Reliability of Measuring the Proximal Humeral Bone Mineral Density Using Dual-Energy X-ray Absorptiometry
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
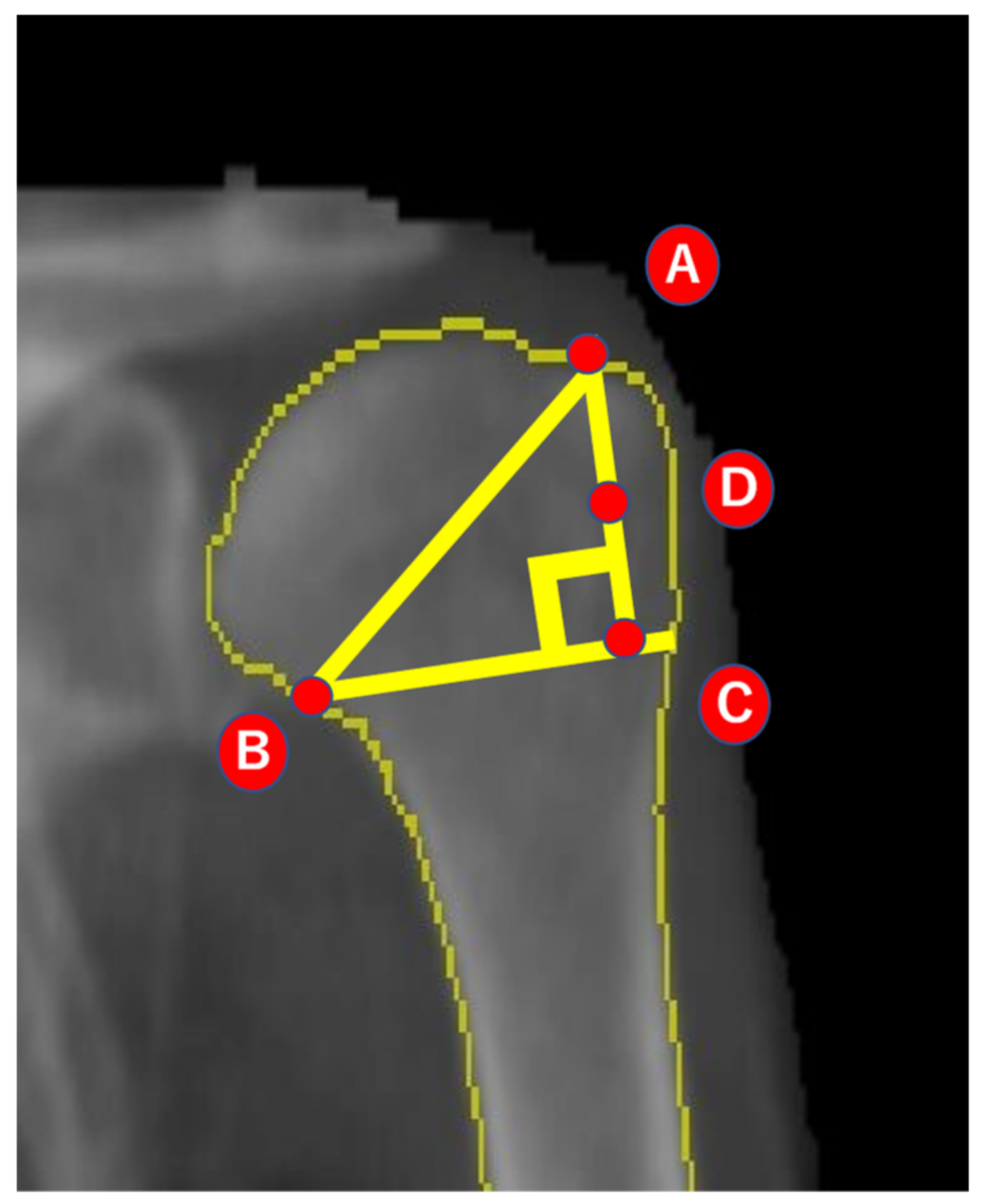
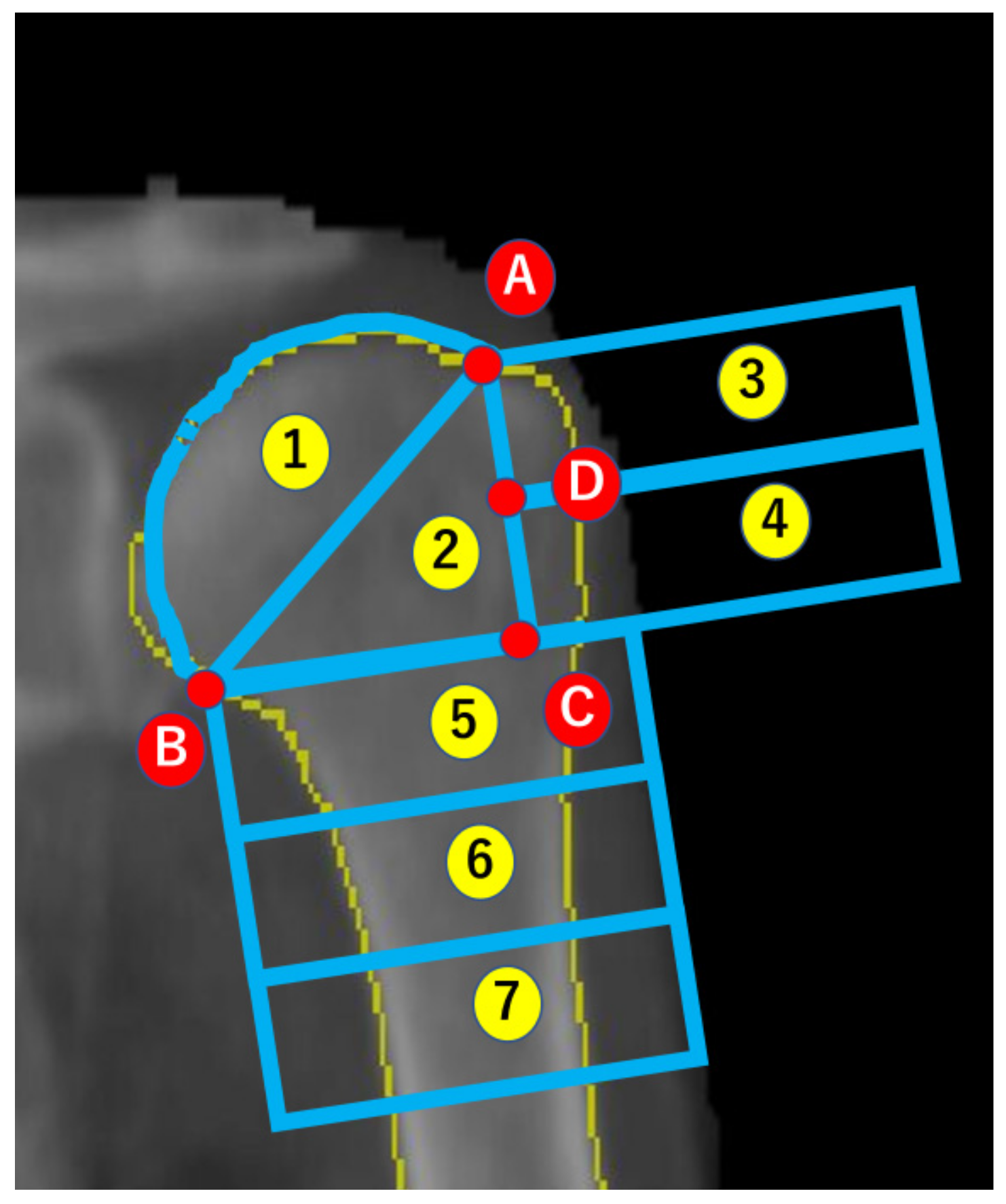
2.2. Analysis of BMD
2.3. Reliability of Imaging and Analysis
2.4. Statistical Analysis
3. Results
3.1. Correlation of the BMD with the ROIs and Age
3.2. Reliability
3.2.1. DXA Imaging Method
3.2.2. BMD Analysis According to ROI
3.2.3. Post hoc Power Analysis of the Correlation
4. Discussion
4.1. Reliability
4.2. BMD of the Humerus
4.3. Correlations of BMD
4.4. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yin, L.; Xu, Z.; Wang, L.; Li, W.; Zhao, Y.; Su, Y.; Sun, W.; Liu, Y.; Yang, M.; Yu, A.; et al. Associations of Muscle Size and Density With Proximal Femur Bone in a Community Dwelling Older Population. Front. Endocrinol. 2020, 11, 503. [Google Scholar] [CrossRef]
- Miyamoto, K.; Hirayama, A.; Sato, Y.; Ikeda, S.; Maruyama, M.; Soga, T.; Tomita, M.; Nakamura, M.; Matsumoto, M.; Yoshimura, N.; et al. A Metabolomic Profile Predictive of New Osteoporosis or Sarcopenia Development. Metabolites 2021, 11, 278. [Google Scholar] [CrossRef]
- Checa-Betegón, P.; Luque-Pérez, R.; Oñate Martínez-Olascoaga, D.; Pérez-González, J.L.; Domínguez-Esteban, I. Osteoporotic Vertebral Fractures: Natural History and Impact. Rev. Esp. Cir. Ortop. Traumatol. 2024, in press. [Google Scholar] [CrossRef]
- Gielen, E.; Aldvén, M.; Kanis, J.A.; Borgström, F.; Senior, E.; Willems, D. Cost-effectiveness of Romosozumab for the Treatment of Postmenopausal Women with Osteoporosis at High Risk of Fracture in Belgium. Osteoporos. Int. 2024, ahead of print. [Google Scholar] [CrossRef]
- Sing, C.W.; Lin, T.C.; Bartholomew, S.; Bell, J.S.; Bennett, C.; Beyene, K.; Bosco-Levy, P.; Bradbury, B.D.; Chan, A.H.Y.; Chandran, M.; et al. Global Epidemiology of Hip Fractures: Secular Trends in Incidence Rate, Post-Fracture Treatment, and All-Cause Mortality. J. Bone Miner. Res. 2023, 38, 1064–1075. [Google Scholar] [CrossRef]
- Genant, H.K.; Engelke, K.; Fuerst, T.; Glüer, C.C.; Grampp, S.; Harris, S.T.; Jergas, M.; Lang, T.; Lu, Y.; Majumdar, S.; et al. Noninvasive Assessment of Bone Mineral and Structure: State of the Art. J. Bone Miner. Res. 1996, 11, 707–730. [Google Scholar] [CrossRef]
- Mirsky, E.C.; Einhorn, T.A. Bone Densitometry in Orthopaedic Practice. J. Bone Jt. Surg. Am. 1998, 80, 1687–1698. [Google Scholar] [CrossRef]
- Court-Brown, C.M.; Caesar, B. Epidemiology of Adult Fractures: A Review. Injury 2006, 37, 691–697. [Google Scholar] [CrossRef]
- Court-Brown, C.M.; Garg, A.; McQueen, M.M. The Epidemiology of Proximal Humeral Fractures. Acta. Orthop. Scand. 2001, 72, 365–371. [Google Scholar] [CrossRef] [PubMed]
- Oh, J.H.; Song, B.W.; Kim, S.H.; Choi, J.-A.; Lee, J.W.; Chung, S.W.; Rhie, T.-Y. The Measurement of Bone Mineral Density of Bilateral Proximal Humeri Using DXA in Patients with Unilateral Rotator Cuff Tear. Osteoporos. Int. 2014, 25, 2639–2648. [Google Scholar] [CrossRef] [PubMed]
- Chan, Y.H. Biostatistics 104: Correlational Analysis. Singap. Med. J. 2003, 44, 614–619. [Google Scholar]
- Shrout, P.E.; Fleiss, J.L. Intraclass Correlations: Uses in Assessing Rater Reliability. Psychol. Bull. 1979, 86, 420–428. [Google Scholar] [CrossRef] [PubMed]
- Bayar, A.; Sarikaya, S.; Keser, S.; Ozdolap, S.; Tuncay, I.; Ege, A. Regional Bone Density Changes in Anterior Cruciate Ligament Deficient Knees: A DEXA Study. Knee 2008, 15, 373–377. [Google Scholar] [CrossRef] [PubMed]
- Fuller, J.T.; Archer, J.; Buckley, J.D.; Tsiros, M.D.; Thewlis, D. The Reliability of Dual-Energy X-ray Absorptiometry Measurements of Bone Mineral Density in the Metatarsals. Skelet. Radiol. 2016, 45, 135–140. [Google Scholar] [CrossRef]
- Krappinger, D.; Roth, T.; Gschwentner, M.; Suckert, A.; Blauth, M.; Hengg, C.; Kralinger, F. Preoperative Assessment of the Cancellous Bone Mineral Density of the Proximal Humerus using CT data. Skelet. Radiol. 2012, 41, 299–304. [Google Scholar] [CrossRef] [PubMed]
- Barvencik, F.; Gebauer, M.; Beil, F.T.; Vettorazzi, E.; Mumme, M.; Rupprecht, M.; Pogoda, P.; Wegscheider, K.; Rueger, J.M.; Pueschel, K.; et al. Age- and Sex-related Changes of Humeral Head Microarchitecture: Histomorphometric Analysis of 60 Human Specimens. J. Orthop. Res. 2010, 28, 18–26. [Google Scholar] [CrossRef] [PubMed]
- Tingart, M.J.; Apreleva, M.; von Stechow, D.; Zurakowski, D.; Warner, J.J. The Cortical Thickness of the Proximal Humeral Diaphysis Predicts Bone Mineral Density of the Proximal Humerus. J. Bone Jt. Surg. Br. 2003, 85, 611–617. [Google Scholar] [CrossRef] [PubMed]
- Doetsch, A.M.; Faber, J.; Lynnerup, N.; Wätjen, I.; Bliddal, H.; Danneskiold-Samsøe, B. Bone Mineral Density Measurement over the Shoulder Region. Calcif. Tissue Int. 2002, 71, 308–314. [Google Scholar] [CrossRef]
- Mather, J.; MacDermid, J.C.; Faber, K.J.; Athwal, G.S. Proximal Humerus Cortical Bone Thickness Correlates with Bone Mineral Density and can Clinically Rule Out Osteoporosis. J. Shoulder Elb. Surg. 2013, 22, 732–738. [Google Scholar] [CrossRef]
- Oh, J.H.; Song, B.W.; Lee, Y.S. Measurement of Volumetric Bone Mineral Density in Proximal Humerus using Quantitative Computed Tomography in Patients with Unilateral Rotator Cuff Tear. J. Shoulder Elb. Surg. 2014, 23, 993–1002. [Google Scholar] [CrossRef]
- Kawashima, I.; Ishizuka, S.; Oba, H.; Sakaguchi, T.; Nakashima, H.; Takegami, Y.; Imagama, S. Prevalence and Treatment Rates of Osteoporosis Among Individuals with Rotator Cuff Tears. J. Shoulder Elb. Surg. 2024, in press. [Google Scholar] [CrossRef]
- Chan, M.Y.; Center, J.R.; Eisman, J.A.; Nguyen, T.V. Bone Mineral Density and Association of Osteoarthritis with Fracture Risk. Osteoarthr. Cartil. 2014, 22, 1251–1258. [Google Scholar] [CrossRef]

| Age (years) | 78.2 ± 6.8 |
| Height (cm) | 147.0 ± 6.1 |
| Weight (kg) | 47.1 ± 7.6 |
| Body Mass Index (kg/m2) | 21.8 ± 3.0 |
| Lumbar spine (L1–L4) | 0.84 ± 0.17 | |
| Proximal femur | Total hip | 0.67 ± 0.14 |
| Femoral neck | 0.63 ± 0.13 | |
| Ward’s triangle | 0.44 ± 0.17 | |
| Trochanter | 0.54 ± 0.12 | |
| Intertrochanter | 0.80 ± 0.17 | |
| Proximal humerus | Region 1 | 0.60 ± 0.15 |
| Region 2 | 0.35 ± 0.13 | |
| Region 3 | 0.42 ± 0.12 | |
| Region 4 | 0.35 ± 0.13 | |
| Region 5 | 0.40 ± 0.14 | |
| Region 6 | 0.54 ± 0.16 | |
| Region 7 | 0.64 ± 0.14 | |
| Proximal Humerus | ||||||||
|---|---|---|---|---|---|---|---|---|
| Region 1 | Region 2 | Region 3 | Region 4 | Region 5 | Region 6 | Region 7 | ||
| Lumbar spine (L1–L4) | r | 0.19 | 0.27 | 0.28 | 0.34 | 0.29 | 0.33 | 0.33 |
| p | <0.05 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | |
| Total hip | r | 0.27 | 0.38 | 0.34 | 0.39 | 0.38 | 0.49 | 0.51 |
| p | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | |
| Femoral neck | r | 0.21 | 0.34 | 0.28 | 0.31 | 0.32 | 0.41 | 0.44 |
| p | <0.05 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | |
| Ward’s triangle | r | 0.22 | 0.32 | 0.24 | 0.25 | 0.31 | 0.39 | 0.41 |
| p | <0.05 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | |
| Trochanter | r | 0.28 | 0.38 | 0.40 | 0.44 | 0.35 | 0.44 | 0.46 |
| p | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | |
| Intertrochanter | r | 0.25 | 0.35 | 0.30 | 0.37 | 0.38 | 0.49 | 0.49 |
| p | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | |
| Proximal Humerus | ||||||||
|---|---|---|---|---|---|---|---|---|
| Region 1 | Region 2 | Region 3 | Region 4 | Region 5 | Region 6 | Region 7 | ||
| Age | r | −0.09 | −0.12 | −0.14 | −0.07 | −0.11 | −0.14 | −0.12 |
| p | 0.353 | 0.200 | 0.133 | 0.480 | 0.268 | 0.135 | 0.233 | |
| Lumber spine (L1–L4) | Total hip | Femoral neck | Ward’s triangle | Trochanter | Intertrochanter | |||
| Age | r | −0.03 | 0.10 | 0.10 | 0.09 | 0.07 | 0.11 | |
| p | 0.76 | 0.316 | 0.295 | 0.366 | 0.474 | 0.316 | ||
| ICC (1.1) | 95% CI | |
|---|---|---|
| Region 1 | 0.93 | 0.76–0.98 |
| Region 2 | 0.96 | 0.87–0.99 |
| Region 3 | 0.93 | 0.76–0.98 |
| Region 4 | 0.88 | 0.60–0.97 |
| Region 5 | 0.85 | 0.58–0.97 |
| Region 6 | 0.87 | 0.71–0.98 |
| Region 7 | 0.92 | 0.72–0.98 |
| ICC (1.1) | 95% CI | ICC (2.1) | 95% CI | |
|---|---|---|---|---|
| Region 1 | 0.98 | 0.93–0.99 | 0.98 | 0.93–0.99 |
| Region 2 | 0.98 | 0.94–0.99 | 0.98 | 0.94–0.99 |
| Region 3 | 0.98 | 0.93–0.99 | 0.98 | 0.90–0.99 |
| Region 4 | 0.99 | 0.96–0.99 | 0.99 | 0.96–0.99 |
| Region 5 | 0.99 | 0.96–0.99 | 0.98 | 0.92–0.99 |
| Region 6 | 0.99 | 0.98–0.99 | 0.99 | 0.96–0.99 |
| Region 7 | 0.99 | 0.98–0.99 | 0.99 | 0.97–0.99 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kamiyama, M.; Shitara, H.; Tajika, T.; Shimoyama, D.; Hashimoto, S.; Ichinose, T.; Sasaki, T.; Hamano, N.; Chikuda, H. Reliability of Measuring the Proximal Humeral Bone Mineral Density Using Dual-Energy X-ray Absorptiometry. Osteology 2024, 4, 88-97. https://doi.org/10.3390/osteology4020007
Kamiyama M, Shitara H, Tajika T, Shimoyama D, Hashimoto S, Ichinose T, Sasaki T, Hamano N, Chikuda H. Reliability of Measuring the Proximal Humeral Bone Mineral Density Using Dual-Energy X-ray Absorptiometry. Osteology. 2024; 4(2):88-97. https://doi.org/10.3390/osteology4020007
Chicago/Turabian StyleKamiyama, Masataka, Hitoshi Shitara, Tsuyoshi Tajika, Daisuke Shimoyama, Shogo Hashimoto, Tsuyoshi Ichinose, Tsuyoshi Sasaki, Noritaka Hamano, and Hirotaka Chikuda. 2024. "Reliability of Measuring the Proximal Humeral Bone Mineral Density Using Dual-Energy X-ray Absorptiometry" Osteology 4, no. 2: 88-97. https://doi.org/10.3390/osteology4020007
APA StyleKamiyama, M., Shitara, H., Tajika, T., Shimoyama, D., Hashimoto, S., Ichinose, T., Sasaki, T., Hamano, N., & Chikuda, H. (2024). Reliability of Measuring the Proximal Humeral Bone Mineral Density Using Dual-Energy X-ray Absorptiometry. Osteology, 4(2), 88-97. https://doi.org/10.3390/osteology4020007






