5.85 | <0.001 | 5.01 | <0.001 |
| (2.60–13.12) | (1.97–12.71) | |||
| lifestyle-related diseases | 4.83 | <0.05 | 2.99 | 0.29 |
| (1.17–19.95) | (0.40–22.52) | |||
| Fall-ability | 2.75 | <0.01 | 1.46 | 0.40 |
| (1.28–5.91) | (0.61–3.51) | |||
| cognitive impairment | 2.23 | <0.05 | 0.71 | 0.51 |
| (1.04–4.80) | (0.25–1.97) | |||
| anti-osteoporotic drug administration | 0.92 | 0.8 | ||
| (0.50–1.72) | ||||
| GCS administration | 1.71 | 0.08 | ||
| (0.94–3.09) | ||||
| higher serum albumin level at baseline | 0.6 | 0.24 | ||
| (0.26–1.40) | ||||
| higher mean serum albumin level after baseline | 0.52 | 0.12 | ||
| (0.23–1.17) | ||||
| higher PNI at baseline | 0.97 | 0.2 | ||
| (0.93–1.01) | ||||
| higher mean PNI after baseline | 0.97 | 0.12 | ||
| (0.93–1.01) | ||||
| RA specific | ||||
| longer disease duration of RA | 0.98 | 0.31 | ||
| (0.94–1.02) | ||||
| higher ACPA titer | 1.00 | <0.01 | 1.0007 | <0.01 |
| (1.00–1.00) | (1.00–1.00) | |||
| higher RF titer | 0.83 | 0.12 | ||
| (0.66–1.05) | ||||
| higher SDAI score at baseline | 1.02 | 0.27 | ||
| (0.99–1.05) | ||||
| higher HAQ-DI at baseline | 1.43 | 0.09 | ||
| (0.95–2.17) | ||||
| higher SHS score at baseline | 1 | 0.7 | ||
| (0.996–1.004) | ||||
| higher PS-VAS at baseline | 1.01 | <0.05 | 1.00 | 0.94 |
| (1.00–1.02) | (0.98–1.02) | |||
| higher Vx at baseline | 1.36 | 0.63 | ||
| (0.39–4.73) | ||||
| higher Vy at baseline | 1.24 | 0.67 | ||
| (0.46–3.35) | ||||
| higher Vxy at baseline | 1.13 | 0.78 | ||
| (0.48–2.66) | ||||
| higher Vz at baseline | 2.39 | 12 | ||
| (0.79–7.12) | ||||
| higher mean SDAI score after baseline | 1.05 | <0.05 | 0.99 | 0.99 |
| (1.00–1.10) | (0.91–1.08) | |||
| higher mean HAQ-DI after baseline | 1.36 | 0.17 | ||
| (0.87–2.11) | ||||
| higher mean PS-VAS after baseline | 1.02 | <0.001 | 1.03 | <0.05 |
| (1.01–1.04) | (1.00–1.05) | |||
| higher mean Vx after baseline | 2.22 | 0.4 | ||
| (0.35–14.09) | ||||
| higher mean Vy after baseline | 1.67 | 0.6 | ||
| (0.25–11.28) | ||||
| higher mean Vxy after baseline | 1.66 | 0.47 | ||
| (0.42–6.54) | ||||
| higher mean Vz after baseline | 5.22 | <0.05 | 2.21 | 0.59 |
| (1.01–33.30) | (0.12–39.62) | |||
| RA Group (N = 278) | ||||
|---|---|---|---|---|
| Univariate Model | Multivariate Model | |||
| Candidate Risk Factors | Hazard Ratio | p-Value | Hazard Ratio | p-Value |
| (95% CI) | (95% CI) | |||
| General Factor | ||||
| female | 6.42 | 0.07 | ||
| (0.88–46.70) | ||||
| older age | 1.01 | 0.33 | ||
| (0.99–1.04) | ||||
| lower T-score | 0.83 | 0.12 | ||
| (0.66–1.05) | ||||
| prevalent bone fragility fractures | 5.85 | <0.001 | 4.72 | <0.001 |
| (2.60–13.12) | (2.06–10.79) | |||
| lifestyle-related diseases | 4.83 | <0.05 | 2.83 | 0.16 |
| (1.17–19.95) | (0.67–12.06) | |||
| Fall-ability | 2.75 | <0.01 | 1.73 | 0.4 |
| (1.28–5.91) | (0.78–3.84) | |||
| cognitive impairment | 2.23 | <0.05 | 1.16 | 0.71 |
| (1.04–4.80) | (0.53–2.56) | |||
| anti-osteoporotic drug administration | 0.92 | 0.8 | ||
| (0.50–1.72) | ||||
| GCS administration | 1.71 (0.94–3.09) | 0.08 | ||
| non-RA group (N = 278) | ||||
| General factor | ||||
| female | 0.99 | 0.98 | ||
| (0.42–2.34) | ||||
| older age | 1.01 | 0.39 | ||
| (0.98–1.04) | ||||
| lower T-score | 1.15 | 0.24 | ||
| (0.91–1.45) | ||||
| prevalent bone fragility fractures | 2.76 | <0.01 | 2.1 | 0.06 |
| (1.40–5.45) | (0.98–4.50) | |||
| lifestyle-related diseases | 2.87 | <0.05 | 1.64 | 0.36 |
| (1.13–7.30) | (0.57–4.69) | |||
| Fall-ability | 1.99 | 0.08 | ||
| (0.93–4.26) | ||||
| cognitive impairment | 2.68 | <0.01 | 2.04 | 0.06 |
| (1.29–5.56) | (0.97–4.29) | |||
| anti-osteoporotic drug administration | 1.75 | 0.07 | ||
| (0.97–3.16) | ||||
| GCS administration | 0.92 (0.36–2.32) | 0.85 | ||
| Together (N = 556) | ||||
| General factor | ||||
| presenting RA | 0.9 | 0.62 | ||
| (0.60–1.36) | ||||
| female | 1.76 | 0.15 | ||
| (0.82–3.82) | ||||
| older age | 1.01 | 0.19 | ||
| (0.99–1.04) | ||||
| lower T-score | 1.18 | 0.06 | ||
| (1.00–1.39) | ||||
| prevalent bone fragility fractures | 3.93 | <0.001 | 2.94 | <0.001 |
| (2.34–6.59) | (1.69–5.12) | |||
| lifestyle-related diseases | 3.26 | <0.001 | 1.7 | 0.18 |
| (1.58 –6.74) | (0.79–3.68) | |||
| Fall-ability | 2.39 | <0.01 | 1.72 | 0.06 |
| (2.39–4.10) | (0.99–3.01) | |||
| cognitive impairment | 2.46 | <0.001 | 1.49 | 0.15 |
| (1.45–4.17) | (0.86–2.57) | |||
| anti-osteoporotic drug administration | 1.74 | <0.05 | 1.14 | 0.62 |
| (1.05–2.90) | (0.68–1.94) | |||
| GCS administration | 1.32 | 0.23 | ||
| (0.84–2.08) | ||||
| ROC | Kaplan–Meier | |||||
|---|---|---|---|---|---|---|
| Factor | COI | AUC (95%CI) | p-Value | Prevalence (+/−) | Hazard Ratio (95%CI) | p-Value |
| pr-BFF | present | 0.700 (0.637–0.764) | <0.001 | 26.4%/5.1% | 5.83 (3.22–10.53) | <0.001 |
| LSD | present | 0.586 (0.545–0.627) | <0.001 | 18.7%/3.8% | 4.82 (2.26–10.30) | <0.05 |
| Fall | present | 0.610 (0.544–0.676) | <0.001 | 20.5%/7.8% | 2.75 (1.49–5.05) | <0.01 |
| CI | present | 0.552 (0.492–0.613) | 0.09 | 30.8%/14.3% | 2.23 (0.80–6.23) | <0.05 |
| ACPA | >0.9 | 0.551 (0.451–0.652) | 0.32 | 21.2%/7.1% | 3.07 (1.39–6.80) | 0.05 |
| PS-VAS@BL | >21.0 | 0.601 (0.508–0.695) | <0.05 | 22.7%/11.3% | 2.09 (1.14–3.83) | <0.05 |
| PS-VAS@FU | ≥25.5 | 0.658 (0.568–0.748) | <0.001 | 26.1%/8.6% | 3.36 (1.83–6.16) | <0.001 |
| SDAI@FU | ≥2.11 | 0.654 (0.568–0.739) | <0.001 | 21.0%/5.4% | 4.06 (2.18–7.56) | <0.01 |
| Vz@FU | >0.01 | 0.604 (0.515–0.693) | <0.05 | 22.9%/7.1% | 3.33 (1.83–6.05) | <0.01 |
| Kaplan–Meier | |||
|---|---|---|---|
| Combining Factors | Prevalence (+/−) | Hazard Ratio (95%CI) | p-Value |
| PS-VAS at baseline and PS-VAS after baseline | 26.9%/11.5% | 2.48 (1.27–4.83) | <0.01 |
| PS-VAS at baseline and SDAI after baseline | 26.1%/10.8% | 2.49 (1.32–4.67) | <0.01 |
| PS-VAS at baseline and Vz after baseline | 33.3%/11.4% | 3.10 (1.50–6.38) | <0.001 |
| PS-VAS after baseline and SDAI after baseline | 28.6%/8.1% | 3.87 (2.09–7.17) | <0.001 |
| PS-VAS after baseline and Vz after baseline | 35.7%/9.5% | 4.25 (2.12–8.53) | <0.001 |
| SDAI after baseline and Vz after baseline | 26.6%/7.5% | 3.71 (2.05–6.71) | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yoshii, I.; Sawada, N.; Chijiwa, T. Validation of Combined Indicator Using Joint Index Vector and Pain Score for Risk Weight Calculation of Incident Bone Fragility Fracture in Patients with Rheumatoid Arthritis. Osteology 2025, 5, 35. https://doi.org/10.3390/osteology5040035
Yoshii I, Sawada N, Chijiwa T. Validation of Combined Indicator Using Joint Index Vector and Pain Score for Risk Weight Calculation of Incident Bone Fragility Fracture in Patients with Rheumatoid Arthritis. Osteology. 2025; 5(4):35. https://doi.org/10.3390/osteology5040035
Chicago/Turabian StyleYoshii, Ichiro, Naoya Sawada, and Tatsumi Chijiwa. 2025. "Validation of Combined Indicator Using Joint Index Vector and Pain Score for Risk Weight Calculation of Incident Bone Fragility Fracture in Patients with Rheumatoid Arthritis" Osteology 5, no. 4: 35. https://doi.org/10.3390/osteology5040035
APA StyleYoshii, I., Sawada, N., & Chijiwa, T. (2025). Validation of Combined Indicator Using Joint Index Vector and Pain Score for Risk Weight Calculation of Incident Bone Fragility Fracture in Patients with Rheumatoid Arthritis. Osteology, 5(4), 35. https://doi.org/10.3390/osteology5040035





