Abstract
Background/Objectives: Periprosthetic joint infection (PJI) is a challenging problem in orthopedic surgery and is often associated with high morbidity. The treatment becomes even more challenging whenever the microorganism is virulent and/or not widely known as a causative organism on these occasions. This study aims to report on the clinical outcomes of hip hemiarthroplasty prosthetic hip joint infection by an atypical, rare microorganism, Morganella morganii (M. morganii), focusing on morbidity, revisions, and mortality. Methods: This is a retrospective series of four cases of prosthetic joint infections with Morganella morganii, a rare Gram-negative opportunistic facultative anaerobic pathogen, in four patients who received hip hemiarthroplasty for displaced femoral neck fractures at a level 1 trauma center. Clinical notes, laboratory findings, and radiographs were reviewed to extract relevant information regarding the history and outcomes. Results: The patients were four females, with a mean age of 84.27 years at the time of surgery. Two cases (50%) underwent surgical debridement and implant retention, followed by lifelong antibiotic suppression for symptomatic control of persistent wound drainage, and the other two underwent implant removal and resection arthroplasty (one patient) or received an antibiotic spacer (one patient), followed by chronic antibiotic therapy until wound closure. Conclusions: Periprosthetic hemiarthroplasty infection secondary to M. morganii was associated with overall poor outcomes. Antibiotic suppression could be a reasonable option after the surgical debridement or implant removal in M. morganii PJI to control the symptoms.
1. Introduction
Morganella morganii (M. morganii) is an enteric, facultative anaerobic, motile, Gram-negative microorganism. It is an opportunistic bacterium that is known to cause infections in immunocompromised patients [1,2] and is notable for increasing drug resistance [2] and occasional biofilm formation [3]. The most common manifestations of M. morganii infections are abscesses, urinary tract infections, and bacteremia [2]. Osteoarticular infections secondary to M. morganii are rare and reported in a few articles, mostly case reports, including osteomyelitis [3,4,5,6,7,8,9,10], and only in sporadic cases of prosthetic joint infections of total hip and knee arthroplasty [11,12].
Prosthetic joint infection (PJI) is a serious complication that is associated with significant morbidity and mortality [11,13]. Studies have shown that PJI following total hip and knee arthroplasty occurs with an incidence of 1–2% [11], while in hip hemiarthroplasty for trauma patients, which is a different setting given the patients’ comorbidities, the incidence tends to be higher, falling in the range of 1–6% [13,14,15]. With Staphylococcus being the most common causative organism of PJI, accounting for up to two-thirds of cases [16], treatment of PJI falls under two broad choices, namely debridement, antibiotics, implant retention (DAIR), or implant removal (which includes either implant revision or resection arthroplasty). The decision to perform DAIR or implant removal in PJI depends on many factors, including the timing and severity of infection, the causative organism's virulence, medical comorbidities, and the host. DAIR is usually utilized as a primary choice in acute PJI, while implant removal with two-stage revision arthroplasty remains the gold-standard treatment in late PJI. Specific antibiotic therapy is a crucial step in successful PJI management, whether DAIR, implant removal, or conservative antibiotic suppression is utilized.
Hip hemiarthroplasty (HA) is a widely utilized treatment option for displaced femoral neck fractures, especially in the elderly, with reported successful outcomes [17,18]. In this study, we report on a series of deep periprosthetic infections with M. morganii after hip hemiarthroplasty, which has not been reported before. We aim to add to the existing literature a reference for scenarios in which a physician might face a similar case caused by this rare pathogen.
2. Materials and Methods
2.1. Study Protocol
This is a single-center retrospective analysis of patients who underwent hemiarthroplasty between 1988 and 2024 as a treatment for femoral neck fracture and developed PJI with Morganella morganii. After Institutional Review Board (IRB) approval, electronic medical records were retrospectively evaluated using the procedural codes for surgery against our selection criteria to identify the cases and data relevant to this study. Data collected included patients’ demographics, clinical notes, laboratory results, and radiographs.
2.2. Inclusion Criteria
- Patients who underwent hip hemiarthroplasty, either primarily after an acute hip fracture or for the management of femoral neck fracture nonunion.
- Patients diagnosed with postoperative hematoma or postoperative prosthetic joint infection.
2.3. Exclusion Criteria
- Patients with bacterial cultures indicating no M. morganii bacterial growth.
- Patients who had negative bacterial cultures.
2.4. Statistical Analysis
Statistical analysis was performed using Microsoft Excel (version 2503, Microsoft, Redmond, WA, USA). Values were reported as means, with standard deviations, and percentages of the total number of PJI cases.
3. Results
3.1. Patients
Sixty-two HA hematoma/PJI cases were retrieved from our medical database. Out of them, four patients (6.4%) with M. morganii infection were identified, and their clinical data and radiographs were reviewed for this study. The patients included four females, with a mean age of 84.27 years (range, 63.98–93.2) at the time of hemiarthroplasty. Two patients had their left side and two cases had the right side involved. Two patients received cemented and two received cementless bipolar hemiarthroplasty, all for displaced femoral neck fractures. Three patients had chronic renal disease, with one case having end-stage renal disease under dialysis. The mean body mass index (BMI) for all the patients was 33.5 (range, 23–45.2). Cases presented with wound inflammation, swelling, and copious wound discharge, as well as hip pain, from the second postoperative (post-op) day in two patients, two weeks post-op in one, and after 14 months post-op in one patient.
3.2. Diagnosis
Given the retrospective nature of the study, the patients were identified as having PJI as documented in their clinical notes. The diagnosis of PJI was established by the providers based on the symptoms, signs, and laboratory investigations (cultures from surgical tissue biopsies and/or implant sonication). The original Musculoskeletal Infection Society (MSIS) criteria [19] could be retrospectively applicable in all cases (Table 1), since all of them had more than a single positive bacterial culture indicating M. morganii or Gram-negative bacillus growth, one of them had a sinus tract connected with the joint (major criteria), and all of them met other minor criteria (purulence, elevated ESR and CRP). The Gram-stained films of M. morganii show a moderate polymorphonuclear lymphocytic infiltrate and Gram-negative bacilli (Figure 1) that grew on blood, chocolate, and MacConkey agar cultures (Figure 2). M. morganii typically grows on MacConkey agar media (a selective medium for Gram-negative bacilli), while showing non-swarming growth on blood agar, which distinguishes it from the closely related Proteus species. Bacterial cultures frequently showed multiple-organism infections.

Table 1.
Case presentations, management, and clinical outcomes.
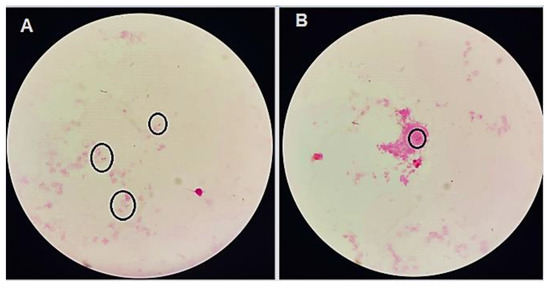
Figure 1.
(A,B) Gram stain films showing Gram-negative bacilli (rods inside the circles).

Figure 2.
Bacterial growth on blood agar (A), chocolate agar (B), and MacConkey agar media (C). The culture showed the pure growth of convex, pale, non-lactose fermenting colonies on MacConkey agar (C), and there was no swarming on blood agar (A).
All patients underwent surgical exploration for debridement (Table 1). The eventual outcomes were resection arthroplasty (one patient, Girdlestone procedure), receiving an antibiotic spacer (one patient), and implant retention in another two patients, while all cases required postoperative antibiotic therapy (Table 1). Based on the infectious disease consultation, different antibiotics were prescribed, considering the culture results, the patients’ associated medical comorbidities, and administration feasibility (Table 2).
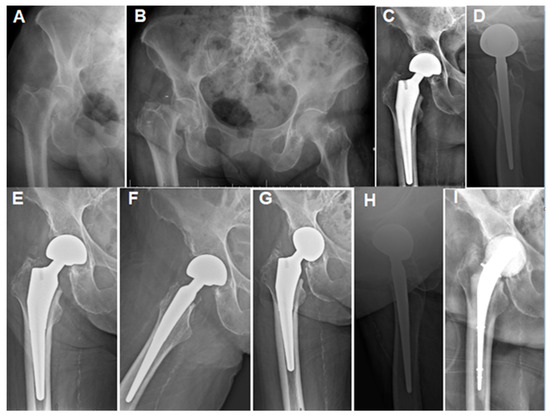

Figure 3.
Case 4 (Table 1): (A,B) Plain hip radiographs of a 93.2-year-old female showing displaced right femoral neck fractures. (C,D) Immediate postoperative hip radiographs showing cementless bipolar HA with stable components. (E,F) Three-month plain radiographs showing stable components and mild radiological erosion. (G,H) Fourteen-month postoperative radiograph showing acetabular erosion. The patient presented at this stage with a 2-week history of hip pain and a discharging wound sinus. (I) AP radiograph after debridement, implant removal, and spacer insertion. The patient received a 6-week IV antibiotic regimen and was followed for a total of 9 months after spacer insertion (including the antibiotic period) before being lost to follow-up.

Table 2.
Results of bacterial cultures and sensitivity in all patients. R: resistant. S: sensitive. I: intermediate sensitivity.
4. Discussion
This is a case series of four patients who underwent several interventions due to PJI with Morganella morganii following hip hemiarthroplasty. The outcomes in all patients showed significant morbidity, with chronic antibiotic suppression following unsuccessful surgical debridement in two patients, implant removal and spacer insertion in one patient, and resection arthroplasty (Girdlestone procedure) in one patient. All patients required postoperative antibiotic therapy, including the implant removal cases, to maintain symptomatic relief of the local and systemic manifestations of PJI. To the best of our knowledge, this is the largest series of periprosthetic arthroplasty infections with this bacterium and the only series that reports infection by M. morganii in hip hemiarthroplasty.
M. morganii, a non-capsulated, non-spore-forming, motile Gram-negative rod from the Enterobacteriaceae family, primarily resides in the human gastrointestinal tract and typically causes infections in immunocompromised patients [1,3,12,21]. M. morganii grows on ordinary media such as blood agar and grows on selective media such as MacConkey’s agar, which inhibits Gram-positive bacteria [22]. It lacks the swarming phenomenon when grown on blood agar, which distinguishes it from the closely related Proteus species [22]. It was first identified by Morgan in 1906 and has since been associated with urinary tract infections and soft tissue abscesses, particularly in individuals receiving chronic antibiotic treatment [1,12,23,24,25,26]. Notably, M. morganii has been reported in various osteoarticular infections, including septic arthritis, psoas abscess, and osteomyelitis [3,4,5,6,7,8,9,10,24,27,28,29], with sporadic case reports related to periprosthetic arthroplasty infections [11,12]. Identified risk factors for M. morganii infections include immunosuppression, diabetes, chronic alcoholism, and a history of trauma or surgical intervention [3,4,5,6,7,8,9,10]. In the reviewed cases, additional factors such as chronic kidney disease and high BMI were present.
M. morganii has been reported as a causative organism in native and prosthetic bone and joint infections. In cases of osteomyelitis and native septic arthritis caused by M. morganii, the available literature recommends extensive surgical debridement (single or multiple sessions) followed by specific antibiotic therapy for 4–6 weeks [3,4,5,6,7,8,9,10,27,28,29]. Only one study [28] reported successful eradication of infection with closed drainage of indolent native hip septic arthritis by M. morganii in a 53-year-old woman, who then underwent a successful THA with 4.5 years of follow-up. As for total joint PJI, M. morganii has seldom been reported as a causative organism, mainly in case reports or among larger series of PJI caused by other microorganisms. Fehring et al. [11] reported one case of infected total knee arthroplasty with M. morganii that was managed with repeated two-stage revision (two-stage revision after failed previous two-stage revision) and chronic suppressive antibiotic therapy, with no recurrence of infection after 27 months. Similarly, Anagnostakos et al. [12] reported one case of an infected total hip arthroplasty by M. morganii that was treated with two-stage revision and antibiotic suppression therapy. Total joint arthroplasty, however, is a different entity from HA, since HA patients tend to be older, frail, and have several medical comorbidities. These factors, besides the different surgical settings and the presence of trauma, may render HA patients more vulnerable to PJI and their related morbid outcomes. This could explain the reported higher incidence of PJI in HA than in elective total hip arthroplasty (1.6–10% vs. 0.2–0.7%, respectively) [13,14,15].
These previous two reports about M. morganii PJI [11,12] and the current cases in our study highlight the high morbidity of M. morganii prosthetic joint infection, which seems to necessitate multiple debridement surgeries and prolonged specific antibiotic suppression. This may be attributed to the biofilm formation ability and possession of antimicrobial resistance [3,30]. Notably, M morganii strains inherently produce inducible AmpC β-lactamases, rendering them intrinsically resistant to ampicillin and first-generation cephalosporins. Consequently, the use of third-generation cephalosporins or aztreonam may select for mutants that exhibit resistance to all β-lactam antibiotics—including those combined with β-lactamase inhibitors—except carbapenems and ceftazidime-avibactam [31]. Resistance to carbapenems can further arise through AmpC overexpression and alterations in porin proteins. Additionally, M. morganii strains producing extended-spectrum β-lactamases (ESBLs) or carbapenemases (such as VIM-1, KPC-2, and NDM-1) have been documented in the literature [31,32,33,34].For all of the reasons described, it is important to perform antimicrobial susceptibility testing to avoid antimicrobial resistance. Identification of the causative microorganism and its antibiotic susceptibility is a crucial step for the successful treatment of periprosthetic infections [35]. Surgeons should be aware of the characteristics, behavior, and antibiotic susceptibility of different causative pathogens to achieve satisfactory clinical results.
Prosthetic joint infection is recognized as a devastating complication that is associated with high morbidity and mortality and requires proper prophylaxis to decrease its occurrence [36]. The literature reveals a contrast in the mortality risk of PJI compared with that of common cancers [37], and it shows that the relative five-year survival rates of prostate cancer, melanoma, breast cancer, colorectal cancer, and lung cancer are 99%, 91%, 89%, 64%, and 16%, respectively, compared with 73.9% for prosthetic joint infection. When focusing on hip hemiarthroplasty, PJI has an overall DAIR success rate that reaches 42.3% [38]. Compared to the M. morganii outcomes in our study, which indicated overall unsuccessful DAIR, staphylococcal HA infection, being the most common, has a successful DAIR rate of about 31% [16].
Considering the limited available evidence, M. morganii prosthetic joint infection may require chronic suppressive antibiotic therapy, whether surgical debridement with modular part exchange or two-stage revision is performed. Early recognition and specific antimicrobial management are essential to improving outcomes. Chronic infection at presentation or a delay in the surgical debridement may be associated with guarded outcomes, likely requiring implant removal.
This study has several limitations. First, the small number of cases is a major limitation that may necessitate careful interpretation of the outcomes. The absence of a comparative patient group is also another potential limitation. A study comparing the survival and outcomes of M. Morganii PJI to those of other causative organisms in a large group of patients would be ideal for generating stronger evidence. The rarity of this condition, however, makes a larger series more challenging. A multicenter study may be more appropriate to better understand the outcomes of such a rare condition.
5. Conclusions
M. morganii hip hemiarthroplasty infections are associated with high morbidity. This highly resistant organism is associated with a high failure rate of surgical debridement and usually requires chronic antibiotic suppression therapy or resection arthroplasty.
Author Contributions
Conceptualization, A.N.M. and D.S.H.; methodology, A.N.M., A.O.-B., D.G.-C. and J.P.P.; software, A.N.M.; validation, J.P.P., M.S., D.S.H. and G.M.; formal analysis, A.N.M. and A.O.-B.; investigation, A.N.M., A.O.-B. and D.G.-C.; resources, M.S. and D.S.H.; data curation, A.N.M., A.O.-B., G.M. and J.D.B.; writing—original draft preparation, A.N.M.; writing—review and editing, A.O.-B., D.G.-C., A.N.M. and D.S.H.; visualization, D.S.H. and M.S.; supervision, D.S.H.; project administration, A.N.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
This study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Review Board of Geisinger Review Board (protocol code 2024-0166 and date of approval: 27 February 2025).
Informed Consent Statement
Informed consent was waived due to the retrospective nature of the study.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Morgan, H.D.R. Report XCV. Upon the Bacteriology of the Summer Diarrhoea of Infants. Br. Med. J. 1906, 1, 908–912. [Google Scholar] [CrossRef][Green Version]
- Liu, H.; Zhu, J.; Hu, Q.; Rao, X. Morganella morganii, a Non-Negligent Opportunistic Pathogen. Int. J. Infect. Dis. 2016, 50, 10–17. [Google Scholar] [CrossRef] [PubMed]
- De, A.; Raj, H.J.; Maiti, P.K. Biofilm in Osteomyelitis Caused by a Rare Pathogen, Morganella morganii: A Case Report. J. Clin. Diagn. Res. 2016, 10, DD06–DD08. [Google Scholar] [CrossRef] [PubMed]
- Koyuncu, S.; Ozan, F. Morganella morganii Osteomyelitis Complicated by Secondary Septic Knee Arthritis: A Case Report. Acta Orthop. Traumatol. Turc. 2012, 46, 464–467. [Google Scholar] [CrossRef] [PubMed]
- Smithson Amat, A.; Perelló Carbonell, R.; Arenillas Rocha, L.; Soriano Viladomiu, A. Osteomielitis Costal por Morganella morganii. An. Med. Interna 2004, 21, 464. [Google Scholar] [CrossRef]
- Zhu, J.; Li, H.; Feng, L.; Yang, M.; Yang, R.; Yang, L.; Li, L.; Li, R.; Liu, M.; Hou, S.; et al. Severe Chronic Osteomyelitis Caused by Morganella morganii with High Population Diversity. Int. J. Infect. Dis. 2016, 50, 44–47. [Google Scholar] [CrossRef][Green Version]
- Mody-Bailey, N.; Ezeokoli, E.U.; Hill, J. Tibial Osteomyelitis Caused by Morganella morganii after External Fixation for Limb Length Discrepancy in a Pediatric Patient: A Case Report and Literature Review. J. Am. Acad. Orthop. Surg. Glob. Res. Rev. 2022, 6, e22.00171. [Google Scholar] [CrossRef]
- Staudt, M.D.; Etarsky, D.; Ranger, A. Infected cephalohematomas and underlying osteomyelitis: A case-based review. Child’s Nervous System 2016, 32, 1363–1369. [Google Scholar] [CrossRef]
- Cetin, M.; Ocak, S.; Kuvandik, G.; Aslan, B.; Temiz, M.; Aslan, A. Morganella morganii-Associated Arthritis in a Diabetic Patient. Adv. Ther. 2008, 25, 240–244. [Google Scholar] [CrossRef]
- Harris, M.C.; DeRosa, D.C.; West, P.A. Subacute Osteomyelitis of the Pediatric Talus: A First Report of Brodie’s Abscess from Morganella morganii. Case Rep. Orthop. 2019, 2019, 7108047. [Google Scholar] [CrossRef]
- Fehring, K.A.; Abdel, M.P.; Ollivier, M.; Mabry, T.M.; Hanssen, A.D. Repeat Two-Stage Exchange Arthroplasty for Periprosthetic Knee Infection Is Dependent on Host Grade. J. Bone Jt. Surg. 2017, 99, 19–24. [Google Scholar] [CrossRef]
- Anagnostakos, K.; Grzega, C.; Sahan, I.; Geipel, U.; Becker, S.L. Occurrence of Rare Pathogens at the Site of Periprosthetic Hip and Knee Joint Infections: A Retrospective, Single-Center Study. Antibiotics 2021, 10, 882. [Google Scholar] [CrossRef]
- Ahmed, S.S.; Haddad, F.S. Prosthetic joint infection. Bone Jt. Res. 2019, 8, 570–572. [Google Scholar] [CrossRef] [PubMed]
- De Jong, L.; Klem, T.M.A.L.; Kuijper, T.M.; Roukema, G.R. Factors affecting the rate of surgical site infection in patients after hemiarthroplasty of the hip following a fracture of the neck of the femur. Bone Jt. J. 2017, 99, 1088–1094. [Google Scholar] [CrossRef] [PubMed]
- Masters, J.; Metcalfe, D.; Ha, J.S.; Judge, A.; Costa, M.L. Surgical site infection after hip fracture surgery: A systematic review and meta-analysis of studies published in the UK. Bone Jt. Res. 2020, 9, 554–562. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, A.N.; Brule, N.R.; Suk, M.; Horwitz, D.S. Outcomes of Staphylococcal Prosthetic Joint Infection After Hip Hemiarthroplasty: Single Center Retrospective Study. Medicina 2025, 61, 602. [Google Scholar] [CrossRef]
- Mahmoud, A.N.; Suk, M.; Horwitz, D.S. Symptomatic Acetabular Erosion After Hip Hemiarthroplasty: Is It a Major Concern? A Retrospective Analysis of 2477 Hemiarthroplasty Cases. J. Clin. Med. 2024, 13, 6756. [Google Scholar] [CrossRef]
- Mahmoud, A.N.; Doyle, C.M.; Kline, C.M.; Brule, N.; Sams, K.B.; Nye, A.; Horwitz, D.S. Hip Fracture in Blind Patients: Outcomes of Hip Hemiarthroplasty. Arch. Trauma Res. 2024, 13, 209–215. [Google Scholar]
- Parvizi, J.; Zmistowski, B.; Berbari, E.F.; Bauer, T.W.; Springer, B.D.; Della Valle, C.J.; Garvin, K.L.; Mont, M.A.; Wongworawat, M.D.; Zalavras, C.G. New definition for periprosthetic joint infection: From the Workgroup of the Musculoskeletal Infection Society. Clin. Orthop. Relat. Res. 2011, 469, 2992–2994. [Google Scholar] [CrossRef]
- Mahmoud, A.N.; Echeverry-Martinez, M.F.; Horwitz, D.S. Adequate bone healing after supplementary fixation of periprosthetic total knee arthroplasty fractures using Luque cerclage wiring: A retrospective case series. Eur. J. Orthop. Surg. Traumatol. 2024, 34, 389–395. [Google Scholar] [CrossRef]
- Gebhart-Mueller, Y.; Mueller, P.; Nixon, B. Unusual Case of Postoperative Infection Caused by Morganella morganii. J. Foot Ankle Surg. 1998, 37, 145–147. [Google Scholar] [CrossRef]
- Freeman, B.A. The Enteric Bacilli: Klebsiella, Enterobacter, Serratia, Proteus, Providentia, and Morganella. In The Burrow Textbook of Microbiology, 22nd ed.; W.B. Saunders: Philadelphia, PA, USA, 1985; p. 483. [Google Scholar]
- Sevin, A.; Buttiaux, R. The Characters and Position of Morgan’s Bacillus. J. Pathol. Bacteriol. 1939, 49, 457–466. [Google Scholar] [CrossRef]
- Nakajima, M.; Shirokawa, M.; Miyakuni, Y.; Nakano, T.; Goto, H. Giant Iliopsoas Abscess Caused by Morganella morganii. Am. J. Case Rep. 2017, 18, 395–398. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Penner, J.L.; Hennessy, J.N. O Antigen Grouping of Morganella morganii (Proteus Morganii) by Slide Agglutination. J. Clin. Microbiol. 1979, 10, 1023. [Google Scholar] [CrossRef] [PubMed]
- Braunstein, H.; Tomasulo, M. Identification of Proteus Morganii and Distinction from Other Proteus Species. Am. J. Clin. Pathol. 1975, 69, 905–908. [Google Scholar][Green Version]
- Schonwetter, R.S.; Orson, F.M. Chronic Morganella morganii Arthritis in an Elderly Patient. J. Clin. Microbiol. 1988, 26, 1414–1415. [Google Scholar] [CrossRef]
- Katz, L.M.; Lewis, R.J.; Borenstein, D.G. Successful Joint Arthroplasty Following Proteus Morganii (Morganella morganii) Septic Arthritis: A Four-Year Study. Arthritis Rheum. 1987, 30, 583–585. [Google Scholar] [CrossRef]
- Nazarian, D.G.; de Jesus, D.; McGuigan, F.; Booth, R.E., Jr. A Two-Stage Approach to Primary Knee Arthroplasty in the Infected Arthritic Knee. J. Arthroplast. 2003, 18, 16–21. [Google Scholar] [CrossRef]
- Poirel, L.; Guibert, M.; Girlich, D.; Naas, T.; Nordmann, P. Cloning, Sequence Analyses, Expression, and Distribution of ampC-ampR from Morganella morganii Clinical Isolates. Antimicrob. Agents Chemother. 1999, 43, 769–776. [Google Scholar] [CrossRef]
- Aschbacher, R.; Pagani, L.; Doumith, M.; Pike, R.; Woodford, N.; Spoladore, G.; Larcher, C.; Livermore, D.M. Metallo-β-Lactamases among Enterobacteriaceae from Routine Samples in an Italian Tertiary-Care Hospital and Long-Term Care Facilities during 2008. Clin. Microbiol. Infect. 2011, 17, 181–189. [Google Scholar] [CrossRef]
- Cai, J.C.; Yang, W.; Hu, Y.Y.; Zhang, R.; Zhou, H.W.; Chen, G.X. Detection of KPC-2 and qnrS1 in Clinical Isolates of Morganella morganii from China. Diagn. Microbiol. Infect. Dis. 2012, 73, 207–209. [Google Scholar] [CrossRef] [PubMed]
- Meletis, G.; Malousi, A.; Tychala, A.; Kassomenaki, A.; Vlachodimou, N.; Mantzana, P.; Metallidis, S.; Skoura, L.; Protonotariou, E. Probable Three-Species In Vivo Transfer of blaNDM-1 in a Single Patient in Greece: Occurrence of NDM-1-Producing Klebsiella Pneumoniae, Proteus Mirabilis, and Morganella morganii. Antibiotics 2023, 12, 1206. [Google Scholar] [CrossRef] [PubMed]
- Dropa, M.; Balsalobre, L.C.; Lincopan, N.; Mamizuka, E.M.; Murakami, T.; Cassettari, V.C.; Franco, F.; Guida, S.M.; Balabakis, A.J.; Passadore, L.F.; et al. Extended-Spectrum Beta-Lactamases among Enterobacteriaceae Isolated in a Public Hospital in Brazil. Rev. Inst. Med. Trop. São Paulo 2009, 51, 203–209. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Karim, M.A.; Andrawis, J.; Bengoa, F.; Bracho, C.; Compagnoni, R.; Cross, M.; Danoff, J.; Della Valle, C.J.; Foguet, P.; Fraguas, T.; et al. Hip and Knee Section, Diagnosis, Algorithm: Proceedings of International Consensus on Orthopedic Infections. J. Arthroplast. 2019, 34, S339–S350. [Google Scholar] [CrossRef]
- Autorino, C.M.; Battenberg, A.; Blom, A.; Catani, F.; ElGanzoury, I.; Farrell, A.; Giorgini, A.; Goswami, K.; Hernandez, V.; Karas, V.; et al. General Assembly, Prevention, Operating Room-Surgical Attire: Proceedings of International Consensus on Orthopedic Infections. J. Arthroplast. 2019, 34, S117–S125. [Google Scholar] [CrossRef]
- Zmistowski, B.; Karam, J.A.; Durinka, J.B.; Casper, D.S.; Parvizi, J. Periprosthetic joint infection increases the risk of one-year mortality. J. Bone Jt. Surg. 2013, 95, 2177–2184. [Google Scholar] [CrossRef]
- Bourget-Murray, J.; Horton, I.; Morris, J.; Bureau, A.; Garceau, S.; Abdelbary, H.; Grammatopoulos, G. Periprosthetic joint infection following hip hemiarthroplasty: Factors associated with infection and treatment outcome. Bone Jt. Open 2022, 3, 924–932. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).