Discrepancy Between the 10-Year Probability of Major Osteoporotic Fracture with FRAX and the Actual Fracture Prevalence over 10 Years in Japanese
Abstract
1. Introduction
2. Materials and Methods
2.1. Recruiting Patients, Bone Mineral Density Measurement, and Existing Fracture Evaluation
2.2. Risk Factor Selection and Group Comparison
2.3. Following Up
2.4. Fracture Probability Calculation with FRAX and Comparison with Actual Fracture Performance
2.5. Statistical Procedures and Software
2.6. Ethical Considerations
3. Results
3.1. Patient Demographic Characteristics
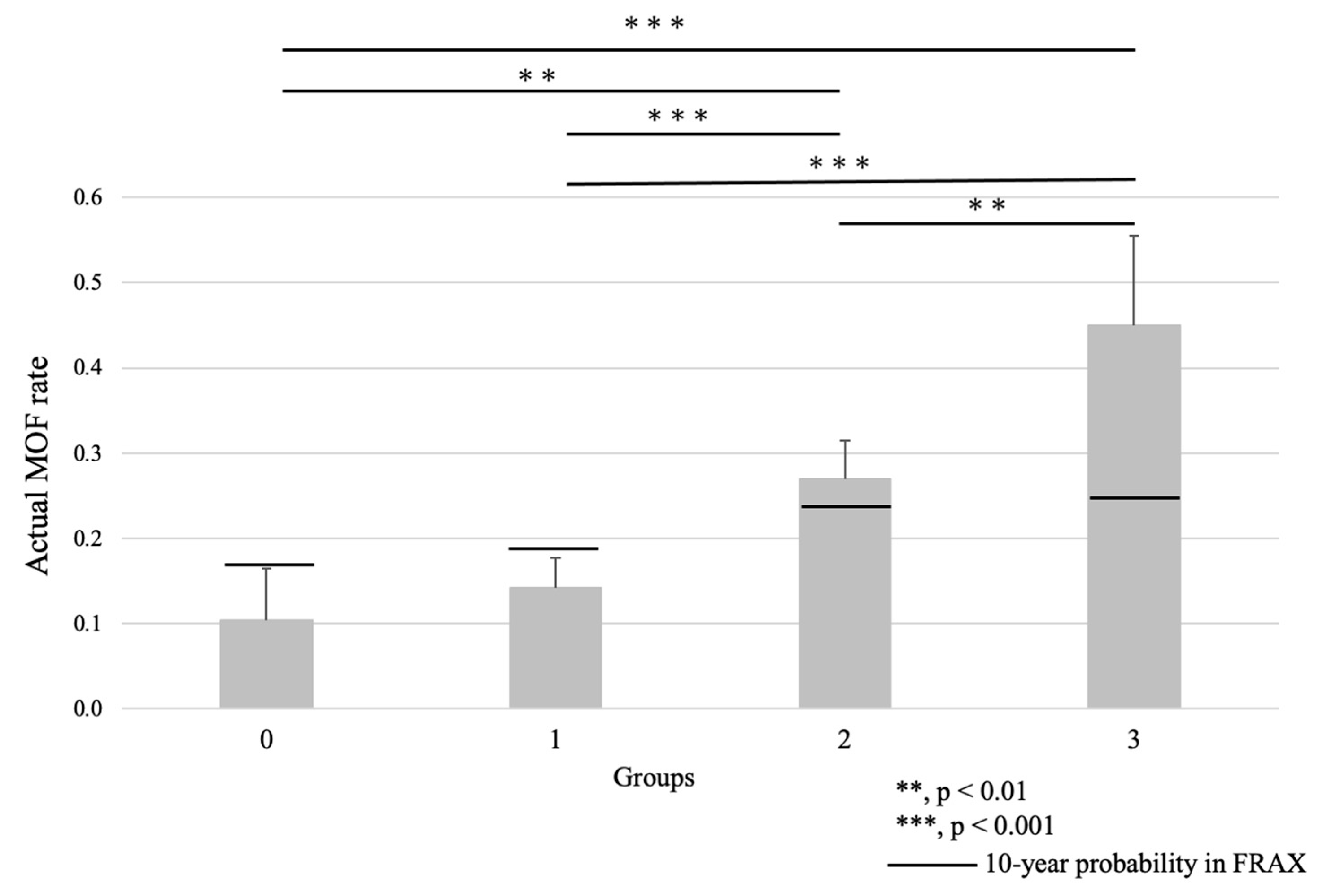
3.2. Group Comparison
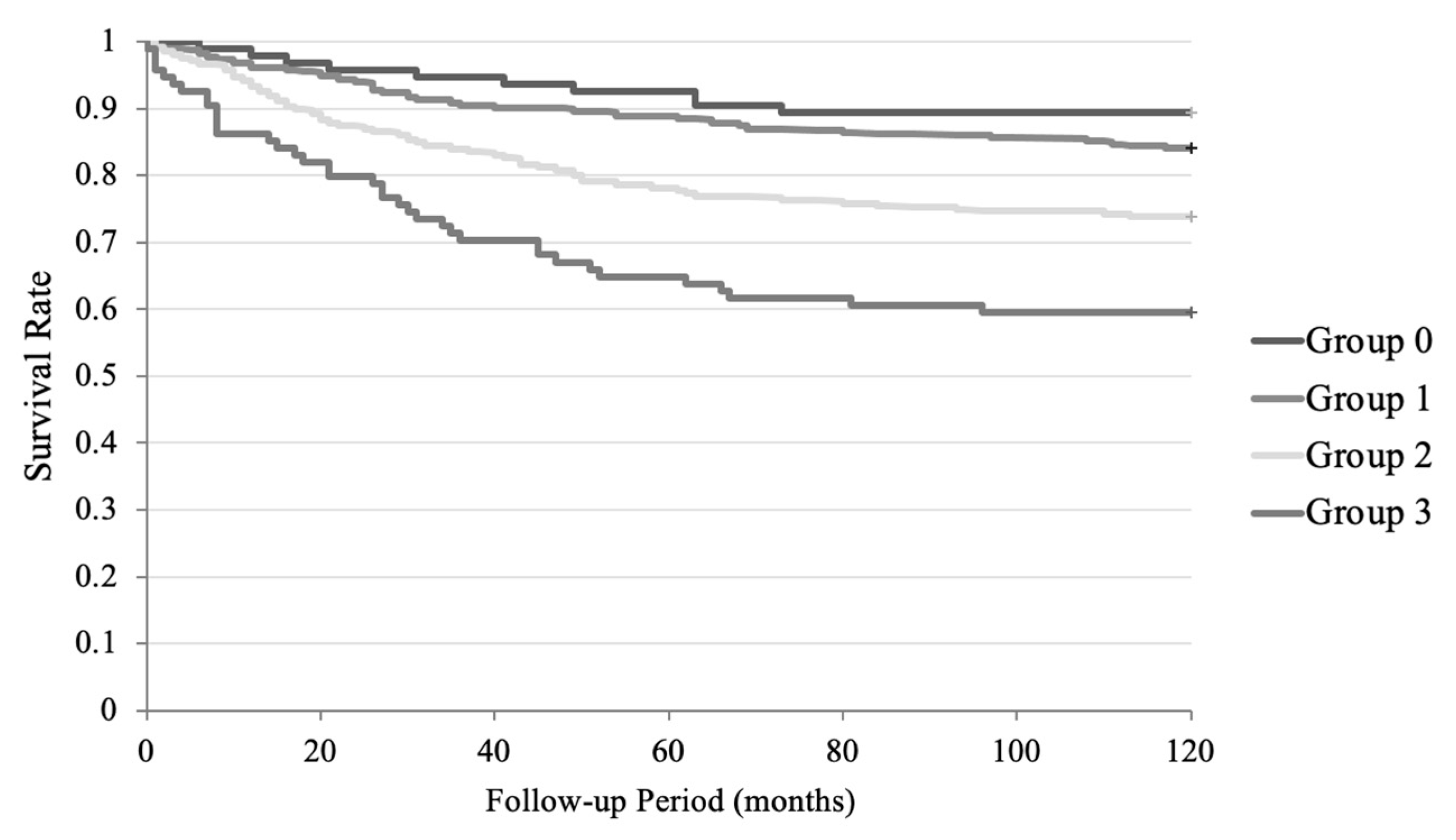
3.3. Cox Regression Analysis in the Follow-Up Period
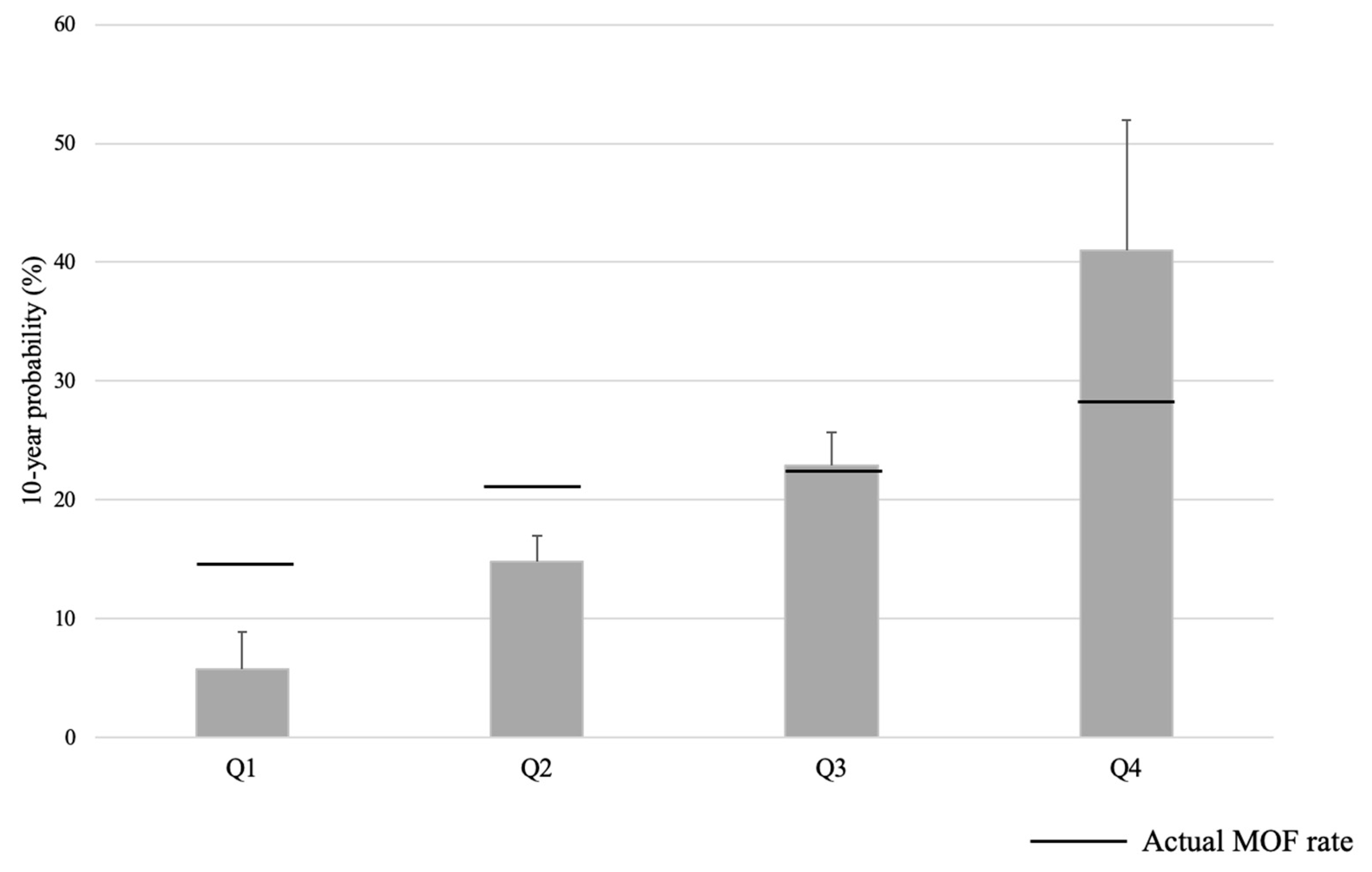
3.4. Comparison Between the 10-Year Probability with FRAX and the Actual Incidence of Fracture Performance
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Curtis, E.M.; Moon, R.J.; Harvey, N.C.; Cooper, C. The impact of fragility fracture and approaches to osteoporosis risk assessment worldwide. Bone 2017, 104, 29–38. [Google Scholar] [CrossRef]
- Hernlund, E.; Svedbom, A.; Ivergård, M.; Compston, J.; Cooper, C.; Stenmark, J.; McCloskey, E.V.; Jönsson, B.; Kanis, J.A. Osteoporosis in the European Union: Medical management, epidemiology and economic burden. A report prepared in collaboration with the International Osteoporosis Foundation (IOF) and the European Federation of Pharmaceutical Industry Associations (EFPIA). Arch. Osteoporos. 2013, 8, 136. [Google Scholar] [CrossRef] [PubMed]
- Ballane, G.; Cauley, J.A.; Luckey, M.M.; El-Hajj Fuleihan, G. Worldwide prevalence and incidence of osteoporotic vertebral fractures. Osteoporos. Int. 2017, 28, 1531–1542. [Google Scholar] [CrossRef]
- Tamaki, J.; Fujimori, K.; Ikehara, S.; Kamiya, K.; Nakatoh, S.; Okimoto, N.; Ogawa, S.; Ishii, S.; Iki, M.; Working Group of Japan Osteoporosis Foundation. Estimates of hip fracture incidence in Japan using the National Health Insurance Claim Database in 2012–2015. Osteoporos. Int. 2019, 30, 975–983. [Google Scholar] [CrossRef]
- Tsukutani, Y.; Hagino, H.; Ito, Y.; Nagashima, H. Epidemiology of fragility fractures in Sakaiminato, Japan: Incidence, secular trends, and prognosis. Osteoporos. Int. 2015, 26, 2249–2255. [Google Scholar] [CrossRef]
- Iihara, N.; Ohara, E.; Bando, Y.; Yoshida, T.; Ohara, M.; Kirino, Y. Fragility Fractures in Older People in Japan Based on the National Health Insurance Claims Database. Biol. Pharm. Bull. 2019, 42, 778–785. [Google Scholar] [CrossRef]
- Hagino, H. Locomotive Syndrome and Osteoporosis—Importance of Bone Stroke Prevention—The Bone. 2017. Available online: https://www.jstage.jst.go.jp/article/jjrmc/58/1/58_58.59/_pdf (accessed on 10 January 2021).
- Kanis, J.A.; Oden, A.; Johansson, H.; Borgström, F.; Ström, O.; McCloskey, E. FRAX and its applications to clinical practice. Bone 2009, 44, 734–743. [Google Scholar] [CrossRef] [PubMed]
- Kanis, J.A.; Johansson, H.; Harvey, N.C.; McCloskey, E.V. A brief history of FRAX. Arch. Osteoporos. 2018, 13, 118. [Google Scholar] [CrossRef] [PubMed]
- De Laet, C.; Kanis, J.A.; Odén, A.; Johanson, H.; Johnell, O.; Delmas, P.; Eisman, J.A.; Kroger, H.; Fujiwara, S.; Garnero, P.; et al. Body mass index as a predictor of fracture risk: A meta-analysis. Osteoporos. Int. 2005, 16, 1330–1338. [Google Scholar] [CrossRef]
- Johnell, O.; Kanis, J.A.; Oden, A.; Johansson, H.; De Laet, C.; Delmas, P.; Eisman, J.A.; Fujiwara, S.; Kroger, H.; Mellstrom, D.; et al. Predictive value of BMD for hip and other fractures. J. Bone Miner. Res. 2005, 20, 1185–1194. [Google Scholar] [CrossRef]
- Kanis, J.A.; Johnell, O.; Oden, A.; Johansson, H.; De Laet, C.; Eisman, J.A.; Fujiwara, S.; Kroger, H.; McCloskey, E.V.; Mellstrom, D.; et al. Smoking and fracture risk: A meta-analysis. Osteoporos. Int. 2005, 16, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Kanis, J.A.; Johansson, H.; Johnell, O.; Oden, A.; De Laet, C.; Eisman, J.A.; Pols, H.; Tenenhouse, A. Alcohol intake as a risk factor for fracture. Osteoporos. Int. 2005, 16, 737–742. [Google Scholar] [CrossRef] [PubMed]
- Kanis, J.A.; Johansson, H.; Oden, A.; Johnell, O.; de Laet, C.; Melton, L.J., III; Tenenhouse, A.; Reeve, J.; Silman, A.J.; Pols, H.A.; et al. A meta-analysis of prior corticosteroid use and fracture risk. J. Bone Miner. Res. 2004, 19, 893–899. [Google Scholar] [CrossRef]
- Kanis, J.A.; Johnell, O.; De Laet, C.; Johansson, H.; Oden, A.; Delmas, P.; Eisman, J.; Fujiwara, S.; Garnero, P.; Kroger, H.; et al. A meta-analysis of previous fracture and subsequent fracture risk. Bone 2004, 35, 375–382. [Google Scholar] [CrossRef]
- Kanis, J.A.; Johansson, H.; Oden, A.; Johnell, O.; De Laet, C.; Eisman, J.A.; McCloskey, E.V.; Mellstrom, D.; Melton, L.J., 3rd; Pols, H.A.; et al. A family history of fracture and fracture risk: A meta-analysis. Bone 2004, 35, 1029–1037. [Google Scholar] [CrossRef] [PubMed]
- Clinical Indications for Bone Mass Measurements. A report from the Scientific Advisory Board of the National Osteoporosis Foundation. J. Bone Miner. Res. 1989, 4 (Suppl. 2), 1–28. [Google Scholar] [CrossRef]
- Kanis, J.A.; Melton, L.J., 3rd; Christiansen, C.; Johnston, C.C.; Khaltaev, N. The diagnosis of osteoporosis. J. Bone Miner. Res. 1994, 9, 1137–1141. [Google Scholar] [CrossRef]
- Sugimoto, T.; Sato, M.; Dehle, F.C.; Brnabic, A.J.; Weston, A.; Burge, R. Lifestyle-Related Metabolic Disorders, Osteoporosis, and Fracture Risk in Asia: A Systematic Review. Value Health Reg. Issues 2016, 9, 49–56. [Google Scholar] [CrossRef]
- Pouresmaeili, F.; Kamalidehghan, B.; Kamarehei, M.; Goh, Y.M. A comprehensive overview on osteoporosis and its risk factors. Ther. Clin. Risk Manag. 2018, 14, 2029–2049. [Google Scholar] [CrossRef]
- Han, D.; Fan, Z.; Chen, Y.S.; Xue, Z.; Yang, Z.; Liu, D.; Zhou, R.; Yuan, H. Retrospective study: Risk assessment model for osteoporosis-a detailed exploration involving 4,552 Shanghai dwellers. PeerJ 2023, 11, e16017. [Google Scholar] [CrossRef]
- Hofbauer, L.C.; Brueck, C.C.; Singh, S.K.; Dobnig, H. Osteoporosis in patients with diabetes mellitus. J. Bone Miner. Res. 2007, 22, 1317–1328. [Google Scholar] [CrossRef]
- de Paula, F.J.A.; Horowitz, M.C.; Rosen, C.J. Novel insights into the relationship between diabetes and osteoporosis. Diabetes Metab. Res. Rev. 2010, 26, 622–630. [Google Scholar] [CrossRef] [PubMed]
- Kurra, S.; Siris, E. Diabetes and bone health: The relationship between diabetes and osteoporosis-associated fractures. Diabetes Metab. Res. Rev. 2011, 27, 430–435. [Google Scholar] [CrossRef]
- Biskobing, D.M. COPD and osteoporosis. Chest 2002, 121, 609–620. [Google Scholar] [CrossRef]
- Graat-Verboom, L.; Wouters, E.F.M.; Smeenk, F.W.J.M.; van den Borne, B.E.E.M.; Lunde, R.; Spruit, M.A. Current status of research on osteoporosis in COPD: A systematic review. Eur. Respir. J. 2009, 34, 209. [Google Scholar] [CrossRef]
- Inoue, D.; Watanabe, R.; Okazaki, R. COPD and osteoporosis: Links, risks, and treatment challenges. Int. J. Chronic Obstr. Pulm. Dis. 2016, 29, 637–648. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.M.; Long, J.; Montez-Rath, M.; Leonard, M.; Chertow, G.M. Hip fracture in patients with non-dialysis-requiring chronic kidney disease. J. Bone Miner. Res. 2016, 31, 1803–1809. [Google Scholar] [CrossRef] [PubMed]
- Khairallah, P.; Nickolas, T.L. Management of Osteoporosis in CKD. Clin. J. Am. Soc. Nephrol. 2018, 13, 962–969. [Google Scholar] [CrossRef]
- Schwartz, A.V.; Vittinghoff, E.; Bauer, D.C.; Hillier, T.A.; Strotmeyer, E.S.; Ensrud, K.E.; Donadson, M.G.; Cauley, J.A.; Harris, T.B.; Koster, A.K.; et al. Association of BMD and FRAX score with risk of fracture in older adults with type 2 diabetes. JAMA 2011, 305, 2184–2192. [Google Scholar] [CrossRef]
- Szulc, P. Vascular calcification and fracture risk. Clin. Cases Miner. Bone Metab. 2015, 12, 139–141. [Google Scholar] [CrossRef]
- Szulc, P. Abdominal aortic calcification: A reappraisal of epidemiological and pathophysiological data. Bone 2016, 84, 25–37. [Google Scholar] [CrossRef] [PubMed]
- Lewis, J.R.; Eggermont, C.J.; Schousboe, J.T.; Lim, W.H.; Wong, G.; Khoo, B.; Sim, M.; Yu, M.X.; Ueland, T.; Bollersley, J.; et al. Association between abdominal aortic calcification, bone mineral density, and fracture in older women. J. Bone Miner. Res. 2019, 34, 2052–2060. [Google Scholar] [CrossRef]
- Saito, M.; Marumo, K. Collagen cross-links as a determinant of bone quality: A possible explanation for bone fragility in aging, osteoporosis, and diabetes mellitus. Osteoporos. Int. 2010, 21, 195–214. [Google Scholar] [CrossRef]
- Saito, M.; Marumo, K. Effecs of collagen crosslinking on bone material properties in health and disease. Calcif. Tissue Int. 2015, 97, 242–261. [Google Scholar] [CrossRef] [PubMed]
- Ishibashi, H. Fall risk and fracture. Locomotive syndrome and fall. Clin. Calcium 2013, 23, 669–677. (In Japanese) [Google Scholar]
- Kerschan-Schindl, K. Prevention and rehabilitation of osteoporosis. Wien. Med. Wochenschr. 2016, 166, 22–27. [Google Scholar] [CrossRef]
- Nilsson, M.; Eriksson, J.; Larsson, B.; Odén, A.; Johansson, H.; Lorentzon, M. Fall Risk Assessment Predicts Fall-Related Injury, Hip Fracture, and Head Injury in Older Adults. J. Am. Geriatr. Soc. 2016, 64, 2242–2250. [Google Scholar] [CrossRef]
- Vottis, C.T.; Mitsiokapa, E.; Igoumenou, V.G.; Megaloikonomos, P.D.; Galanopoulos, I.P.; Georgoudis, G.; KouLouvaris, P.; Papagelopoulos, P.J.; Mavrojenis, A.F. Fall Risk Assessment Metrics for Elderly Patients With Hip Fractures. Orthopedics 2018, 41, 142–156. [Google Scholar] [CrossRef] [PubMed]
- Genant, H.K.; Jergas, M.; Palermo, L.; Nevitt, M.; Valentin, R.S.; Black, D.; Cummings, S.R. Comparison of semiquantitative visual and quantitative morphometric assessment of prevalent and incident vertebral fractures in osteoporosis The Study of Osteoporotic Fractures Research Group. J. Bone Miner. Res. 1996, 11, 984–996. [Google Scholar] [CrossRef]
- FRAX® Fracture Risk Assessment Tool. 2008. Available online: https://www.sheffield.ac.uk/FRAX/tool.aspx?lang=jp (accessed on 5 May 2021).
- Yoshii, I.; Chijiwa, T.; Sawada, N.; Kokei, S. Musculoskeletal ambulation disability symptom complex as a risk factor of incident bone fragility fracture. Osteoporos. Sarcopenia 2021, 7, 115–120. [Google Scholar] [CrossRef]
- Kumar, S.; Smith, C.; Clifton-Bligh, R.J.; Beck, B.R.; Girgis, C.M. Exercise for Postmenopausal Bone Health—Can We Raise the Bar? Curr. Osteoporos. Rep. 2025, 23, 20. [Google Scholar] [CrossRef]
- Ito, H. Diagnosis of musculoskeletal ambulation disability symptom complex (MADS). Clin. Calcium 2008, 18, 1560–1565. (In Japanese) [Google Scholar] [PubMed]
- Shrestha, P.; Basida, B.; Elam, R.E.; Hajj, J.E.; Le, B.; Fink, H.A.; Bethel, M.; Tharrington, S.G.; Carbone, L.D. Disparities in the Diagnosis and Treatment of Osteoporosis in Persons with Cognitive Impairment and Dementia. Curr. Osteoporos. Rep. 2025, 23, 31. [Google Scholar] [CrossRef] [PubMed]
- Ensrud, K.E.; Schousboe, J.T.; Crandall, C.J.; Leslie, W.D.; Fink, H.A.; Cawthon, P.M.; Kado, D.M.; Lane, N.E.; Caulley, J.A.; Langsetmo, L. Hip Fracture Risk Assessment Tools for Adults Aged 80 Years and Older. JAMA Netw. Open 2024, 7, e2418612. [Google Scholar] [CrossRef] [PubMed]
- Jain, R.K.; Liu, J.Y.; Grant, R.W.; Haider, S.; Huang, E.S.; Laiteerapong, N.; Lipska, K.J.; Lo, J.C.; Moffet, H.H.; Parker, M.M.; et al. Biases in the performance of FRAX without BMD in predicting fracture risk in a multiethnic population with diabetes: The Diabetes and Aging Study. J. Bone Miner. Res. 2025, 40, 478–491. [Google Scholar] [CrossRef]



| Cases | 931 |
|---|---|
| sex (male:female, female%) | 131:802, 86.1% |
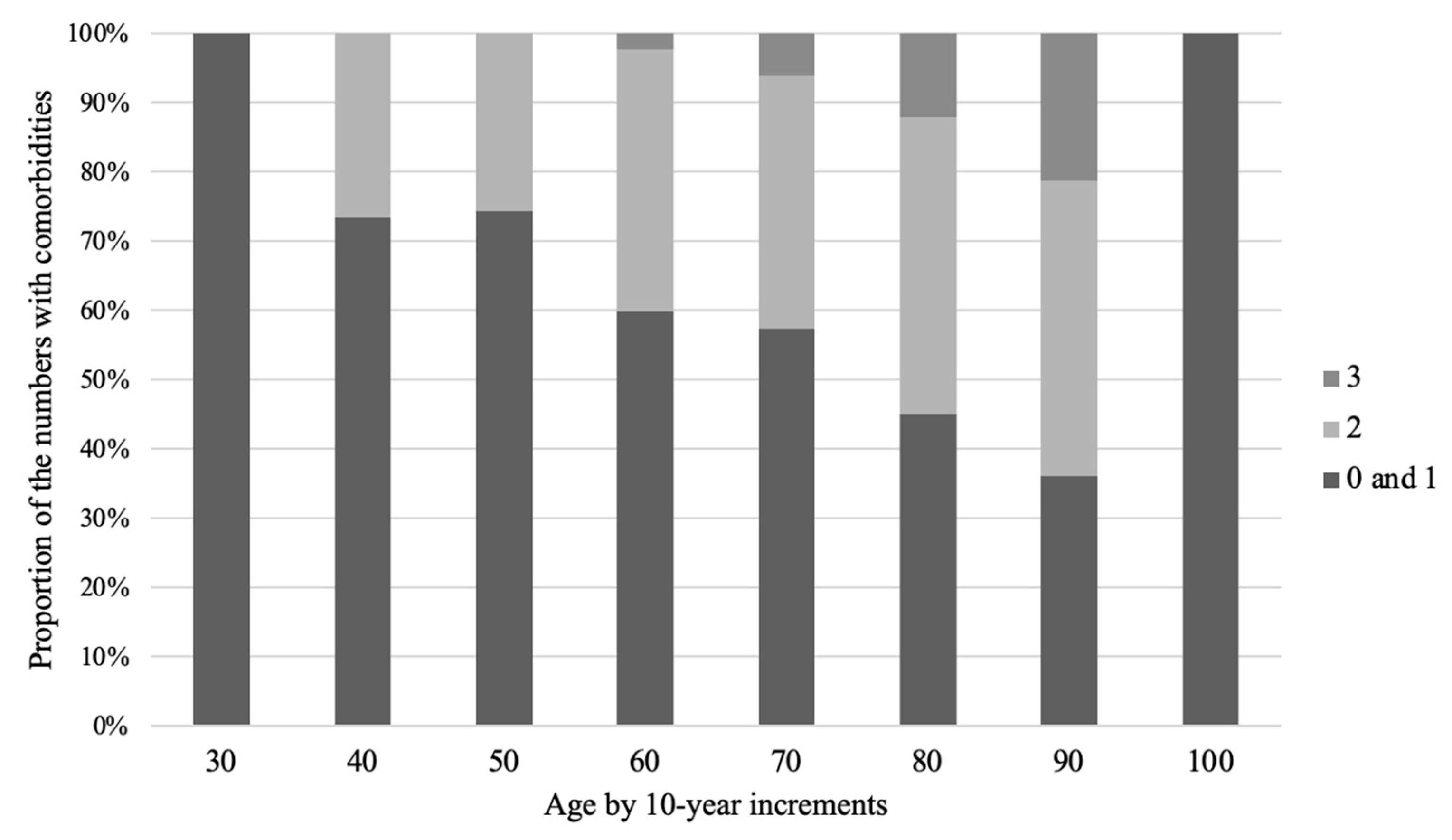
| age (mean, S.D.) (30s, 40s, 50s, 60s, 70s, 80s, ≥90s,) | 78.6, 10.7 (1, 15, 35, 124, 246, 387, 123) |
| follow-up period (mean, S.D.) | 49.1, 25.8 |
| existing VF | 181 (19.4%) |
| existing HF | 31 (3.3%) |
| existing SF | 10 (1.1%) |
| existing WF | 11 (1.2%) |
| existing MOF | 223 (24.0%) |
| Tscore_LS (mean, S.D.) | −2.29, 1.69 |
| Tscore_FN (mean, S.D.) | −2.05, 1.16 |
| BMI | 22.5, 3.9 |
| current smoking habit | 24 (2.7%) |
| alcohol habit | 13 (1.5%) |
| type 2 DM | 202 (21.7%) |
| hypertension | 468 (50.3%) |
| hyperlipidemia | 247 (26.5%) |
| chronic heart failure | 209 (22.4%) |
| COPD | 79 (8.5%) |
| CKD ≥ Stage 3a | 358 (38.5%) |
| insomnia | 197 (21.2%) |
| lifestyle-related diseases | 622 (66.9%) |
| MADS | 197 (21.2%) |
| osteoarthritis | 528 (56.7%) |
| contracture | 91 (9.8%) |
| Disuse | 65 (7.0%) |
| Parkinsonism | 25 (2.7%) |
| Fall-ability | 617 (66.3%) |
| cognitive disorder | 146 (15.7%) |
| RA | 284 (30.5%) |
| parental history of hip fracture | 0 (0%) |
| secondary osteoporosis | 0 (0%) |
| anti-osteoporotic drug administration | 574 (61.7%) |
| GCS administration | 168 (18.0%) |
| vitamin-D supplementation | 547 (58.8%) |
| Variable | MOF Group (N = 203) | Non-MOF Group (N = 728) | p-Value |
|---|---|---|---|
| sex (male:female, female%) | 14:189, 93.1% | 115:613, 84.2% | <0.01 |
| age (mean, S.D.) | 78.6, 9.9 | 78.6, 10.9 | 0.88 |
| Age by teens (30s, 40s, 50s, 60s, 70s, 80s, ≥90) | 0, 2, 8, 25, 58, 87, 23 | 1, 13, 27, 99, 188, 300, 100 | 0.92 |
| follow-up period (mean, S.D.) | 34.8, 28.7 | 120, 0 | <0.001 |
| existing VF | 57 (28.1%) | 124 (17.0%) | <0.01 |
| existing HF | 10 (4.9%) | 21 (2.9%) | 0.20 |
| existing SF | 5 (2.5%) | 5 (0.7%) | 0.06 |
| existing WF | 4 (2.0%) | 7 (1.0%) | 0.09 |
| existing MOF | 70 (34.5%) | 153 (21.0%) | <0.001 |
| Tscore_LS (mean, S.D.) | −2.59, 1.40 | −2.20, 1.75 | <0.05 |
| Tscore_FN (mean, S.D.) | −2.22, 1.05 | −2.01, 1.18 | <0.05 |
| BMI | 21.3, 4.0 | 22.8, 3.8 | 0.07 |
| current smoking habit | 8 (4.0%) | 17 (2.3%) | 0.15 |
| alcohol habit | 2 (1.0%) | 12 (1.6%) | 0.31 |
| type 2 DM | 53 (26.1%) | 149 (20.4%) | 0.09 |
| hypertension | 128 (63.1%) | 340 (46.7%) | <0.001 |
| hyperlipidemia | 76 (37.4%) | 171 (23.5%) | <0.001 |
| chronic heart failure | 70 (34.5%) | 139 (19.1%) | <0.001 |
| COPD | 24 (11.8%) | 55 (7.6%) | 0.05 |
| CKD ≥ Stage 3a | 65 (45.1%) | 137 (36.0%) | 0.05 |
| insomnia | 57 (28.1%) | 140 (19.2%) | <0.01 |
| lifestyle-related diseases | 162 (79.8%) | 460 (63.2%) | <0.001 |
| MADS | 69 (40.0%) | 128 (17.6%) | <0.001 |
| osteoarthritis | 130 (64.0%) | 398 (54.7%) | <0.05 |
| contracture | 37 (18.2%) | 54 (7.4%) | <0.001 |
| Disuse | 27 (13.3%) | 38 (5.2%) | <0.001 |
| Parkinsonism | 9 (4.4%) | 16 (2.2%) | 0.08 |
| Fall-ability | 161 (79.3%) | 456 (62.6%) | <0.001 |
| cognitive disorder | 52 (25.6%) | 94 (12.9%) | <0.001 |
| RA | 56 (27.6%) | 228 (31.3%) | 0.31 |
| parental history of hip fracture | 0 (0%) | 0 (0%) | N/A |
| secondary osteoporosis | 0 (0%) | 0 (0%) | N/A |
| anti-osteoporotic drug administration | 63 (31.0%) | 149 (20.5%) | <0.01 |
| GCS administration | 45 (22.2%) | 123 (16.9%) | 0.08 |
| vitamin-D supplementation | 122 (60.1%) | 425 (58.4%) | 0.66 |
| estimated 10-year probability of MOF with FRAX | 23.3, 13.9 | 20.4, 14.4 | <0.05 |
| estimated 10-year probability of HF with FRAX | 11.2, 10.1 | 9.5, 10.4 | <0.05 |
| Variable | Risk Ratio (95% CI) | p-Value |
|---|---|---|
| female gender | 2.21 (1.29~3.81) | <0.01 |
| older age | 1.00 (0.99~1.01) | 0.91 |
| heavier body weight | 0.96 (0.92~0.99) | <0.05 |
| taller height | 0.97 (0.94~1.01) | 0.11 |
| higher BMI | 0.90 (0.82~0.99) | <0.05 |
| existing MOF | 1.81 (1.36~2.42) | <0.001 |
| existing VF | 1.73 (1.27~2.35) | <0.001 |
| parental history of hip fracture | – | – |
| current smoking habit | 1.49 (0.73~3.02) | 0.27 |
| GCS administration | 1.32 (0.95~1.84) | 0.10 |
| presence of RA | 0.85 (0.63~1.16) | 0.31 |
| secondary osteoporosis | – | – |
| alcohol habit | 0.63 (0.16~2.55) | 0.52 |
| higher T-score in the proximal femur | 0.87 (0.78~0.96) | <0.01 |
| hypertension | 1.83 (1.38~2.44) | <0.001 |
| hyperlipidemia | 1.73 (1.30~2.30) | <0.001 |
| chronic heart failure | 2.00 (1.50~2.67) | <0.001 |
| insomnia | 1.55 (1.14~2.10) | <0.01 |
| lifestyle-related diseases | 2.13 (1.51~3.00) | <0.001 |
| MADS | 2.16 (1.62~2.89) | <0.001 |
| osteoarthritis | 1.41 (1.06~1.88) | <0.05 |
| contracture | 2.33 (1.63~3.33) | <0.001 |
| Disuse | 2.41 (1.61~3.62) | <0.001 |
| Fall-ability | 2.12 (1.51~2.97) | <0.001 |
| cognitive impairment | 2.10 (1.53~2.87) | <0.001 |
| MOF Number | Q1 | Q2 | Q3 | Q4 | Total | |
| number of presented comorbidities, such as lifestyle-related diseases, Fall-ability, and cognitive impairment | 0 | 3 | 2 | 4 | 1 | 10 |
| 1 | 15 | 17 | 21 | 8 | 61 | |
| 2 | 13 | 26 | 43 | 12 | 94 | |
| 3 | 4 | 8 | 21 | 5 | 38 | |
| Total | 35 | 53 | 89 | 26 | 203 | |
| Total Number | Q1 | Q2 | Q3 | Q4 | Total | |
| 0 | 29 | 27 | 31 | 7 | 94 | |
| 1 | 111 | 99 | 125 | 49 | 384 | |
| 2 | 77 | 104 | 86 | 92 | 359 | |
| 3 | 12 | 21 | 32 | 29 | 94 | |
| Total | 229 | 251 | 217 | 234 | 931 | |
| MOF Rate | Q1 | Q2 | Q3 | Q4 | Total | |
| 0 | 10.34% | 7.41% | 12.90% | 14.29% | 10.64% | |
| 1 | 13.51% | 17.17% | 16.80% | 16.33% | 15.89% | |
| 2 | 16.88% | 25.00% | 50.00% | 13.04% | 26.18% | |
| 3 | 33.33% | 38.10% | 65.63% | 17.24% | 40.43% | |
| Total | 15.28% | 21.12% | 41.01% | 11.11% | 21.80% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yoshii, I.; Sawada, N.; Chijiwa, T. Discrepancy Between the 10-Year Probability of Major Osteoporotic Fracture with FRAX and the Actual Fracture Prevalence over 10 Years in Japanese. Osteology 2025, 5, 28. https://doi.org/10.3390/osteology5040028
Yoshii I, Sawada N, Chijiwa T. Discrepancy Between the 10-Year Probability of Major Osteoporotic Fracture with FRAX and the Actual Fracture Prevalence over 10 Years in Japanese. Osteology. 2025; 5(4):28. https://doi.org/10.3390/osteology5040028
Chicago/Turabian StyleYoshii, Ichiro, Naoya Sawada, and Tatsumi Chijiwa. 2025. "Discrepancy Between the 10-Year Probability of Major Osteoporotic Fracture with FRAX and the Actual Fracture Prevalence over 10 Years in Japanese" Osteology 5, no. 4: 28. https://doi.org/10.3390/osteology5040028
APA StyleYoshii, I., Sawada, N., & Chijiwa, T. (2025). Discrepancy Between the 10-Year Probability of Major Osteoporotic Fracture with FRAX and the Actual Fracture Prevalence over 10 Years in Japanese. Osteology, 5(4), 28. https://doi.org/10.3390/osteology5040028






