Vitamin D Deficiency in Orthopedic Patients in Different Latitudes—First Study Comparing German and Greek Populations
Abstract
1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Maier, G.S.; Jakobs, P.; Roth, K.E.; Kurth, A.A.; Maus, U. Is there an epidemic vitamin D deficiency in German orthopaedic patients? Clin. Orthop. Relat. Res. 2013, 471, 3029–3035. [Google Scholar] [CrossRef] [PubMed]
- Grossman, D.C.; Curry, S.J.; Owens, D.K.; Barry, M.J.; Caughey, A.B.; Davidson, K.W.; Doubeni, C.A.; Epling, J.W.; Kemper, A.R.; Krist, A.H.; et al. Vitamin D, Calcium, or Combined Supplementation for the Primary Prevention of Fractures in Community-Dwelling Adults: US Preventive Services Task Force Recommendation Statement. JAMA 2018, 319, 1592–1599. [Google Scholar] [PubMed]
- Zhao, J.-G.; Zeng, X.-T.; Wang, J.; Liu, L. Association Between Calcium or Vitamin D Supplementation and Fracture Incidence in Community-Dwelling Older Adults: A Systematic Review and Meta-analysis. JAMA 2017, 318, 2466–2482. [Google Scholar] [CrossRef] [PubMed]
- Wacker, M.; Holick, M.F. Vitamin D—Effects on skeletal and extraskeletal health and the need for supplementation. Nutrients 2013, 5, 111–148. [Google Scholar] [CrossRef] [PubMed]
- Goltzman, D. Functions of vitamin D in bone. Histochem. Cell Biol. 2018, 149, 305–312. [Google Scholar] [CrossRef] [PubMed]
- Medrano, M.; Carrillo-Cruz, E.; Montero, I.; Perez-Simon, J.A. Vitamin D: Effect on Haematopoiesis and Immune System and Clinical Applications. Int. J. Mol. Sci. 2018, 19, 2663. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Zhu, X.; Gu, L.; Zhan, Y.; Chen, L.; Li, X. Association Between Vitamin D and Influenza: Meta-Analysis and Systematic Review of Randomized Controlled Trials. Front. Nutr. 2021, 8, 799709. [Google Scholar] [CrossRef] [PubMed]
- Grant, W.B.; Lahore, H.; McDonnell, S.L.; Baggerly, C.A.; French, C.B.; Aliano, J.L.; Bhattoa, H.P. Evidence that Vitamin D Supplementation Could Reduce Risk of Influenza and COVID-19 Infections and Deaths. Nutrients 2020, 12, 988. [Google Scholar] [CrossRef]
- Entrenas Castillo, M.; Entrenas Costa, L.M.; Vaquero Barrios, J.M.; Alcalá Díaz, J.F.; López Miranda, J.; Bouillon, R.; Quesada Gomez, J.M. Effect of calcifediol treatment and best available therapy versus best available therapy on intensive care unit admission and mortality among patients hospitalized for COVID-19: A pilot randomized clinical study. J. Steroid Biochem. Mol. Biol. 2020, 203, 105751. [Google Scholar] [CrossRef]
- Geng, C.; Shaikh, A.S.; Han, W.; Chen, D.; Guo, Y.; Jiang, P. Vitamin D and depression: Mechanisms, determination and application. Asia Pac. J. Clin. Nutr. 2019, 28, 689–694. [Google Scholar]
- Cosentino, N.; Campodonico, J.; Milazzo, V.; de Metrio, M.; Brambilla, M.; Camera, M.; Marenzi, G. Vitamin D and Cardiovascular Disease: Current Evidence and Future Perspectives. Nutrients 2021, 13, 3603. [Google Scholar] [CrossRef] [PubMed]
- Charoenngam, N.; Shirvani, A.; Holick, M.F. Vitamin D for skeletal and non-skeletal health: What we should know. J. Clin. Orthop. Trauma 2019, 10, 1082–1093. [Google Scholar] [CrossRef] [PubMed]
- de La Puente Yagüe, M.; Collado Yurrita, L.; Ciudad Cabañas, M.J.; Cuadrado Cenzual, M.A. Role of Vitamin D in Athletes and Their Performance: Current Concepts and New Trends. Nutrients 2020, 12, 579. [Google Scholar] [CrossRef] [PubMed]
- de Martinis, M.; Allegra, A.; Sirufo, M.M.; Tonacci, A.; Pioggia, G.; Raggiunti, M.; Ginaldi, L.; Gangemi, S. Vitamin D Deficiency, Osteoporosis and Effect on Autoimmune Diseases and Hematopoiesis: A Review. Int. J. Mol. Sci. 2021, 22, 8855. [Google Scholar] [CrossRef]
- Chevalley, T.; Brandi, M.L.; Cashman, K.D.; Cavalier, E.; Harvey, N.C.; Maggi, S.; Cooper, C.; Al-Daghri, N.; Bock, O.; Bruyère, O.; et al. Role of vitamin D supplementation in the management of musculoskeletal diseases: Update from an European Society of Clinical and Economical Aspects of Osteoporosis, Osteoarthritis and Musculoskeletal Diseases [ESCEO] working group. Aging Clin. Exp. Res. 2022, 34, 2603–2623. [Google Scholar] [CrossRef]
- Giustina, A.; Bouillon, R.; Dawson-Hughes, B.; Ebeling, P.R.; Lazaretti-Castro, M.; Lips, P.; Marcocci, C.; Bilezikian, J.P. Vitamin D in the older population: A consensus statement. Endocrine 2022, 79, 31–44. [Google Scholar] [CrossRef]
- Gallagher, J.C. Vitamin D and aging. Endocrinol. Metab. Clin. N. Am. 2013, 42, 319–332. [Google Scholar] [CrossRef]
- Caccamo, D.; Ricca, S.; Currò, M.; Ientile, R. Health Risks of Hypovitaminosis D: A Review of New Molecular Insights. Int. J. Mol. Sci. 2018, 19, 892. [Google Scholar] [CrossRef]
- Lamberg-Allardt, C. Vitamin D in foods and as supplements. Prog. Biophys. Mol. Biol. 2006, 92, 33–38. [Google Scholar] [CrossRef]
- Jungert, A.; Neuhäuser-Berthold, M. Dietary vitamin D intake is not associated with 25-hydroxyvitamin D3 or parathyroid hormone in elderly subjects, whereas the calcium-to-phosphate ratio affects parathyroid hormone. Nutr. Res. 2013, 33, 661–667. [Google Scholar] [CrossRef]
- Mithal, A.; Wahl, D.A.; Bonjour, J.-P.; Burckhardt, P.; Dawson-Hughes, B.; Eisman, J.A.; El-Hajj Fuleihan, G.; Josse, R.G.; Lips, P.; Morales-Torres, J. Global vitamin D status and determinants of hypovitaminosis D. Osteoporos. Int. 2009, 20, 1807–1820. [Google Scholar] [CrossRef]
- Glowacki, J.; Hurwitz, S.; Thornhill, T.S.; Kelly, M.; LeBoff, M.S. Osteoporosis and vitamin-D deficiency among postmenopausal women with osteoarthritis undergoing total hip arthroplasty. J. Bone Jt. Surg. 2003, 85, 2371–2377. [Google Scholar] [CrossRef]
- Foo, L.H.; Zhang, Q.; Zhu, K.; Ma, G.; Trube, A.; Greenfield, H.; Fraser, D.R. Relationship between vitamin D status, body composition and physical exercise of adolescent girls in Beijing. Osteoporos. Int. 2009, 20, 417–425. [Google Scholar] [CrossRef] [PubMed]
- Hill, T.R.; Cotter, A.A.; Mitchell, S.; Boreham, C.A.; Dubitzky, W.; Murray, L.; Strain, J.J.; Flynn, A.; Robson, P.J.; Wallace, J.M.W.; et al. Vitamin D status and its determinants in adolescents from the Northern Ireland Young Hearts 2000 cohort. Br. J. Nutr. 2008, 99, 1061–1067. [Google Scholar] [CrossRef] [PubMed]
- Hintzpeter, B.; Mensink, G.B.M.; Thierfelder, W.; Müller, M.J.; Scheidt-Nave, C. Vitamin D status and health correlates among German adults. Eur. J. Clin. Nutr. 2008, 62, 1079–1089. [Google Scholar] [CrossRef] [PubMed]
- Kechichian, E.; Ezzedine, K. Vitamin D and the Skin: An Update for Dermatologists. Am. J. Clin. Dermatol. 2018, 19, 223–235. [Google Scholar] [CrossRef]
- Smith, N.; Sievert, L.L.; Muttukrishna, S.; Begum, K.; Murphy, L.; Sharmeen, T.; Gunu, R.; Chowdhury, O.; Bentley, G.R. Mismatch: A comparative study of vitamin D status in British-Bangladeshi migrants. Evol. Med. Public Health 2021, 9, 164–173. [Google Scholar] [CrossRef]
- Schilling, S. Epidemic vitamin D deficiency among patients in an elderly care rehabilitation facility. Dtsch. Arztebl. Int. 2012, 109, 33–38. [Google Scholar] [CrossRef]
- Moon, A.S.; Boudreau, S.; Mussell, E.; He, J.K.; Brabston, E.W.; Ponce, B.A.; Momaya, A.M. Current concepts in vitamin D and orthopaedic surgery. Orthop. Traumatol. Surg. Res. OTSR 2019, 105, 375–382. [Google Scholar] [CrossRef]
- Jones, G. Interpreting vitamin D assay results: Proceed with caution. Clin. J. Am. Soc. Nephrol. CJASN 2015, 10, 331–334. [Google Scholar] [CrossRef]
- Hollis, B.W. Circulating 25-hydroxyvitamin D levels indicative of vitamin D sufficiency: Implications for establishing a new effective dietary intake recommendation for vitamin D. J. Nutr. 2005, 135, 317–322. [Google Scholar] [CrossRef]
- Grant, W.B.; Holick, M.F. Benefits and requirements of vitamin D for optimal health: A review. Altern. Med. Rev. J. Clin. Ther. 2005, 10, 94–111. [Google Scholar]
- Scharla, S.H. Prevalence of subclinical vitamin D deficiency in different European countries. Osteoporos. Int. 1998, 8 (Suppl. S2), S7–S12. [Google Scholar] [CrossRef] [PubMed]
- Hernigou, P.; Sitbon, J.; Dubory, A.; Auregan, J.C. Vitamin D history part III: The “modern times”-new questions for orthopaedic practice: Deficiency, cell therapy, osteomalacia, fractures, supplementation, infections. Int. Orthop. 2019, 43, 1755–1771. [Google Scholar] [CrossRef] [PubMed]
- Kawashima, I.; Hiraiwa, H.; Ishizuka, S.; Kawai, R.; Hoshino, Y.; Kusaka, Y.; Tsukahara, T. Comparison of vitamin D sufficiency between indoor and outdoor elite male collegiate athletes. Nagoya J. Med. Sci. 2021, 83, 219–226. [Google Scholar]
- Priemel, M.; von Domarus, C.; Klatte, T.O.; Kessler, S.; Schlie, J.; Meier, S.; Proksch, N.; Pastor, F.; Netter, C.; Streichert, T.; et al. Bone mineralization defects and vitamin D deficiency: Histomorphometric analysis of iliac crest bone biopsies and circulating 25-hydroxyvitamin D in 675 patients. J. Bone Miner. Res. Off. J. Am. Soc. Bone Miner. Res. 2010, 25, 305–312. [Google Scholar] [CrossRef]
- Smith, J.M.; Cancienne, J.M.; Brockmeier, S.F.; Werner, B.C. Vitamin D deficiency and total shoulder arthroplasty complications. Shoulder Elb. 2021, 13, 99–105. [Google Scholar] [CrossRef]
- Jamal, A.B.; Hasan Khan, M.N.; Sadiq, M. Intertrochanteric Hip Fractures And Vitamin D Deficiency; A Significant Association. J. Ayub Med. Coll. Abbottabad JAMC 2021, 33, 257–261. [Google Scholar]
- Webb, A.R. Who, what, where and when-influences on cutaneous vitamin D synthesis. Prog. Biophys. Mol. Biol. 2006, 92, 17–25. [Google Scholar] [CrossRef]
- Sizar, O.; Khare, S.; Goyal, A.; Bansal, P.; Givler, A. StatPearls. In Vitamin D Deficiency; StatPearls Publishing LLC: Treasure Island, FL, USA, 2021. [Google Scholar]
- Rizzoli, R. Vitamin D supplementation: Upper limit for safety revisited? Aging Clin. Exp. Res. 2021, 33, 19–24. [Google Scholar] [CrossRef]
| Both Groups | Group Regensburg | Group Patras | |||
|---|---|---|---|---|---|
| 25-OH-D mean value (Sign.) | 25-OH-D mean value (Sign.) | ||||
| Patients (n) | 1000 | 500 | - | 500 | |
| Men (n) | 433 (43.3%) | 230 (46%) | 17.70 | 204 (40.7%) | 18.20 |
| Women (n) | 567 (56.7%) | 270 (54%) | 18.10 | 297 (59.3%) | 18.40 |
| Age (years) | 59 (SD ±± 18.2) | 60 (SD ±± 18.1) | - | 59 (SD ±± 18.2) | |
| Osteoporosis | 165 (16.5%) | 57 (11.4%) | 23.50 | 108 (21.6%) | 23.95 |
| Obesity (BMI) | 248 (24.8%) | 133 (26.7%) | 16.00 | 115 (23%) | 15.40 |
| Thyroid disease | 129 (12.9%) | 64 (12.8%) | 16 | 65 (13%) | 17.60 |
| Psychiatric diseases | 171 (17.1%) | 91 (18.2%) | 16.9 | 80 (16%) | 18.15 |
| Infectious diseases | 24 (2.4%) | 13 (2.6%) | 15.50 | 11 (2.2%) | 16.40 |
| Renal failure | 92 (9.2%) | 57 (11.4%) | 17.80 | 35 (7%) | 14.60 |
| Pulmonary disease | 85 (8.5%) | 48 (9.6%) | 18.50 | 37 (7.4%) | 17.30 |
| Cardiovascular disease | 238 (23.8%) | 133 (26.7%) | 17.50 | 105 (21%) | 17.30 |
| Diabetes | 146 (14.6%) | 87 (17.4%) | 16.50 | 59 (11.8%) | 14.30 |
| Hypertension | 456 (45.6%) | 253 (50.7%) | 16.80 | 203 (40.5%) | 17.10 |
| Carcinoma | 176 (17.6%) | 96 (9.2%) | 16.35 | 80 (16%) | 17.20 |
| Bone fracture | 81 (8.1%) | 46 (9.2%) | 18.85 | 35 (7%) | 18.50 |
| Supplement VitD | 132 (13.2%) | 51 (10.2%) | 24.50 | 81 (16.2%) | 23.50 |
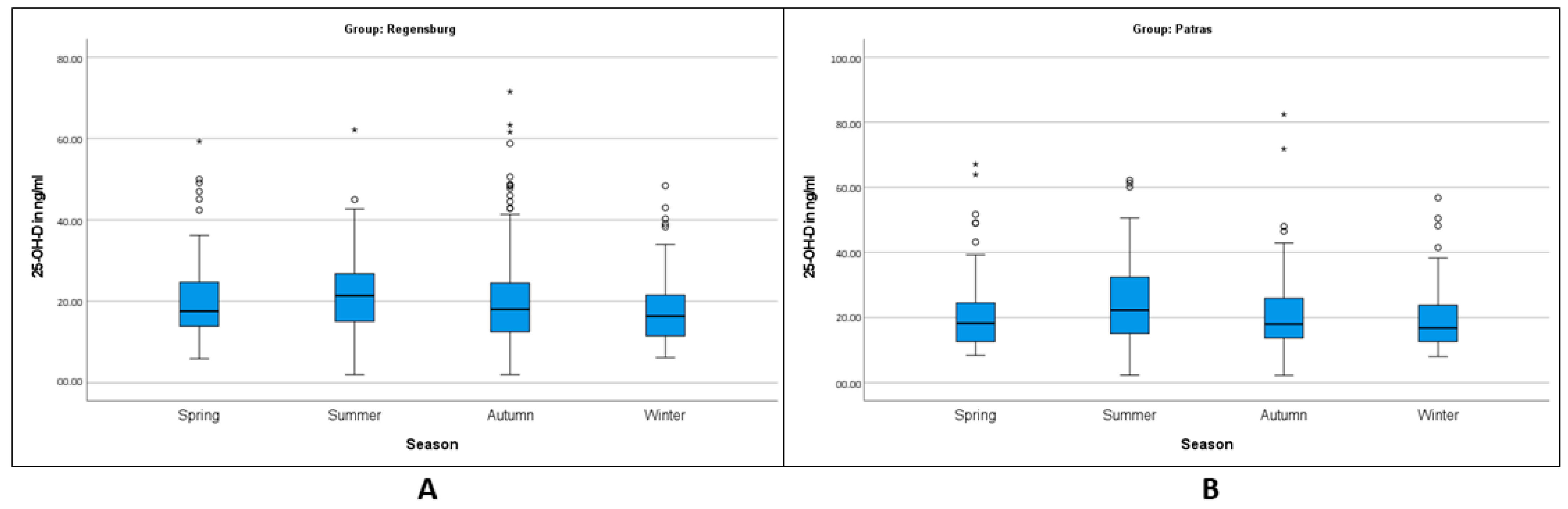
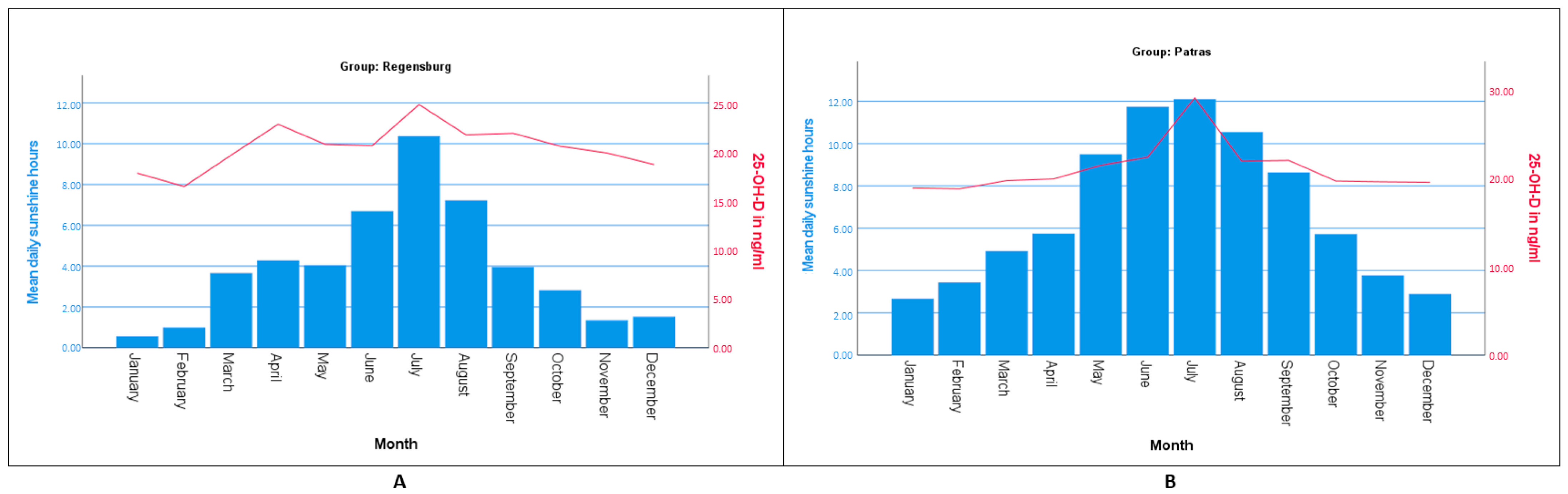
| Spring | 198 (19.8%) | 92 (18.4%) | 20.64 | 106 (21.2%) | 20.39 |
| Summer | 206 (20.6%) | 85 (17%) | 21.89 | 121 (24.2%) | 20.8 |
| Autumn | 353 (35.5%) | 194 (38.8%) | 20.62 | 159 (31.8%) | 20.52 |
| Winter | 243 (24.3%) | 129 (25.8%) | 17.72 | 114 (20.8%) | 19.17 |
| I | |||||
|---|---|---|---|---|---|
| Age Group | Mean Value | SD | 95% Confidence Interval | Median | |
| Lower Limit | Upper Limit | ||||
| 30 years and younger | 19.40 | ±±8.26 | 16.89 | 21.91 | 18.60 |
| 31–50 years | 21.05 | ±±10.58 | 18.83 | 23.27 | 19.20 |
| 51–69 years | 19.55 | ±±9.84 | 18.09 | 21.02 | 18.00 |
| 70 years and older | 20.28 | ±±11.75 | 18.60 | 21.96 | 17.00 |
| II | |||||
| Age Group | Mean Value | SD | 95% Confidence Interval | Median | |
| Lower Limit | Upper Limit | ||||
| 30 years and younger | 20.56 | ±±8.8 | 17.80 | 23.33 | 17.65 |
| 31–50 years | 20.72 | ±±9.44 | 18.98 | 22.45 | 19.20 |
| 51–69 years | 21.07 | ±±10.44 | 19.47 | 22.66 | 18.70 |
| 70 years and older | 21.87 | ±±12.79 | 19.97 | 23.79 | 17.30 |
| I | |||||
|---|---|---|---|---|---|
| Season | Mean Value | SD | 95% Confidence Interval | Median | |
| Lower Limit | Upper Limit | ||||
| Spring | 20.65 | ±±10.38 | 18.50 | 22.80 | 17.60 |
| Summer | 21.89 | ±±10.42 | 19.63 | 24.14 | 21.40 |
| Autumn | 20.61 | ±±11.87 | 18.93 | 22.29 | 18.05 |
| Winter | 17.72 | ±±8.34 | 16.26 | 19.16 | 16.40 |
| II | |||||
| Season | Mean Value | SD | 95% Confidence Interval | Median | |
| Lower Limit | Upper Limit | ||||
| Spring | 20.39 | ±±10.95 | 18.28 | 22.50 | 18.25 |
| Summer | 24.80 | ±±12.25 | 22.59 | 27.00 | 22.30 |
| Autumn | 20.52 | ±±10.49 | 18.89 | 22.17 | 18.00 |
| Winter | 19.19 | ±±9.40 | 17.44 | 20.94 | 16.65 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mamilos, A.; Matzaroglou, C.; Maier, G.S.; Zawy Alsofy, S.; Drees, P.; Kafchitsas, K. Vitamin D Deficiency in Orthopedic Patients in Different Latitudes—First Study Comparing German and Greek Populations. Osteology 2023, 3, 11-20. https://doi.org/10.3390/osteology3010002
Mamilos A, Matzaroglou C, Maier GS, Zawy Alsofy S, Drees P, Kafchitsas K. Vitamin D Deficiency in Orthopedic Patients in Different Latitudes—First Study Comparing German and Greek Populations. Osteology. 2023; 3(1):11-20. https://doi.org/10.3390/osteology3010002
Chicago/Turabian StyleMamilos, Andreas, Charalambos Matzaroglou, Gerrit S. Maier, Samer Zawy Alsofy, Philipp Drees, and Konstantinos Kafchitsas. 2023. "Vitamin D Deficiency in Orthopedic Patients in Different Latitudes—First Study Comparing German and Greek Populations" Osteology 3, no. 1: 11-20. https://doi.org/10.3390/osteology3010002
APA StyleMamilos, A., Matzaroglou, C., Maier, G. S., Zawy Alsofy, S., Drees, P., & Kafchitsas, K. (2023). Vitamin D Deficiency in Orthopedic Patients in Different Latitudes—First Study Comparing German and Greek Populations. Osteology, 3(1), 11-20. https://doi.org/10.3390/osteology3010002






