Evaluating the Outcomes of Vertebral Biopsies Performed in Osteoporotic Vertebral Fractures: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Eligibility Criteria
2.2. Literature Strategy and Data Sources
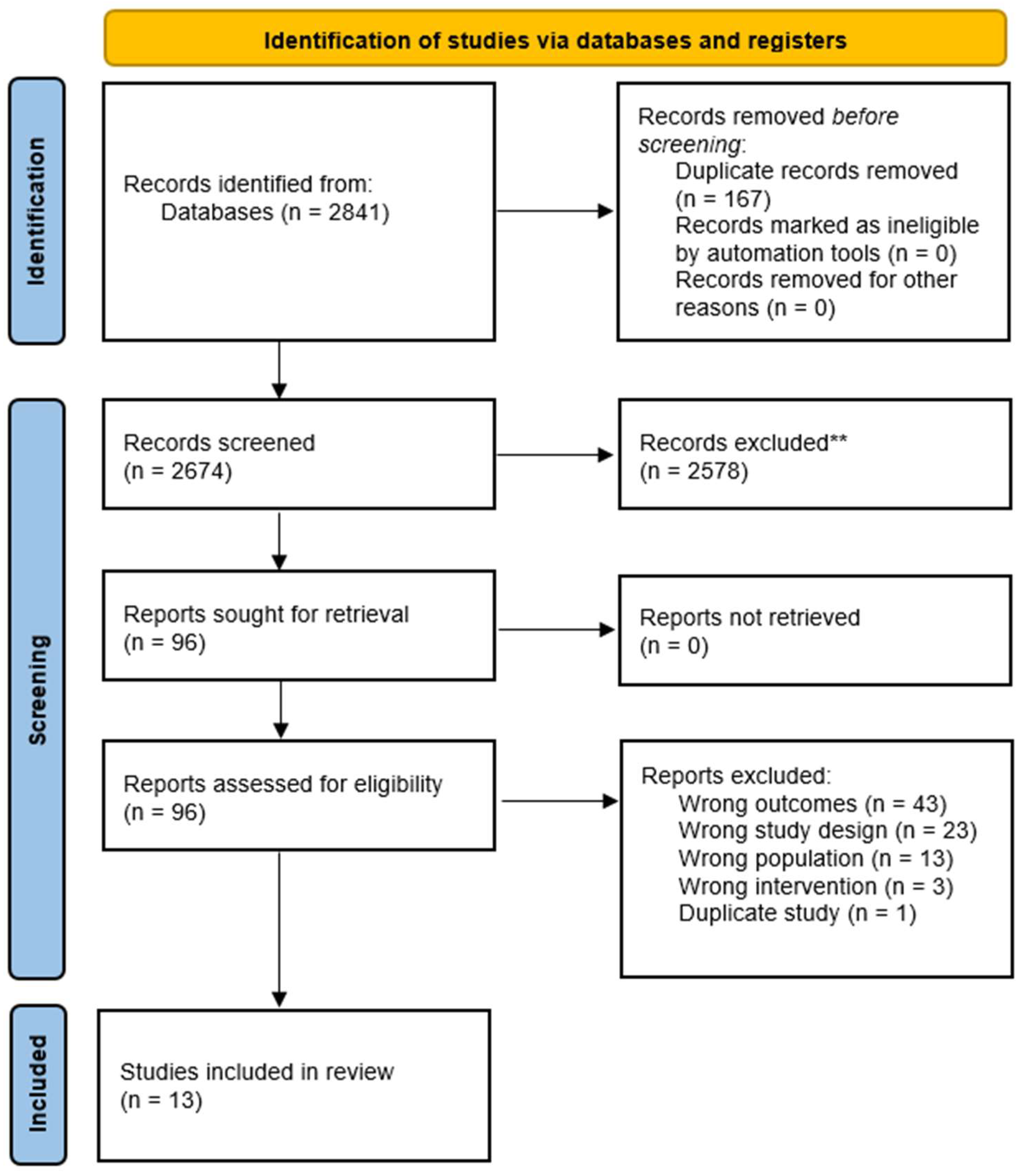
2.3. Study Selection
2.4. Data Extraction
- (1)
- Study characteristics (authors, publication year, country, and design);
- (2)
- Patient demographics (sample size, age, and sex);
- (3)
- Biopsy technique and imaging modalities used;
- (4)
- Fracture location and how diagnosis was confirmed (biopsy vs. radiologic follow-up);
- (5)
- Outcomes of interest, primarily the prevalence and types of malignancy identified (e.g., solid tumors, multiple myeloma, lymphoma).
2.5. Risk of Bias Assessment
2.6. Statistical Analysis
3. Results
3.1. Study Characteristics
3.2. Prevalence of Solid Tumor Metastases
3.3. Prevalence of Multiple Myeloma
3.4. Unsuspected Versus Suspected Biopsy Protocols
3.5. Subgroup Analysis of Suspected Cases
3.6. Complications
3.7. Bias Assessment
4. Discussion
4.1. Principal Findings
4.2. Comparison with Existing Literature
4.3. Biopsy Versus Imaging in Diagnostic Accuracy
4.4. Patient Selection and Triage for Biopsy
4.5. Clinical Benefits of Early Diagnosis for Patient Management
4.6. Health Economics and Policy Considerations
4.7. Future Perspectives
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hernlund, E.; Svedbom, A.; Ivergård, M.; Compston, J.; Cooper, C.; Stenmark, J.; McCloskey, E.V.; Jönsson, B.; Kanis, J.A. Osteoporosis in the European Union: Medical Management, Epidemiology and Economic Burden: A Report Prepared in Collaboration with the International Osteoporosis Foundation (IOF) and the European Federation of Pharmaceutical Industry Associations (EFPIA). Arch. Osteoporos. 2013, 8, 136. [Google Scholar] [CrossRef] [PubMed]
- Osterhoff, G.; Scheyerer, M.J.; Spiegl, U.J.A.; Schnake, K.J. The Role of Routine Transpedicular Biopsies during Kyphoplasty or Vertebroplasty for Vertebral Compression Fractures in the Detection of Malignant Diseases: A Systematic Review. Arch. Orthop. Trauma Surg. 2023, 143, 1887–1893. [Google Scholar] [CrossRef]
- Kafchinski, L.A. Metastasectomy for Oligometastatic Bone Disease of the Appendicular Skeleton: A Concise Review. J. Surg. Oncol. 2023, 128, 438–444. [Google Scholar] [CrossRef]
- Wickstrøm, L.A.; Rafaelsen, S.R.; Andersen, M.; Andresen, A.D.K.; Elmose, S.F.; Carreon, L. Rate of Unexpected Malignancy in Patients Undergoing Percutaneous Vertebroplasty after Implementing a New Scanning Protocol. Spine 2024, 49, E300–E305. [Google Scholar] [CrossRef]
- Schoenfeld, A.J.; DiNicola, N.J.; Ehrler, D.M.; Koerber, A.; Paxos, M.; Shorten, S.D.; Bowers, J.; Jackson, E.; Smith, M.J. Retrospective Review of Biopsy Results Following Percutaneous Fixation of Vertebral Compression Fractures. Injury 2008, 39, 327–333. [Google Scholar] [CrossRef]
- Hershkovich, O.; Bayley, E.; Rudik, O.; Alexandrovsky, V.; Friedlander, A.; Daglen, E.; Lotan, R. Cost-Benefit Analysis of Routine Bone Biopsy During Augmentation of Osteoporotic Vertebral Compression Fractures. Spine 2020, 45, 1634–1638. [Google Scholar] [CrossRef] [PubMed]
- Pagdal, S.S.; Nadkarni, S.; Hardikar, S.M.; Hardikar, M.S. Role of Transpedicular Percutaneous Vertebral Biopsy for Diagnosis of Pathology in Vertebral Compression Fractures. Asian Spine J. 2016, 10, 925. [Google Scholar] [CrossRef]
- Chou, K.N.; Lin, B.J.; Chien, L.Y.; Tsai, W.C.; Ma, H.I.; Hueng, D.Y. Simple Transpedicular Vertebral Biopsy for Diagnosis of Malignancy in Vertebral Compression Fracture. Neurol. India 2013, 61, 587–592. [Google Scholar] [CrossRef] [PubMed]
- Pneumaticos, S.G.; Chatziioannou, S.N.; Savvidou, C.; Pilichou, A.; Rontogianni, D.; Korres, D.S. Routine Needle Biopsy during Vertebral Augmentation Procedures. Is It Necessary? Eur. Spine J. 2010, 19, 1894. [Google Scholar] [CrossRef]
- Venturi, C.; Barbero, S.; Tappero, C.; Ciccone, V.; Mastrogiacomo, F.; Molinaro, L.; Gandini, G. La Biopsia Coassiale in Corso Di Vertebroplastica Percutanea in Pazienti Con Sospetti Cedimenti Vertebrali Porotici: Analisi Retrospettiva Dei Risultati Bioptici. Radiol. Medica 2011, 116, 302–309. [Google Scholar] [CrossRef]
- Muijs, S.P.J.; Akkermans, P.A.; Van Erkel, A.R.; Dijkstra, S.D. The Value of Routinely Performing a Bone Biopsy during Percutaneous Vertebroplasty in Treatment of Osteoporotic Vertebral Compression Fractures. Spine 2009, 34, 2395–2399. [Google Scholar] [CrossRef]
- Joseph, R.N.; Swift, A.J.; Maliakal, P.J. Single Centre Prospective Study of the Efficacy of Percutaneous Cement Augmentation in the Treatment of Vertebral Compression Fractures. Br. J. Neurosurg. 2013, 27, 459–464. [Google Scholar] [CrossRef]
- Jia, J.; Li, J. Age Was a Protective Factor for Unexpected Malignant Diagnoses in Patients with Vertebral Compression Fracture. Clin. Neurol. Neurosurg. 2024, 243, 108377. [Google Scholar] [CrossRef] [PubMed]
- Sozzi, C.; Trentadue, M.; Nicolì, L.; Tavani, F.; Piovan, E. Utility of Vertebral Biopsy before Vertebroplasty in Patients with Diagnosis of Vertebral Compression Fracture. Radiol. Medica 2021, 126, 956–962. [Google Scholar] [CrossRef]
- Nowak, S.; Müller, J.; Schroeder, H.W.S.; Müller, J.U. Incidence of Unexpected Positive Histology in Kyphoplasty. Eur. Spine J. 2018, 27, 847–850. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, 71. [Google Scholar] [CrossRef] [PubMed]
- Lam, C.; Bulut, H.; Ozkan, K. Malignancy Rates in Osteoporotic Vertebral Fractures: A Systematic Review and Meta-Analysis. PROSPERO NIHR. 2025. Available online: https://www.crd.york.ac.uk/PROSPERO/view/CRD420251052182 (accessed on 1 October 2025).
- Ottawa Hospital Research Institute. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-Analyses. Available online: https://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (accessed on 17 August 2025).
- Jamovi—Open Statistical Software for the Desktop and Cloud. Available online: https://www.jamovi.org/ (accessed on 16 August 2025).
- Jia, J.; Chen, C.; Wang, P. Evaluation of biopsy results during vertebral augmentation in patients with a known history of malignancy. Eur. Radiol. 2023, 33, 4422–4428. [Google Scholar] [CrossRef] [PubMed]
- Tariq, M.B.; Obedian, R. Role of Bone Biopsy During Kyphoplasty in the Setting of Known Cancer: A Case Report. Spine 2021, 46, E1220–E1224. [Google Scholar] [CrossRef] [PubMed]
- Öcal, Ö.; Günerhan, G.; Divanlıoğlu, D.; Seçen, A.E.; Gündü, U.K.; Saylak, B. Utility of Routine Needle Biopsy During Kyphoplasty For Osteoporotic Vertebral Fractures. J. Turk. Spinal Surg. 2022, 33, 140–143. [Google Scholar] [CrossRef]
- Zhu, Y.; Yang, K.; Wang, C.; Fan, Y.; Wu, X.; He, S.; Gu, G. Towards optimized biopsy use in vertebral compression fractures: Integrating risk assessment for better clinical decision-making. Int. Orthop. 2025, 49, 203–209. [Google Scholar] [CrossRef]
- Miao, K.H.; Miao, J.H.; Belani, P.; Dayan, E.; Carlon, T.A.; Cengiz, T.B.; Finkelstein, M. Radiological Diagnosis and Advances in Imaging of Vertebral Compression Fractures. J. Imaging. 2024, 10, 244. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, Y.; Zhang, E.; Chen, Y.; Wang, Q.; Liu, K.; Yu, H.J.; Yuan, H.; Lang, N.; Su, M.-Y. Differential diagnosis of benign and malignant vertebral fracture on CT using deep learning. Eur. Radiol. 2021, 31, 9612–9619. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Iwata, E.; Shigematsu, H.; Morita, T.; Tanaka, M.; Okuda, A.; Masuda, K.; Ikejiri, M.; Nakajima, H.; Koizumi, M.; et al. Differential diagnosis between metastatic and osteoporotic vertebral fractures using sagittal T1-weighted magnetic resonance imaging. J. Orthop. Sci. 2020, 25, 763–769. [Google Scholar] [CrossRef]
- Mauch, J.T.; Carr, C.M.; Cloft, H.; Diehn, F.E. Review of the Imaging Features of Benign Osteoporotic and Malignant Vertebral Compression Fractures. AJNR Am. J. Neuroradiol. 2018, 39, 1584–1592. [Google Scholar] [CrossRef]
- Sutcliffe, P.; Connock, M.; Shyangdan, D.; Court, R.; Kandala, N.B.; Clarke, A. A Systematic Review of Evidence on Malignant Spinal Metastases: Natural History and Technologies for Identifying Patients at High Risk of Vertebral Fracture and Spinal Cord Compression. Health Technol. Assess. 2013, 17, 1–274. [Google Scholar] [CrossRef]
- Patel, S.S.; Tao, D.; McMurry, H.S.; Shatzel, J.J. Screening for Occult Cancer after Unprovoked Venous Thromboembolism: Assessing the Current Literature and Future Directions. Eur. J. Haematol. 2023, 110, 24–31. [Google Scholar] [CrossRef] [PubMed]
- Piccioli, A.; Lensing, A.W.A.; Prins, M.H.; Falanga, A.; Scannapieco, G.L.; Ieran, M.; Cigolini, M.; Ambrosio, G.B.; Monreal, M.; Girolami, A.; et al. Extensive Screening for Occult Malignant Disease in Idiopathic Venous Thromboembolism: A Prospective Randomized Clinical Trial. J. Thromb. Haemost. 2004, 2, 884–889. [Google Scholar] [CrossRef]
- Nottebaert, M.; Exner, G.U.; von Hochstetter, A.R.; Schreiber, A. Metastatic Bone Disease from Occult Carcinoma: A Profile. Int. Orthop. 1989, 13, 119–123. [Google Scholar] [CrossRef] [PubMed]
- Barzilai, O.; Versteeg, A.L.; Sahgal, A.; Rhines, L.D.; Bilsky, M.H.; Sciubba, D.M.; Schuster, J.M.; Weber, M.H.; Pal Varga, P.; Boriani, S.; et al. Survival, Local Control, and Health-Related Quality of Life in Patients with Oligometastatic and Polymetastatic Spinal Tumors: A Multicenter, International Study. Cancer 2019, 125, 770–778. [Google Scholar] [CrossRef] [PubMed]
- van Tol, F.R.; Versteeg, A.L.; Verkooijen, H.M.; Öner, F.C.; Verlaan, J.-J. Time to Surgical Treatment for Metastatic Spinal Disease: Identification of Delay Intervals. Glob. Spine J. 2021, 13, 316–323. [Google Scholar] [CrossRef]
- Amelink, J.J.G.J.; van Munster, B.T.; Bindels, B.J.J.; Pierik, R.J.B.; van Tiel, J.; Groot, O.Q.; Kasperts, N.; Tobert, D.G.; Verlaan, J.J. Surgical management of spinal metastases: A cross-continental study in the United States and the Netherlands. J. Bone Oncol. 2025, 52, 100676. [Google Scholar] [CrossRef] [PubMed]
- Fürstenberg, C.H.; Wiedenhöfer, B.; Gerner, H.J.; Putz, C. The effect of early surgical treatment on recovery in patients with metastatic compression of the spinal cord. J. Bone Jt. Surg. Br. 2009, 91, 240–244. [Google Scholar] [CrossRef]
- Hsieh, C.J.; Wu, C.Y.; Lin, Y.H.; Huang, Y.C.; Yang, W.C.; Chen, T.W.; Ma, W.L.; Lin, W.H.; Hsu, F.M.; Xiao, F.; et al. Delay of Surgery for Spinal Metastasis due to the COVID-19 Outbreak Affected Patient Outcomes. Neurospine 2023, 20, 1431–1442. [Google Scholar] [CrossRef]
- Bangash, A.H.; Kirnaz, S.; Fluss, R.; Cao, V.; Alexandrov, A.; Belman, L.; Gelfand, Y.; Murthy, S.G.; Yassari, R.; De la Garza Ramos, R. Short-Term Outcomes in Planned Versus Unplanned Surgery for Spinal Metastases. Cancers 2025, 17, 2403. [Google Scholar] [CrossRef]
- Zeoli, T.; Chanbour, H.; Ahluwalia, R.; Abtahi, A.M.; Stephens, B.F.; Zuckerman, S.L. Does Elective Admission vs. Emergency Department Presentation Affect Surgical Outcomes in Metastatic Spine Surgery? Diagnostics 2024, 14, 1058. [Google Scholar] [CrossRef]
- Ammann, C.; Maqkaj, R.; Schneider, M.A.; Hehl, S.J.; Fritsch, R.; Pohl, D.; Rogler, G.; Gubler, C.; Turina, M.; Scharl, M. Detection rate of colorectal cancer by routine colonoscopy is comparable in patients aged 45–49 and 50–54 years. Schweiz. Med. Wochenschr. 2024, 154, 3769. [Google Scholar] [CrossRef]
- Stratmann, K.; Czerwinska, K.; Filmann, N.; Tacke, W.; Weber, C.; Bock, H.; Blumenstein, I. Prevalence of colorectal cancer and its precursor lesions in symptomatic patients under 55 years of age undergoing total colonoscopy: Results of a large retrospective, multicenter, controlled endoscopy study. Int. J. Colorectal. Dis. 2021, 36, 1695–1700. [Google Scholar] [CrossRef]
- American College of Radiology. Management of Vertebral Compression Fractures. ACR Appropriateness Criteria. Revised 2022. Available online: https://acsearch.acr.org/docs/70545/Narrative (accessed on 16 September 2025).
- Tokuhashi, Y.; Uei, H.; Oshima, M.; Ajiro, Y. Scoring System for Prediction of Metastatic Spine Tumor Prognosis. World J. Orthop. 2014, 5, 262. [Google Scholar] [CrossRef] [PubMed]
- Tomita, K.; Kawahara, N.; Kobayashi, T.; Yoshida, A.; Murakami, H.; Akamaru, T. Surgical strategy for spinal metastases. Spine (Phila Pa 1976) 2001, 26, 298–306. [Google Scholar] [CrossRef] [PubMed]
| Author | Year | Patient Count | Malignant Cases | Malignancy (%) | Age (Mean/Median) | Multiple Myeloma (n) | Primary Bone Tumors (n) | Metastatic Tumors (n) |
|---|---|---|---|---|---|---|---|---|
| Wickstrøm et al. [4] | 2024 | 459 | 27 | 5.88 | 75 | NSM | NSM | NSM |
| Schoenfeld et al. [5] | 2008 | 50 | 4 | 8.00 | 76 | 1 | 0 | 3 |
| Hershkovich et al. [6] | 2020 | 113 | 13 | 11.50 | 71 | 9 | 0 | 4 |
| Chou et al. [8] | 2013 | 450 | 61 | 13.56 | — | 9 | 0 | 52 |
| Joseph et al. [12] | 2012 | 56 | 8 | 14.29 | 60 | NSM | NSM | NSM |
| Nowak et al. [15] | 2018 | 97 | 10 | 10.31 | 68 | — | 0 | 10 |
| Pagdal et al. [7] | 2016 | 84 | 10 | 11.90 | 63 | 8 | 0 | 2 |
| Venturi et al. [10] | 2011 | 98 | 2 | 2.04 | 73 | 0 | 1 (Chondrosarcoma) | 1 |
| Jia et al. [13] | 2024 | 1352 | 44 | 3.25 | 70 | 24 | 0 | 20 |
| Muijs et al. [11] | 2009 | 71 | 3 | 4.23 | 73 | 1 | 0 | 0 |
| Jia et al. [20] | 2023 | 156 | 73 | 46.79 | 66 | 20 | 0 | 53 |
| Sozzi et al. [14] | 2021 | 324 | 20 | 6.17 | 73 | 12 | 0 | 8 |
| Pneumaticos et al. [9] | 2010 | 75 | 11 | 14.67 | 69 | 3 | 0 | 8 |
| Diagnosis Category | Pooled Prevalence (%) | 95% CI (Lower–Upper) | p-Value | Number of Studies (k) |
|---|---|---|---|---|
| Solid Malignancy Metastasis | 4.87 | 2.30–7.44 | <0.001 | 9 |
| Multiple Myeloma | 2.62 | 1.31–3.94 | <0.001 | 8 |
| All Malignant Diagnoses | 8.00 | 5.43–10.60 | <0.001 | 12 |
| Protocol | k | n | Malignant Cases | Pooled % (95% CI) | I2 | Prediction Interval |
|---|---|---|---|---|---|---|
| Unsuspected cases | 9 | 2700 | 72 | 2.74% (1.83–4.09) | 41.7% | 1.21–6.12% |
| Suspected cases | 4 | 271 | 108 | 36.77% (22.06–54.44) | 82.5% | 11.72–71.80% |
| Subgroup | k | n | Malignant Cases | Pooled % (95% CI) | I2 | Prediction Interval |
|---|---|---|---|---|---|---|
| History + Imaging | 2 | 59 | 27 | 45.8% (33.6–58.6) | 0% | 33.6–58.6% |
| Imaging-only suspicion | 1 | 56 | 8 | 14.3% (7.3–26.1) | – | 7.3–26.1% |
| Known malignancy history | 1 | 156 | 73 | 46.8% (39.1–54.6) | – | 39.1–54.6% |
| Study | Representativeness of Exposed Cohort | Selection of Non-Exposed | Ascertainment of Exposure | Outcome Not Present at Start | Comparability | Assessment of Outcome | Adequate Follow-Up Length | Adequacy of Follow-Up | Total Score (Max 9) |
|---|---|---|---|---|---|---|---|---|---|
| Wickstrøm et al. (2024) [4] | ★ | ★ | ★ | ★ | ★★ | ★ | ★ | ★ | 9 |
| Schoenfeld et al. (2008) [5] | ★ | ★ | ★ | ★ | ★★ | ★ | ★ | ★ | 9 |
| Hershkovich et al. (2020) [6] | ★ | ★ | ★ | ★ | ★★ | ★ | ★ | ★ | 9 |
| Chou et al. (2013) [8] | ★ | ★ | ★ | ★★ | ★ | ★ | ★ | 8 | |
| Joseph et al. (2012) [12] | ★ | ★ | ★ | ★★ | ★ | ★ | ★ | 8 | |
| Nowak et al. (2018) [15] | ★ | ★ | ★ | ★★ | ★ | ★ | ★ | 8 | |
| Venturi et al. (2011) [10] | ★ | ★ | ★ | ★ | ★★ | ★ | ★ | ★ | 9 |
| Jia et al. (2024) [13] | ★ | ★ | ★★ | ★ | ★ | ★ | 7 | ||
| Jia et al. (2023) [20] | ★ | ★ | ★★ | ★ | ★ | ★ | 7 | ||
| Muijs et al. (2009) [11] | ★ | ★ | ★ | ★ | ★★ | ★ | ★ | ★ | 9 |
| Sozzi et al. (2021) [14] | ★ | ★ | ★ | ★★ | ★ | ★ | ★ | 8 | |
| Pneumaticos et al. (2010) [9] | ★ | ★ | ★ | ★ | ★★ | ★ | ★ | ★ | 9 |
| Pagdal et al. (2016) [7] | ★ | ★ | ★ | ★ | ★★ | ★ | ★ | ★ | 9 |
| Domain | Suspicion Criterion | Clinical/Diagnostic Rationale | Suspicion Level | Recommendation | Key References |
|---|---|---|---|---|---|
| Clinical | Prior history of malignancy | Patients with known cancer have the highest rate of malignant VCFs (≈45–47%). | High | Always biopsy | [11,13,20] |
| Age/osteoporosis mismatch | Younger patients or those without osteoporosis risk factors should raise suspicion. | Moderate | Biopsy strongly recommended | [12,15] | |
| Persistent, nocturnal, or progressive pain | Uncharacteristic for benign OVFs; often indicates pathological fracture. | Moderate–High | Biopsy | [12] | |
| Neurological deterioration | Suggests epidural/paraspinal involvement by tumor. | High | Biopsy + urgent oncologic workup | [10] | |
| Laboratory | Anemia, high ESR/CRP, hypercalcemia, M-protein spike | Classic for multiple myeloma or systemic malignancy. | Moderate–High | Biopsy to confirm | [7] |
| Imaging (CT/MRI) | Pedicle or posterior element destruction | Rare in benign OVF; strong predictor of malignancy. | High | Biopsy | [8] |
| Convex posterior vertebral wall | Non-osteoporotic feature; indicates infiltration. | High | Biopsy | [8] | |
| Paraspinal or epidural soft-tissue mass | Direct evidence of tumor extension. | High | Biopsy | [8] | |
| Diffuse marrow signal abnormality on MRI | Suggests infiltrative process (myeloma/metastasis). | High | Biopsy | [8] | |
| Multiple non-adjacent lesions | More consistent with metastatic disease than osteoporosis. | High | Biopsy | [11] | |
| Diagnostic performance | “Benign-appearing” MRI but cancer history | 11/427 MRI-benign cases revealed malignancy only on biopsy; sensitivity rose from 59% → 85% when clinical history was added. | Moderate–High | Biopsy despite negative imaging | [4,5] |
| Triage guidance | High suspicion = history of cancer + ≥1 imaging red flag. | High probability of malignant VCF. | High | Definite biopsy | [8] |
| Moderate suspicion = equivocal MRI, clinical/lab mismatch. | Diagnostic uncertainty. | Moderate | Strongly recommend biopsy | [12] | |
| Low–moderate suspicion = typical osteoporotic VCF, no risk factors. | Still 2–3% malignancy detection even in “benign” cases. | Low–Moderate | Opportunistic biopsy during kyphoplasty/vertebroplasty | [2,6,9,14,28] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bulut, H.; Lam, C.; Sheth, V.; Ali, I.; Tsagkaris, C.; Jones, M.; Botchu, R.; Errani, C.; Hamzaoglu, A.; Ozkan, K. Evaluating the Outcomes of Vertebral Biopsies Performed in Osteoporotic Vertebral Fractures: A Systematic Review and Meta-Analysis. Osteology 2025, 5, 30. https://doi.org/10.3390/osteology5040030
Bulut H, Lam C, Sheth V, Ali I, Tsagkaris C, Jones M, Botchu R, Errani C, Hamzaoglu A, Ozkan K. Evaluating the Outcomes of Vertebral Biopsies Performed in Osteoporotic Vertebral Fractures: A Systematic Review and Meta-Analysis. Osteology. 2025; 5(4):30. https://doi.org/10.3390/osteology5040030
Chicago/Turabian StyleBulut, Halil, Chuck Lam, Veer Sheth, Iihan Ali, Christos Tsagkaris, Morgan Jones, Rajesh Botchu, Constantino Errani, Azmi Hamzaoglu, and Korhan Ozkan. 2025. "Evaluating the Outcomes of Vertebral Biopsies Performed in Osteoporotic Vertebral Fractures: A Systematic Review and Meta-Analysis" Osteology 5, no. 4: 30. https://doi.org/10.3390/osteology5040030
APA StyleBulut, H., Lam, C., Sheth, V., Ali, I., Tsagkaris, C., Jones, M., Botchu, R., Errani, C., Hamzaoglu, A., & Ozkan, K. (2025). Evaluating the Outcomes of Vertebral Biopsies Performed in Osteoporotic Vertebral Fractures: A Systematic Review and Meta-Analysis. Osteology, 5(4), 30. https://doi.org/10.3390/osteology5040030







