Static and Dynamic Torque in the Modulation of the Caudal Vertebral Growth
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Model
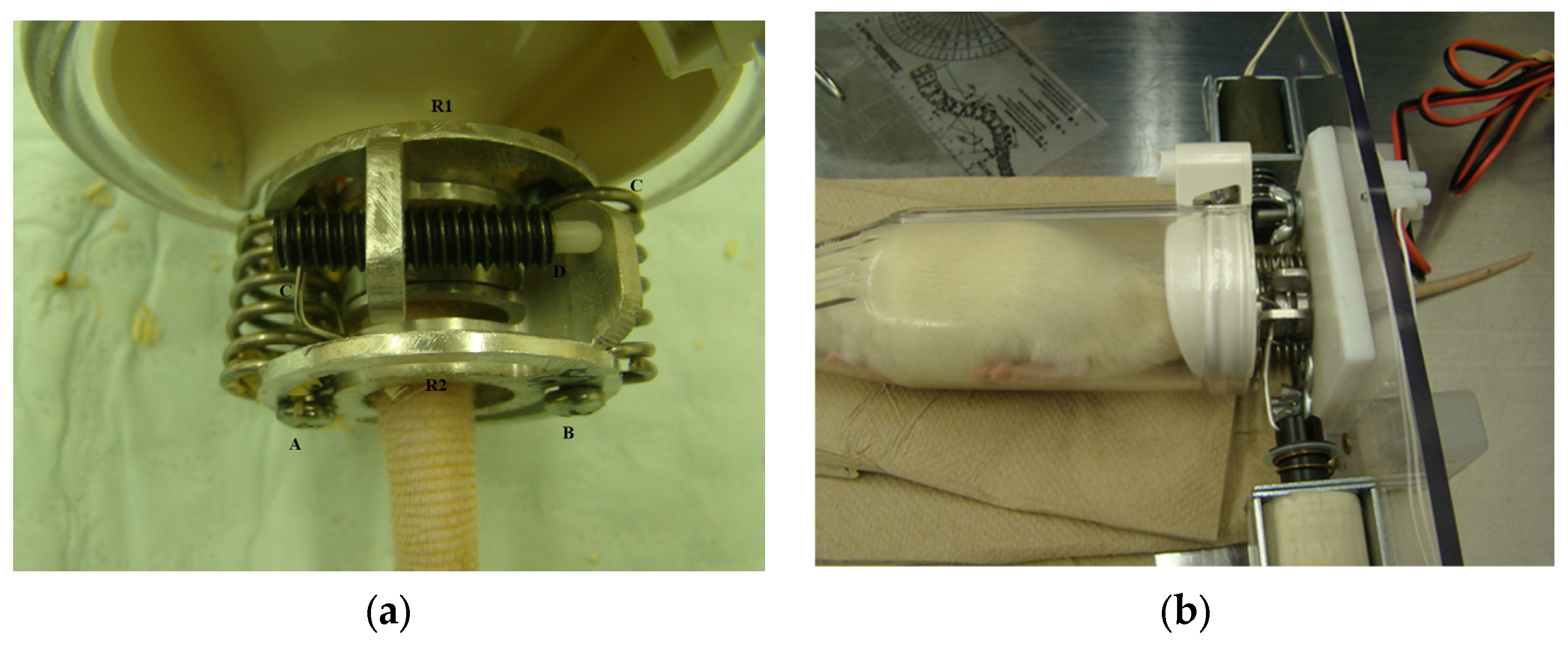
2.2. In Vivo Torque Apparatus with Static Loading and Its Validation
2.3. Caudal Vertebrae Surgery to Mount the In Vivo Torque Apparatus
2.4. Adjusting the In Vivo Torque Device for Static and Dynamic Loading
2.5. Experimental Protocol
2.6. Statistical Analysis
3. Results
3.1. Visual Observation and Radiographic Measurements
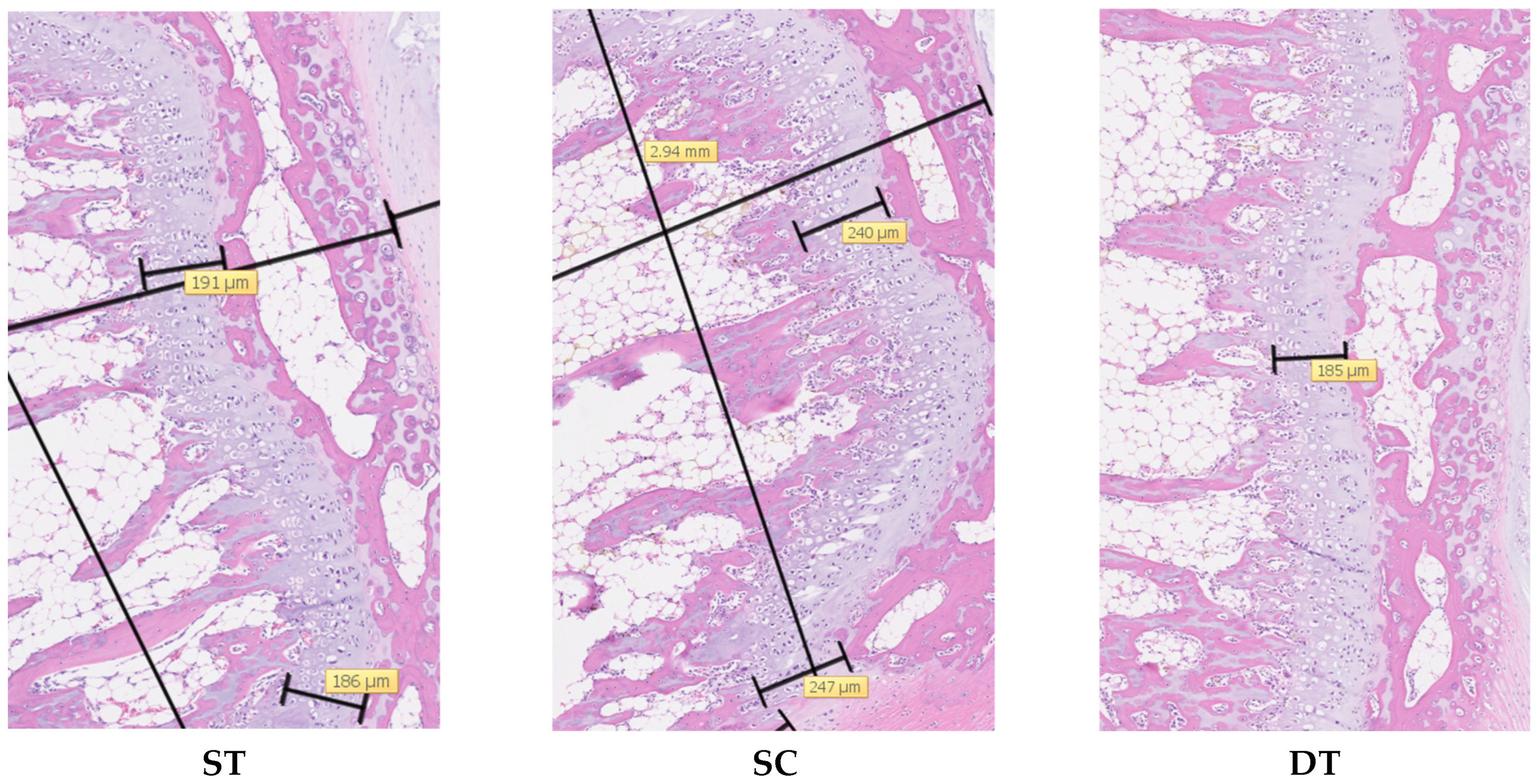
3.2. Histological Analysis-Physeal Height
3.3. Histological Analysis-Physeal Zone Heights
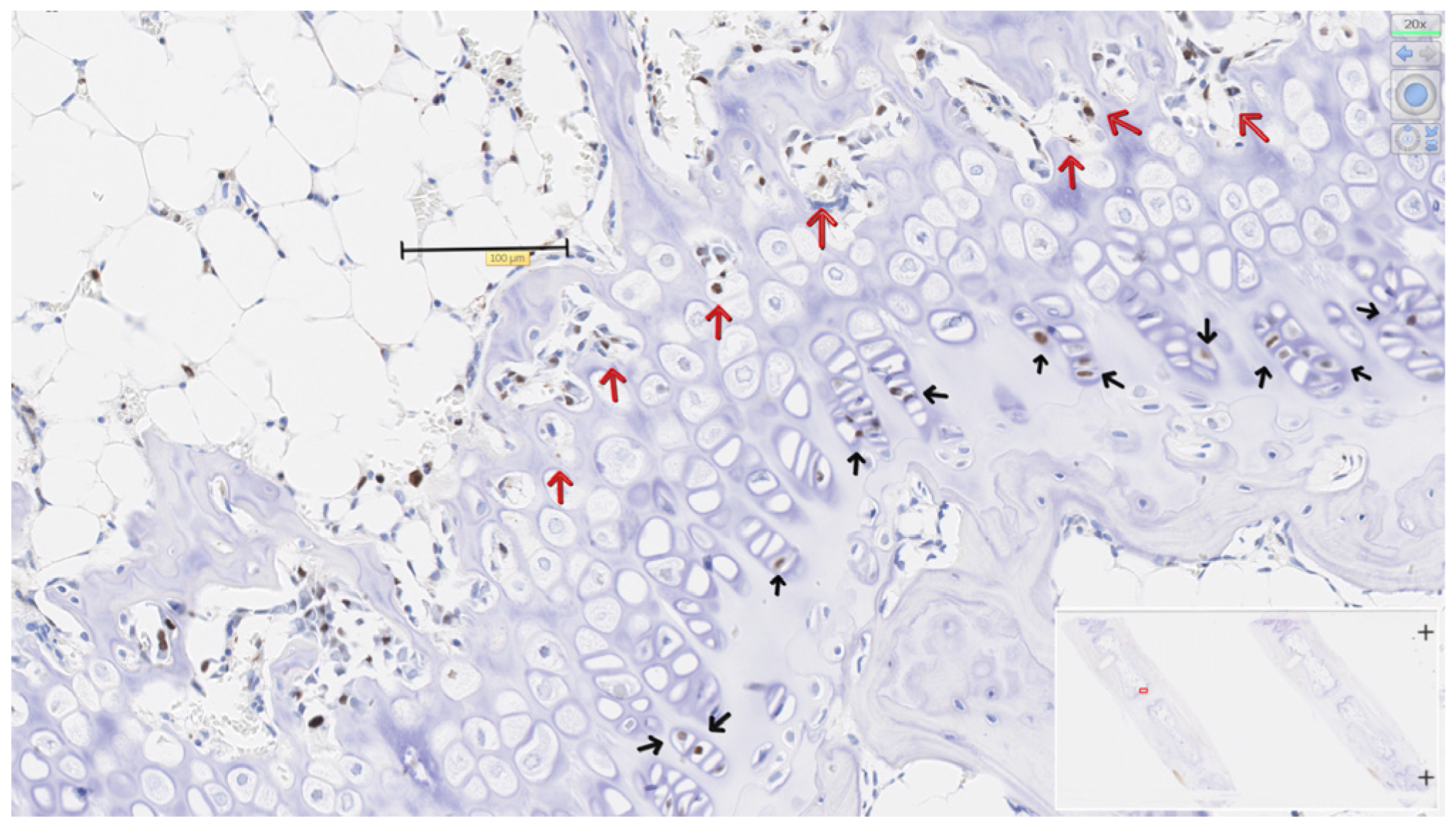
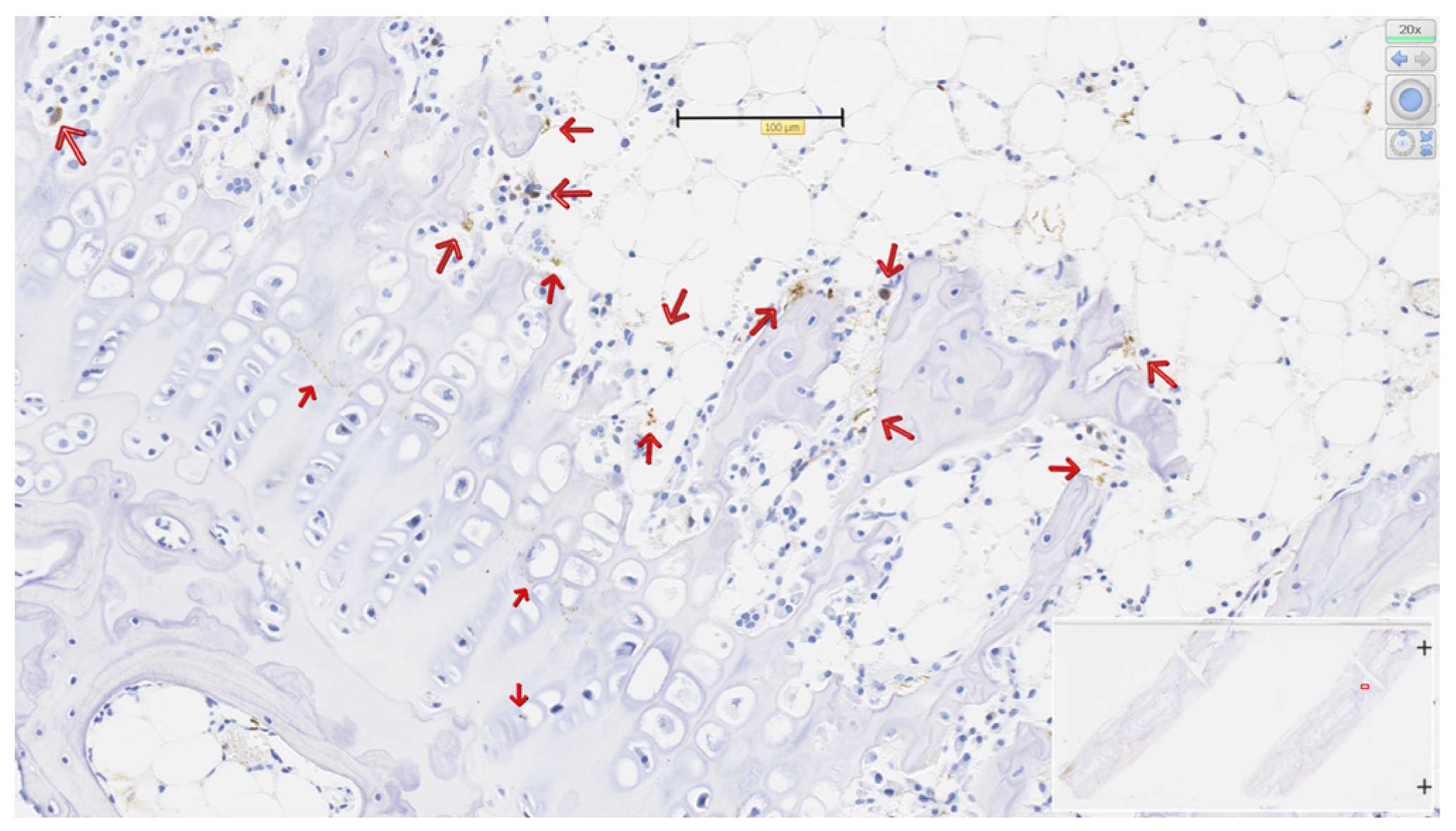
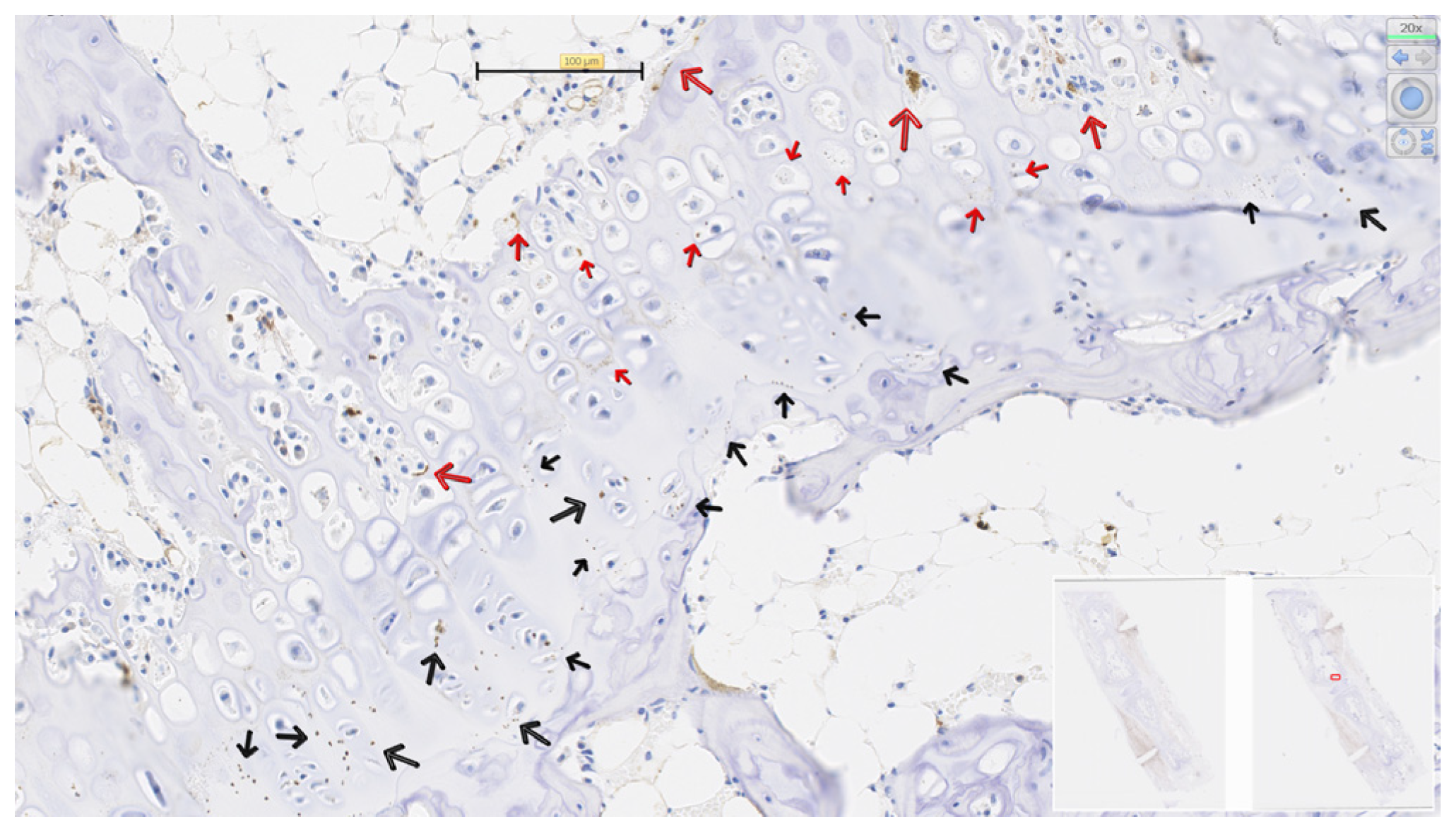
3.4. PCNA in Physeal Zones
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AIS | Adolescent idiopathic scoliosis |
| SD | Sprague-Dawley |
| VBT | Vertebral body tethering |
| MAC | Minimum alveolar concentration |
| PCNA | Proliferating cell nuclear antigen |
| Ca | Caudal vertebral body |
| ANOVA | Analysis of variance |
| ANCOVA | Analysis of covariance |
| SC | Sham control |
| ST | Static torque |
| DT | Dynamic control |
| FSU | Functional spinal unit |
References
- Bagnall, K.M. Using a synthesis of the research literature related to the etiology of adolescent idiopathic scoliosis to provide ideas on future directions for success. Scoliosis 2008, 3, 5. [Google Scholar] [CrossRef]
- Desroches, G.; Aubin, C.-E.; Rivard, C.H. Biomechanical modeling of anterior spine instrumentation in AIS. Stud. Health Technol. Inform. 2006, 123, 415–418. [Google Scholar]
- Gardner-Morse, M.G.; Stokes, I.A.F. Structural behavior of human lumbar spinal motion segments. J. Biomech. 2004, 37, 205–212. [Google Scholar] [CrossRef]
- Stokes, I.A.F.; Burwell, R.G.; Dangerfield, P.H.; IBSE. Biomechanical spinal growth modulation and progressive adolescent scoliosis—A test of the “vicious cycle” pathogenetic hypothesis: Summary of an electronic focus group debate of the IBSE. Scoliosis 2006, 1, 16. [Google Scholar] [CrossRef] [PubMed]
- Alfraihat, A.; Olson, J.C.; Snyder, B.D.; Cahill, P.J.; Balasubramanian, S. Thoracic vertebral morphology in normal and scoliosis deformity in skeletally immature rabbits: A Longitudinal study. JOR Spine 2020, 3, e1118. [Google Scholar] [CrossRef] [PubMed]
- Stokes, I.A.; Aronsson, D.D.; Dimock, A.N.; Cortright, V.; Beck, S. Endochondral growth in growth plates of three species at two anatomical locations modulated by mechanical compression and tension. J. Orthop. Res. 2006, 24, 1327–1334. [Google Scholar] [CrossRef] [PubMed]
- D’Andrea, C.R.; Alfraihat, A.; Singh, A.; Anari, J.B.; Cahill, P.J.; Schaer, T.; Snyder, B.D.; Elliott, D.; Balasubramanian, S. Part 1. Review and meta-analysis of studies on modulation of longitudinal bone growth and growth plate activity: A macro-scale perspective. J. Orthop. Res. 2021, 39, 907–918. [Google Scholar] [CrossRef]
- Brink, R.C.; Homans, J.F.; Schlösser, T.P.C.; van Stralen, M.; Vincken, K.L.; Shi, L.; Chu, W.C.W.; Viergever, M.A.; Castelein, R.M.; Cheng, J.C.Y. CT-based study of vertebral and intravertebral rotation in right thoracic adolescent idiopathic scoliosis. Eur. Spine J. 2019, 28, 3044–3052. [Google Scholar] [CrossRef]
- Kouwenhoven, J.-W.M.; Vincken, K.L.; Bartels, L.W.; Castelein, R.M. Analysis of Preexistent Vertebral Rotation in the Normal Spine. Spine 2006, 31, 1467–1472. [Google Scholar] [CrossRef]
- Barbir, A.; Godburn, K.E.; Michalek, A.J.; Lai, A.; Monsey, R.D.; Iatridis, J.C. Effects of Torsion on Intervertebral Disc Gene Expression and Biomechanics, Using a Rat Tail Model. Spine 2011, 36, 607–614. [Google Scholar] [CrossRef]
- Michalek, A.J.; Funabashi, K.L.; Iatridis, J.C. Needle puncture injury of the rat intervertebral disc affects torsional and compressive biomechanics differently. Eur. Spine J. 2010, 19, 2110–2116. [Google Scholar] [CrossRef]
- Michalek, A.J.; Iatridis, J.C. Height and torsional stiffness are most sensitive to annular injury in large animal intervertebral discs. Spine J. 2012, 12, 425–432. [Google Scholar] [CrossRef] [PubMed]
- Benoit, A.; Mustafy, T.; Londono, I.; Grimard, G.; Aubin, C.E.; Villemure, I. In vivo dynamic compression has less detrimental effect than static compression on newly formed bone of a rat caudal vertebra. J. Musculoskelet Neuronal Interact 2016, 16, 211–220. [Google Scholar]
- Ménard, A.L.; Grimard, G.; Valteau, B.; Londono, I.; Moldovan, F.; Villemure, I. In vivo dynamic loading reduces bone growth without histomorphometric changes of the growth plate: Growth plate dynamic loading. J. Orthop. Res. 2014, 32, 1129–1136. [Google Scholar] [CrossRef] [PubMed]
- Stokes, I.A.; Spence, H.; Aronsson, D.D.; Kilmer, N. Mechanical modulation of vertebral body growth. Implications for scoliosis progression. Spine 1996, 21, 1162–1167. [Google Scholar] [CrossRef] [PubMed]
- Stokes, I.A.; Mente, P.L.; Iatridis, J.C.; Farnum, C.E.; Aronsson, D.D. Enlargement of growth plate chondrocytes modulated by sustained mechanical loading. J. Bone Jt. Surg. Am. 2002, 84, 1842–1848. [Google Scholar] [CrossRef]
- Valteau, B.; Grimard, G.; Londono, I.; Moldovan, F.; Villemure, I. In vivo dynamic bone growth modulation is less detrimental but as effective as static growth modulation. Bone 2011, 49, 996–1004. [Google Scholar] [CrossRef]
- Alberty, A.; Peltonen, J.; Ritsilä, V. Effects of distraction and compression on proliferation of growth plate chondrocytes. A study in rabbits. Acta Orthop. Scand. 1993, 64, 449–455. [Google Scholar] [CrossRef]
- Cancel, M.; Grimard, G.; Thuillard-Crisinel, D.; Moldovan, F.; Villemure, I. Effects of in vivo static compressive loading on aggrecan and type II and X collagens in the rat growth plate extracellular matrix. Bone 2009, 44, 306–315. [Google Scholar] [CrossRef]
- Stokes, I.A.F.; Clark, K.C.; Farnum, C.E.; Aronsson, D.D. Alterations in the growth plate associated with growth modulation by sustained compression or distraction. Bone 2007, 41, 197–205. [Google Scholar] [CrossRef][Green Version]
- Rizza, R.; Liu, X.C.; Thometz, J.; Lyon, R.; Tassone, C. Implementation of a New Torque Device in Ox-Tails. In Summer Bioengineering Conference; American Society of Mechanical Engineers: New York, NY, USA, 2010; Parts A and B:819–820. [Google Scholar] [CrossRef]
- Rizza, R.; Liu, X.C. Mechanics and validation of an in vivo device to apply torsional loading to caudal vertebrae. J. Biomech. Eng. 2013, 135, 81003. [Google Scholar] [CrossRef]
- Lazarus, D.E.; Farnsworth, C.L.; Jeffords, M.E.; Marino, N.; Hallare, J.; Edmonds, E.W. Torsional Growth Modulation of Long Bones by Oblique Plating in a Rabbit Model. J. Pediatr. Orthop. 2018, 38, e97–e103. [Google Scholar] [CrossRef]
- Moreland, M. Morphological effects of torsion applied to growing bone. An in vivo study in rabbits. J. Bone Jt. Surg. 1980, 62, 230–237. [Google Scholar] [CrossRef]
- Driscoll, M.; Aubin, C.É.; Moreau, A.; Wakula, Y.; Amini, S.; Parent, S. Novel Hemi-Staple for the Fusionless Correction of Pediatric Scoliosis: Influence on Intervertebral Disks and Growth Plates in a Porcine Model. Clin. Spine Surg. 2016, 29, 457–464. [Google Scholar] [CrossRef]
- Liu, J.; Li, Z.; Shen, J.; Xue, X. Spinal growth modulation with posterior unilateral elastic tether in immature swine model. Spine J. 2015, 15, 138–145. [Google Scholar] [CrossRef]
- Wong, H.K.; Ruiz, J.N.M.; Newton, P.O.; Liu, K.P.G. Non-Fusion surgical correction of thoracic idiopathic scoliosis using a novel, braided vertebral body tethering device: Minimum follow-up of 4 years. JBJS Open Access 2019, 4, e0026. [Google Scholar] [CrossRef]
- Sun, K.; Liu, F.; Wang, J.; Guo, Z.; Ji, Z.; Yao, M. The effect of mechanical stretch stress on the differentiation and apoptosis of human growth plate chondrocytes. In Vitro Cell Dev. Biol. Anim. 2017, 53, 141–148. [Google Scholar] [CrossRef] [PubMed]
- Irie, T.; Aizawa, T.; Kokubun, S. The role of sex hormones in the kinetics of chondrocytes in the growth plate: A study in the rabbit. J. Bone Jt. Surg. 2005, 87, 1278–1284. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Wu, Y.; Cheng, P.; Zhang, J.; Yang, C.; Pi, B.; Ye, Y.; You, H.; Chen, A.; Xu, T.; et al. YAP and ERK mediated mechanical strain-induced cell cycle progression through RhoA and cytoskeletal dynamics in rat growth plate chondrocytes. J. Orthop. Res. 2016, 34, 1121–1129. [Google Scholar] [CrossRef]
- Akyuz, E.; Braun, J.T.; Brown, N.A.T.; Bachus, K.N. Static Versus Dynamic Loading in the Mechanical Modulation of Vertebral Growth. Spine 2006, 31, E952–E958. [Google Scholar] [CrossRef] [PubMed]
- Mente, P.L.; Aronsson, D.D.; Stokes, I.A.; Iatridis, J.C. Mechanical modulation of growth for the correction of vertebral wedge deformities. J. Orthop. Res. 1999, 17, 518–524. [Google Scholar] [CrossRef] [PubMed]
- González-Magaña, A.; Blanco, F.J. Human PCNA Structure, Function and Interactions. Biomolecules 2020, 10, 570. [Google Scholar] [CrossRef]
- Hirata, H.; Yurube, T.; Kakutani, K.; Maeno, K.; Takada, T.; Yamamoto, J.; Kurakawa, T.; Akisue, T.; Kuroda, R.; Kurosaka, M.; et al. A rat tail temporary static compression model reproduces different stages of intervertebral disc degeneration with decreased notochordal cell. J. Orthop. Res. 2014, 32, 455–463. [Google Scholar] [CrossRef] [PubMed]
- Ménard, A.L.; Grimard, G.; Massol, E.; Londono, I.; Moldovan, F.; Villemure, I. Static and dynamic compression application and removal on the intervertebral discs of growing rats: Disc compression in growing rats. J. Orthop. Res. 2016, 34, 290–298. [Google Scholar] [CrossRef] [PubMed]
- Stokes, I.A.F.; McBride, C.A.; Aronsson, D.D.; Roughley, P.J. Metabolic Effects of Angulation, Compression, and Reduced Mobility on Annulus Fibrosis in a Model of Altered Mechanical Environment in Scoliosis. Spine Deform. 2013, 1, 161–170. [Google Scholar] [CrossRef]
- D’Andrea, C.R.; Alfraihat, A.; Singh, A.; Anari, J.B.; Cahill, P.J.; Schaer, T.; Snyder, B.D.; Elliott, D.; Balasubramanian, S. Part 2. Review and meta-analysis of studies on modulation of longitudinal bone growth and growth plate activity: A micro-scale perspective. J. Orthop. Res. 2021, 39, 919–928. [Google Scholar] [CrossRef]
- Roach, H.I.; Mehta, G.; Oreffo, R.O.; Clarke, N.M.; Cooper, C. Temporal analysis of rat growth plates: Cessation of growth with age despite presence of a physis. J. Histochem. Cytochem. 2003, 51, 373–383. [Google Scholar] [CrossRef]

| Parameter | Static Torque | Sham Control | Dynamic Torque |
|---|---|---|---|
| Mean disc height (mm) | 0.004 ± 0.03 *1 | 0.16 ± 0.29 | 0.001 ± 0.05 *2 |
| Right-sided disc height (mm) | −0.01 ± 0.05 *3 | 0.15 ± 0.29 | 0.03 ± 0.06 *4 |
| Parameter | ST | SC | DT |
|---|---|---|---|
| Ca7 Growth Plate Heights | |||
| Middle of Proximal Growth Plate (mm) | 0.17 ± 0.02 *1 | 0.21 ± 0.03 | 0.16 ± 0.03 *2 |
| Left side of Distal Growth Plate (mm) | 0.18 ± 0.03 *3 | 0.24 ± 0.04 | 0.18 ± 0.06 *4 |
| Ca8 Growth Plate Heights | |||
| Middle of Proximal Growth Plate (mm) | 0.18 ± 0.02 *5 | 0.23 ± 0.03 | 0.17 ± 0.02 *6 |
| Right side of Proximal Growth Plate (mm) | 0.18 ± 0.04 *7 | 0.23 ± 0.05 | 0.16 ± 0.03 *8 |
| Parameter | ST | SC | DT |
|---|---|---|---|
| Ca7 Growth Plate Zones | |||
| Proliferative (R-side) of Proximal Physis (μm) | 66.23 ± 24.85 | 66.3 ± 13.65 | 108.4 ± 21.8 a1,b1 |
| Reserve (L-side) of Distal Physis (μm) | 26.6 ± 12.28 a2 | 47.18 ± 7.88 | 24.96 ± 9.22 a3 |
| Hypertrophic (Middle) of Distal Physis (μm) | 105 ± 16.85 | 77.77 ± 17.75 | 113.6 ± 31.71 a4 |
| Ca8 Growth Plate Zones | |||
| Hypertrophic (R-side) of Proximal Physis (μm) | 75.6 ± 16.94 | 71.27 ± 14.50 | 48.55 ± 12.88 a5,b2 |
| Hypertrophic (Middle) of Distal Physis (μm) | 97.93 ± 28.15 a6 | 64.3 ± 18.28 | 62.22 ± 19.73 b3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, X.-C.; Rizza, R.; Thometz, J.; Allen, A.; Rosol, D.; Tassone, C.; North, P.; Jensen, E. Static and Dynamic Torque in the Modulation of the Caudal Vertebral Growth. Osteology 2025, 5, 31. https://doi.org/10.3390/osteology5040031
Liu X-C, Rizza R, Thometz J, Allen A, Rosol D, Tassone C, North P, Jensen E. Static and Dynamic Torque in the Modulation of the Caudal Vertebral Growth. Osteology. 2025; 5(4):31. https://doi.org/10.3390/osteology5040031
Chicago/Turabian StyleLiu, Xue-Cheng, Robert Rizza, John Thometz, Andrew Allen, Derek Rosol, Channing Tassone, Paula North, and Eric Jensen. 2025. "Static and Dynamic Torque in the Modulation of the Caudal Vertebral Growth" Osteology 5, no. 4: 31. https://doi.org/10.3390/osteology5040031
APA StyleLiu, X.-C., Rizza, R., Thometz, J., Allen, A., Rosol, D., Tassone, C., North, P., & Jensen, E. (2025). Static and Dynamic Torque in the Modulation of the Caudal Vertebral Growth. Osteology, 5(4), 31. https://doi.org/10.3390/osteology5040031






