The Regio- and Stereoselective Synthesis of 1,4-Diarylbut-1-en-3-ynes Having Aryl Groups at the Mutual Syn Positions
Abstract
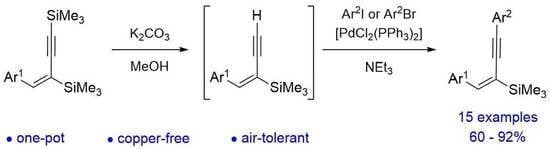
1. Introduction
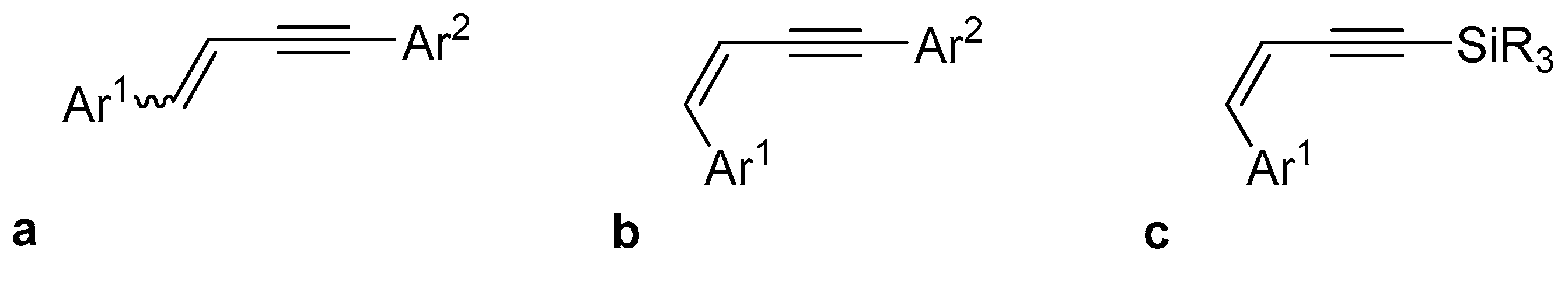
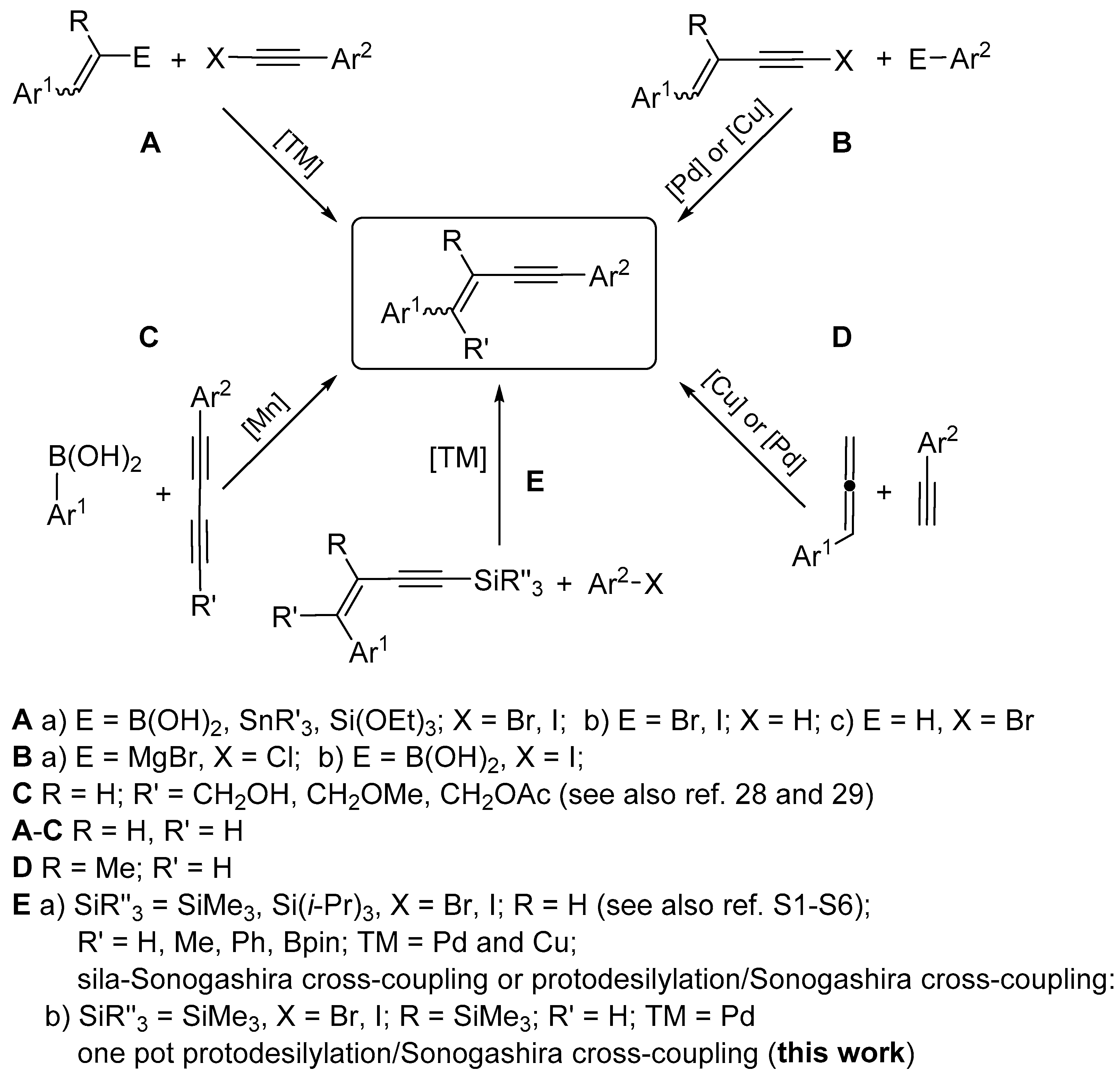
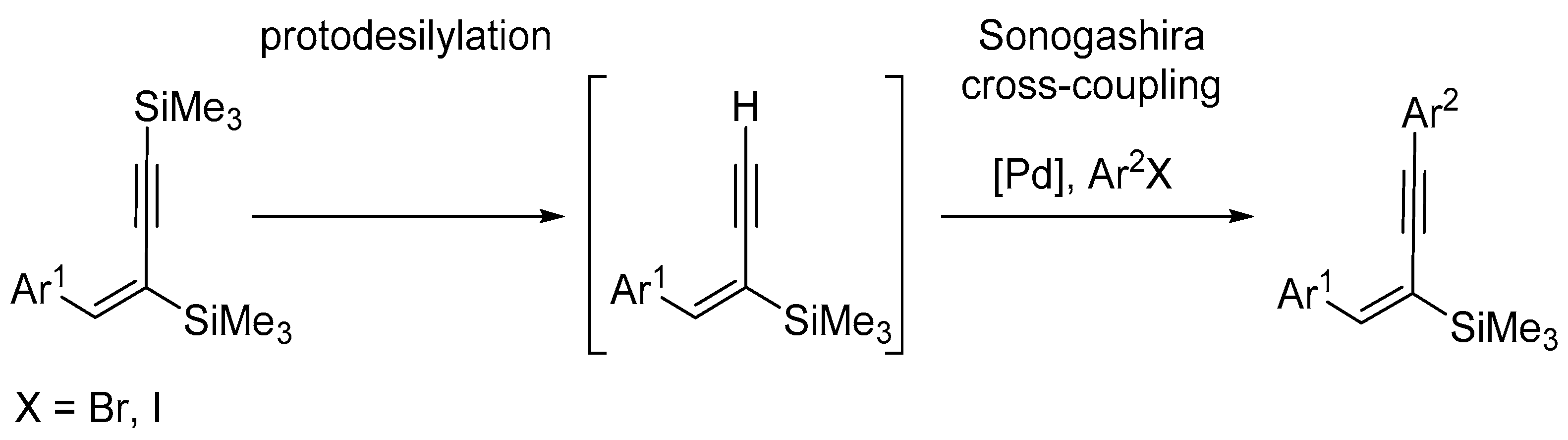
2. Results and Discussion
3. Conclusions
4. Experimental
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Nicolaou, K.C.; Bulger, P.G.; Sarlah, D. Palladium-catalyzed cross-coupling reactions in total synthesis. Angew. Chem. Int. Ed. 2005, 44, 4442–4489. [Google Scholar] [CrossRef] [PubMed]
- Trost, B.M.; Masters, J.T. Transition metal-catalyzed couplings of alkynes to 1,3-enynes: Modern methods and synthetic applications. Chem. Soc. Rev. 2016, 45, 2212–2238. [Google Scholar] [CrossRef] [PubMed]
- Dherbassy, Q.; Manna, S.; Talbot, F.J.T.; Prasitwatcharakorn, W.; Perry, G.J.P.; Procter, D.J. Copper-catalyzed functionalization of enynes. Chem. Sci. 2020, 11, 11380–11393. [Google Scholar] [CrossRef] [PubMed]
- Bao, X.; Ren, J.; Yang, Y.; Ye, X.; Wang, B.; Wang, H. 2-Activated 1,3-enynes in enantioselective synthesis. Org. Biomol. Chem. 2020, 18, 7977–7986. [Google Scholar] [CrossRef]
- Liu, Y.; Luo, P.; Fu, Y.; Hao, T.; Liu, X.; Ding, Q.; Peng, Y. Recent advances in the tandem annulation of 1,3-enynes to functionalized pyridine and pyrrole derivatives. Beilstein J. Org. Chem. 2021, 17, 2462–2476. [Google Scholar] [CrossRef]
- Gevorgyan, V.; Yamamoto, Y. Palladium-catalyzed enyne–yne [4+2] benzannulation as a new and general approach to polysubstituted benzenes. J. Organomet. Chem. 1999, 576, 232–247. [Google Scholar] [CrossRef]
- Iverson, S.L.; Uetrecht, J.P. Identification of a reactive metabolite of terbinafine: Insights into terbinafine-induced hepatotoxicity. Chem. Res. Toxicol. 2001, 14, 175–181. [Google Scholar] [CrossRef]
- Shun, A.L.K.S.; Tykwinski, R.R. Synthesis of naturally occurring polyynes. Angew. Chem. Int. Ed. 2006, 45, 1034–1057. [Google Scholar] [CrossRef]
- Wang, D.; Gao, S. Sonogashira coupling in natural product synthesis. Org. Chem. Front. 2014, 1, 556–566. [Google Scholar] [CrossRef]
- El-Shazly, M.; Barve, B.D.; Korinek, M.; Liou, J.-R.; Chuang, D.-W.; Cheng, Y.-B.; Hou, M.-F.; Wang, J.-J.; Wu, Y.-C.; Chang, F.-R. Insights on the Isolation, biological activity and synthetic protocols of enyne derivatives. Curr. Top. Med. Chem. 2014, 14, 1076–1093. [Google Scholar] [CrossRef]
- Liu, Y.; Nishiura, M.; Wang, Y.; Hou, Z. π-Conjugated aromatic enynes as a single-emitting component for white electroluminescence. J. Am. Chem. Soc. 2006, 128, 5592–5593. [Google Scholar] [CrossRef]
- Peng, T.; Li, G.; Liu, Y.; Wua, Y.; Ye, K.; Yao, D.; Yuan, Y.; Hou, Z.; Wang, Y. High-efficiency and deep-blue fluorescent organic light-emitting diodes with the easily controlled doping concentrations. Org. Electron. 2011, 12, 1068–1072. [Google Scholar] [CrossRef]
- Doucet, H.; Hierso, J.-C. Palladium-based catalytic systems for the synthesis of conjugated enynes by Sonogashira reactions and related alkynylations. Angew. Chem. Int. Ed. 2007, 46, 834–871. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhang, Y.; Wang, J. Recent advances in transition-metal-catalyzed synthesis of conjugated enynes. Org. Biomol. Chem. 2016, 14, 6638–6650. [Google Scholar]
- Fu, L.; Greßies, S.; Chen, P.; Liu, G. Recent advances and perspectives in transition metal-catalyzed 1,4-Functionalizations of unactivated 1,3-enynes for the synthesis of allenes. Chin. J. Chem. 2020, 38, 91–100. [Google Scholar] [CrossRef]
- Mao, L.; Bose, S.K. Hydroboration of enynes and mechanistic insights. Adv. Synth. Catal. 2020, 362, 4174–4188. [Google Scholar] [CrossRef]
- Ahammed, S.; Kundu, D.; Ranu, B.C. Cu-Catalyzed Fe-driven Csp−Csp and Csp−Csp2 cross-coupling: An access to 1,3-diynes and 1,3-enynes. J. Org. Chem. 2014, 79, 7391–7398. [Google Scholar] [CrossRef] [PubMed]
- Shirakawa, E.; Yoshida, H.; Kurahashi, T.; Nakao, Y.; Hiyama, T. Carbostannylation of alkynes catalyzed by an iminophosphine-palladium complex. J. Am. Chem. Soc. 1998, 120, 2975–2976. [Google Scholar] [CrossRef]
- Cornelissen, L.; Lefrancq, M.; Riant, O. Copper-catalyzed cross-coupling of vinylsiloxanes with bromoalkynes: Synthesis of enynes. Org. Lett. 2014, 16, 3024–3027. [Google Scholar] [CrossRef]
- Xie, X.; Xu, X.; Li, H.; Xu, X.; Yang, J.; Li, Y. Iron-catalyzed cross-coupling reactions of terminal alkynes with vinyl iodides. Adv. Synth. Catal. 2009, 351, 1263–1267. [Google Scholar] [CrossRef]
- Huang, M.; Feng, Y.; Wu, Y. Enyne synthesis through a modified Sonogashira cross-coupling reaction catalyzed by cyclopalladated complexes. Tetrahedron 2012, 68, 376–381. [Google Scholar] [CrossRef]
- Lin, Y.-Y.; Wang, Y.-J.; Cheng, J.-H.; Lee, C.-F. Copper-catalyzed coupling of alkynes with alkenyl halides. Synlett 2012, 23, 930–934. [Google Scholar]
- Mi, X.; Huang, M.; Feng, Y.; Wu, Y. Discovery of a novel palladium catalyst for the preparation of enynes with a copper- and ligand-free Sonogashira reaction. Synlett 2012, 23, 1257–1261. [Google Scholar] [CrossRef]
- Mukherjee, N.; Kundu, D.; Ranu, B.C. A co-operative Ni/Cu system for Csp-Csp and Csp-Csp2 cross-coupling providing a direct access to unsymmetrical 1,3-diynes and en-ynes. Chem. Commun. 2014, 50, 15784–15787. [Google Scholar] [CrossRef]
- Wena, Y.; Wang, A.; Jiang, H.; Zhu, S.; Huang, L. Highly regio- and stereoselective synthesis of 1,3-enynes from unactivated ethylenes via palladium-catalyzed cross-coupling. Tetrahedron Lett. 2011, 52, 5736–5739. [Google Scholar] [CrossRef]
- Provot, O.; Giraud, A.; Peyrat, J.-F.; Alami, M.; Brion, J.-D. Synthetic approach to enyne and enediyne analogues of anticancer agents. Tetrahedron Lett. 2005, 46, 8547–8550. [Google Scholar] [CrossRef]
- Tikad, A.; Hamze, A.; Provot, O.; Brion, J.-D.; Alami, M. Suzuki coupling reactions of (E)- and (Z)-chloroenynes with boronic acids:versatile access to functionalized 1,3-enynes. Eur. J. Org. Chem. 2010, 2010, 725–731. [Google Scholar] [CrossRef]
- Yan, Z.; Zhu, C.; Xie, J. Manganese(I)-catalyzed selective functionalization of alkynes. Synlett 2018, 29, 124–128. [Google Scholar]
- Yan, Z.; Yuan, X.-A.; Zhao, Y.; Zhu, C.; Xie, J. Selective hydroarylation of 1,3-diynes using a dimeric manganese catalyst: Modular synthesis of Z-enynes. Angew. Chem. Int. Ed. 2018, 57, 12906–12910. [Google Scholar] [CrossRef]
- Zhu, L.; Guo, H.; Feng, X.; Yamamoto, Y.; Bao, M. Copper-catalyzed one-pot synthesis of 1,3-enynes from 2-chloro-N-(quinolin-8-yl)acetamides and terminal alkynes. J. Org. Chem. 2020, 85, 8740–8748. [Google Scholar] [CrossRef]
- Liu, Z.-K.; Yang, Y.; Zhan, Z.-P. Preparation of (E)-1,3-enyne derivatives through palladium catalyzed hydroalkynylation of allenes. J. Org. Chem. 2022, 87, 1589–1597. [Google Scholar] [CrossRef] [PubMed]
- Jayaraman, A.; Lee, S. Selective mono- and dialkynylation of 1-fluoro-2,2-diiodovinylarenes using Pd-catalyzed decarboxylative coupling reactions. Org. Lett. 2019, 21, 7923–7927. [Google Scholar] [CrossRef] [PubMed]
- Sakai, N.; Komatsu, R.; Uchida, N.; Ikeda, R.; Konakahara, T. A single-step synthesis of enynes: Pd-catalyzed arylalkynylation of aryl iodides, internal alkynes, and alkynylsilanes. Org. Lett. 2010, 12, 1300–1303. [Google Scholar] [CrossRef] [PubMed]
- Cabezas, J.A.; Poveda, R.R.; Brenes, J.A. One-pot conversion of aldehydes and ketones into 1-substituted and 1,4-disubstituted 1,3-enynes. Synthesis 2018, 50, 3307–3321. [Google Scholar] [CrossRef]
- Liu, Y.-W.; Li, L.-J.; Xu, H.; Dai, H.-X. Palladium-catalyzed alkynylation of enones with alkynylsilanes via C−C bond activation. J. Org. Chem. 2022, 87, 6807–6811. [Google Scholar] [CrossRef]
- Shao, L.-X.; Shi, M. Copper-catalyzed coupling reactions of alkenyl halides with alkynes in the absence of palladium and ligand. Tetrahedron 2007, 63, 11938–11942. [Google Scholar] [CrossRef]
- Zhu, Y.; Li, T.; Qu, X.; Sun, P.; Yang, H.; Mao, J. Copper(I)-catalyzed synthesis of 1,3-enynes via coupling between vinyl halides and alkynes or domino coupling of vinyl halides. Org. Biomol. Chem. 2011, 9, 7309–7312. [Google Scholar] [CrossRef]
- Kumar, R.; Zajc, B. Stereoselective synthesis of conjugated fluoro enynes. J. Org. Chem. 2012, 77, 8417–8427. [Google Scholar] [CrossRef]
- Wada, T.; Iwasaki, M.; Kondoh, A.; Yorimitsu, H.; Oshima, K. Palladium-catalyzed addition of silyl-substituted chloroalkynes to terminal alkynes. Chem. Eur. J. 2010, 16, 10671–10674. [Google Scholar] [CrossRef]
- Finkbeiner, P.; Kloeckner, U.; Nachtsheim, B.J. OH-Directed alkynylation of 2-vinylphenols with ethynyl benziodoxolones: A fast access to terminal 1,3-enynes. Angew. Chem. Int. Ed. 2015, 54, 4949–4952. [Google Scholar] [CrossRef]
- Caspers, L.D.; Finkbeiner, P.; Nachtsheim, B.J. Direct electrophilic C–H alkynylation of unprotected 2-vinylanilines. Chem. Eur. J. 2017, 23, 2748–2752. [Google Scholar] [CrossRef]
- Sang, H.L.; Hu, Y.; Ge, S. Cobalt-catalyzed regio- and stereoselective hydrosilylation of 1,3-diynes to access silyl-functionalized 1,3-enynes. Org. Lett. 2019, 21, 5234–5237. [Google Scholar] [CrossRef]
- Cembellín, S.; Dalton, T.; Pinkert, T.; Scha, F.; Glorius, F. Highly selective synthesis of 1,3-enynes, pyrroles, and furans by manganese(I)-catalyzed C−H activation. ACS Catal. 2020, 10, 197–202. [Google Scholar] [CrossRef]
- Guo, L.-Y.; Li, Q.; Liu, Y.-T.; Li, L.; Ni, Y.-Q.; Li, Y.; Pan, F. Palladium-catalyzed alkynylation of alkenes via C–H Activation for the preparation of conjugated 1,3-enynes. Adv. Synth. Catal. 2022, 364, 1109–1116. [Google Scholar] [CrossRef]
- Sang, H.L.; Wu, C.; Phua, G.G.D.; Ge, S. Cobalt-catalyzed regiodivergent stereoselective hydroboration of 1,3-diynes to access boryl-functionalized Enynes. ACS Catal. 2019, 9, 10109–10114. [Google Scholar] [CrossRef]
- Rogalski, S.; Kubicki, M.; Pietraszuk, C. Palladium catalysed regio- and stereoselective synthesis of (E)-4-aryl-1,3-bis(trimethylsilyl)but-3-en-1-ynes. Tetrahedron 2018, 74, 6192–6198. [Google Scholar] [CrossRef]
- Pawluć, P.; Hreczycho, G.; Szudkowska, J.; Kubicki, M.; Marciniec, B. New one-pot synthesis of (E)-β-aryl vinyl halides from styrenes. Org. Lett. 2009, 11, 3390–3393. [Google Scholar] [CrossRef]
- Wang, E.; Fu, X.; Xie, X.; Chen, J.; Gao, H.; Liu, Y. Gold and Bronsted acid catalyzed isomerization of [3]cumulenols and [3]cumulenones: Efficient syntheses of 1,5-dien-3-ynes and furan derivatives. Tetrahedron Lett. 2011, 52, 1968–1972. [Google Scholar] [CrossRef]
- Komiya, S. (Ed.) Synthesis of Organometallic Compounds; John Wiley and Sons: Hoboken, NJ, USA, 1997. [Google Scholar]
- Heck, R.F. Palladium Reagents in Organic Synthesis; Academic: New York, NY, USA, 1985. [Google Scholar]
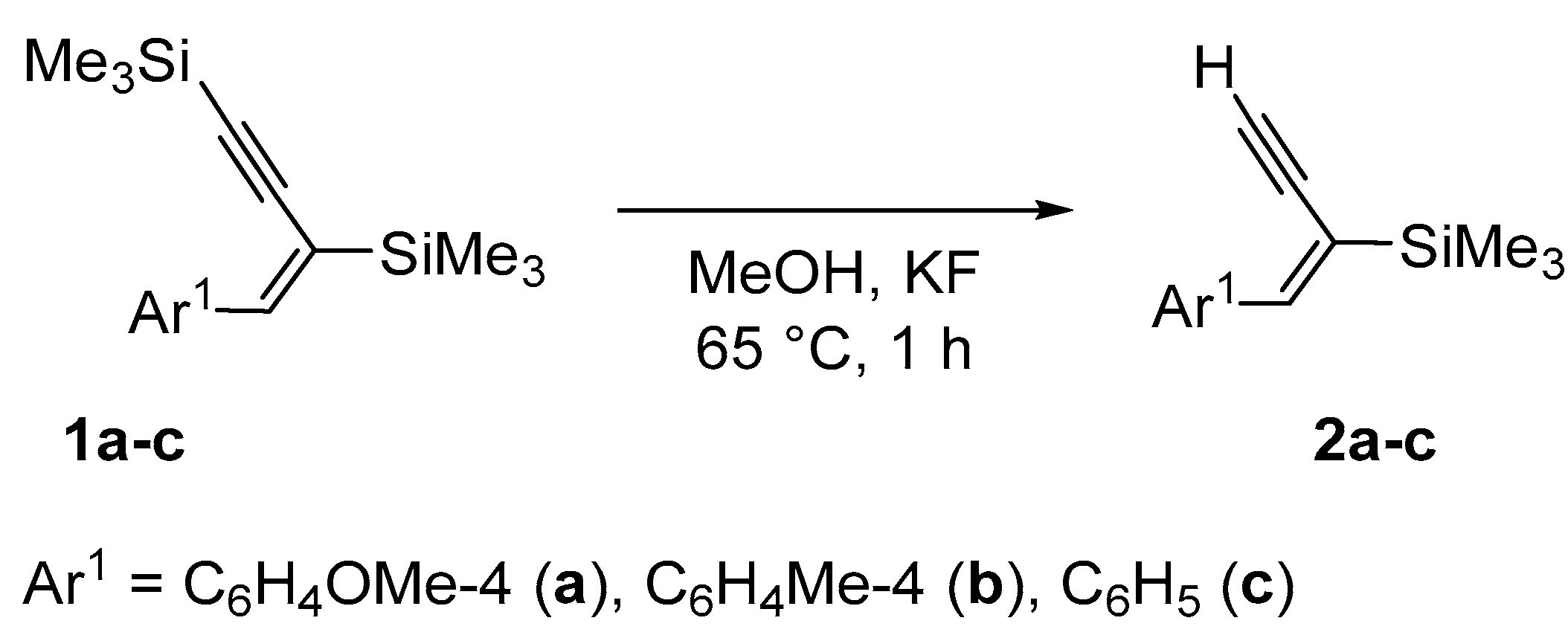

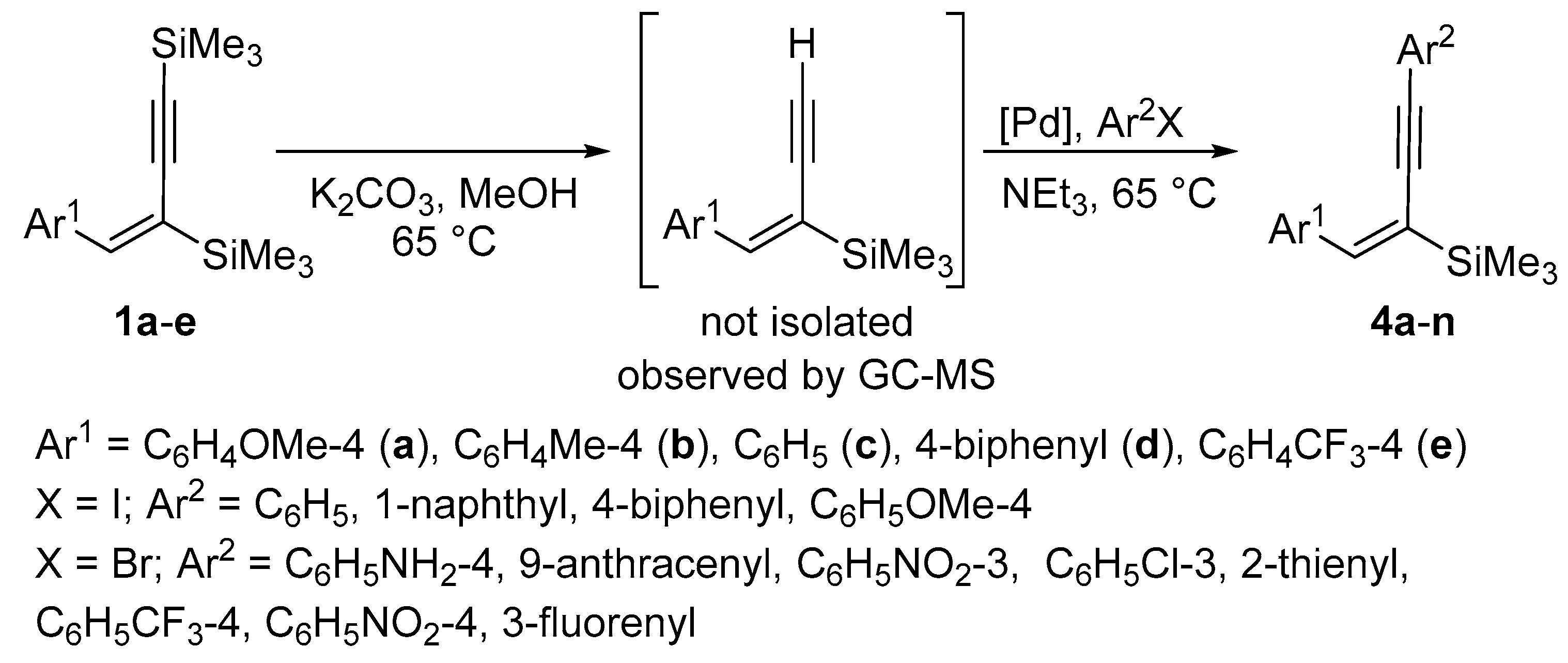
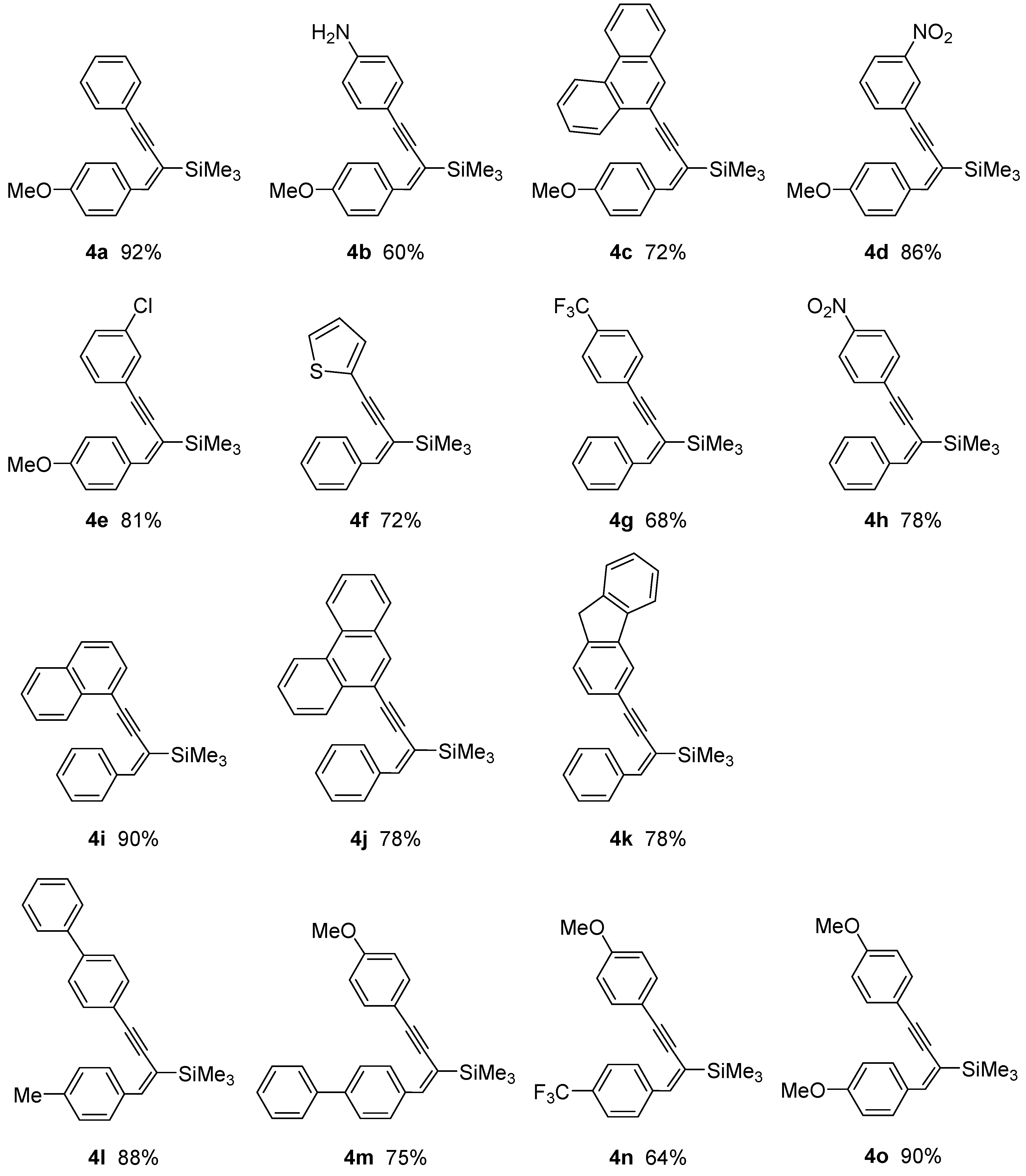
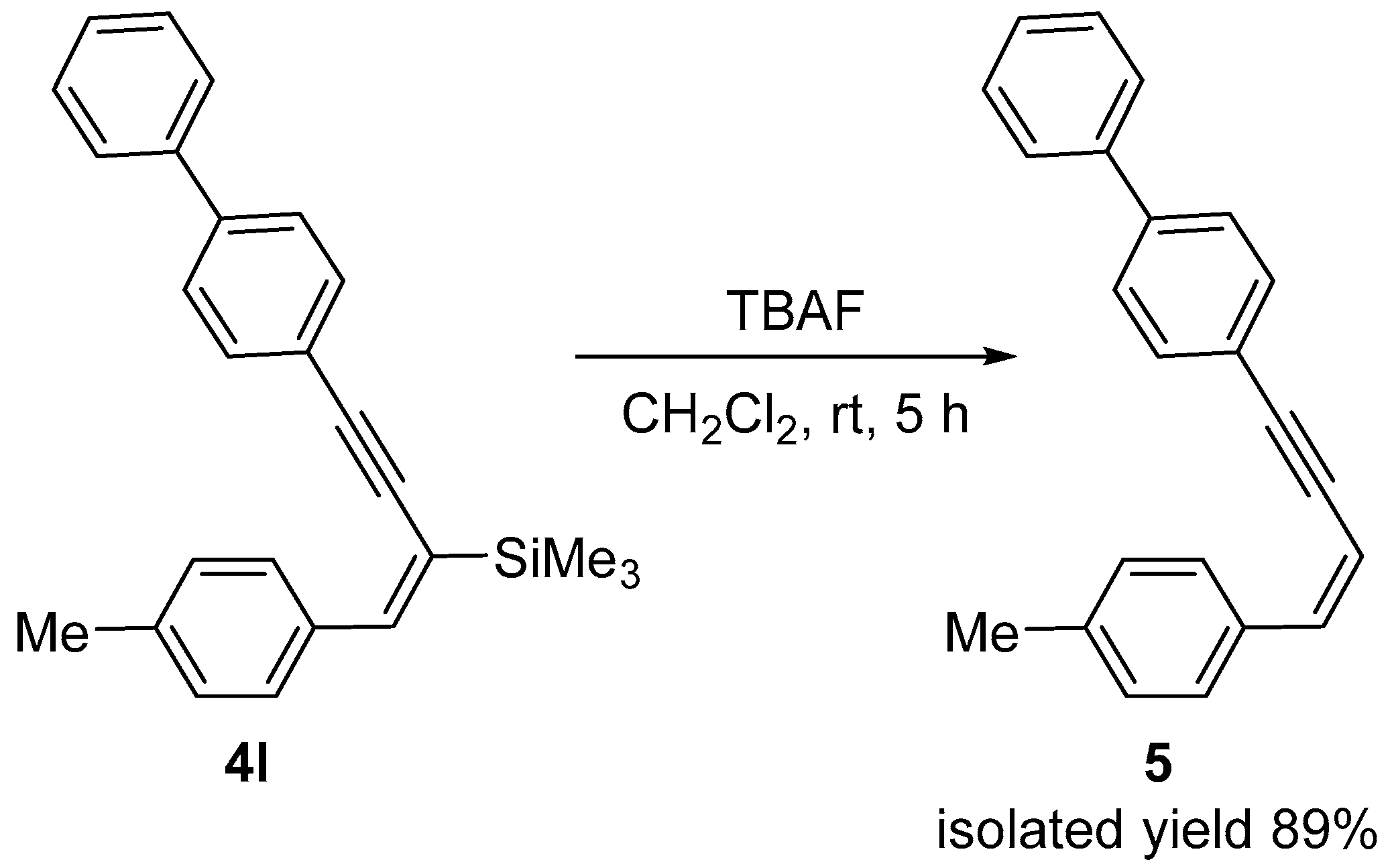

| Enyne | Base | Time [h] | Solvent | Conv. | Yield [%] |
|---|---|---|---|---|---|
| 1a | KF | 3 | toluene | 0 | 0 |
| 1a | K2CO3 | 3 | toluene | 0 | 0 |
| 1a | KF | 3 | DMF | 27 | 22 |
| 1a | KF | 3 | THF | 5 | 5 |
| 1a | KF | 3 | MeOH | 100 a | 99 a |
| 1a | CsF | 2.5 | MeOH | 100 | 99 |
| 1a | NaF | 3 | MeOH | 65 | 65 |
| 1a | K2CO3 | 3 | MeOH | 100 a | 99 a |
| 1a | KF | 1 | MeOH | 100 | 99 |
| 1a | TBAF | 1 | MeOH | 100 | 5 |
| 1a | KOt-Bu | 3 | MeOH | 100 | 30 |
| 1a | KOH | 3 | MeOH | 100 | 22 |
| 1a | K2CO3 | 1 | MeOH | 100 | 99 |
| 1b | K2CO3 | 1 | MeOH | 100 | 98 |
| 1c | K2CO3 | 1 | MeOH | 100 | 96 |
| Cat. | Additive (Amount) a | Base | Conv. [%] | Yield [%] |
|---|---|---|---|---|
| [Pd(PPh3)4] | - | KF | 100 | 99 |
| [Pd(PPh3)4] | - | NEt3 | 100 | 98 |
| [Pd(PPh3)4] | CuI (see text) | KF | 100 | 98 |
| [PdCl2(PPh3)2] | - | KF | 100 | 98 |
| [PdCl2(PPh3)2] | - | NEt3 | 100 | 99 |
| [PdCl2(PPh3)2] | - | NEt3 | 98 a | 98 a |
| [PdCl2(PPh3)2] | CuI (see text) | KF | 100 | 98 |
| [PdCl2(PPh3)2] | - | KF | 97 b | 96 b |
| [PdCl2(PPh3)2] | - | K2CO3 | 90 | 90 |
| PEPPSI-IPr | - | KF | 99 | 95 |
| PEPPSI-IPr | - | NEt3 | 99 | 94 |
| [Pd2(dba)3] | PPh3 (2 equiv) | NEt3 | 99 | 95 |
| [Pd2(dba)3] | SPhos (2 equiv) | NEt3 | 100 | 96 |
| PdCl2 | dppf (1 equiv) | KF | 75 | 75 |
| [PdCl2(PhCN)2] | PPh3 (2 equiv) | KF | 87 | 86 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rogalski, S.; Szymaszek, N.; Pietraszuk, C. The Regio- and Stereoselective Synthesis of 1,4-Diarylbut-1-en-3-ynes Having Aryl Groups at the Mutual Syn Positions. Organics 2023, 4, 206-218. https://doi.org/10.3390/org4020017
Rogalski S, Szymaszek N, Pietraszuk C. The Regio- and Stereoselective Synthesis of 1,4-Diarylbut-1-en-3-ynes Having Aryl Groups at the Mutual Syn Positions. Organics. 2023; 4(2):206-218. https://doi.org/10.3390/org4020017
Chicago/Turabian StyleRogalski, Szymon, Natalia Szymaszek, and Cezary Pietraszuk. 2023. "The Regio- and Stereoselective Synthesis of 1,4-Diarylbut-1-en-3-ynes Having Aryl Groups at the Mutual Syn Positions" Organics 4, no. 2: 206-218. https://doi.org/10.3390/org4020017
APA StyleRogalski, S., Szymaszek, N., & Pietraszuk, C. (2023). The Regio- and Stereoselective Synthesis of 1,4-Diarylbut-1-en-3-ynes Having Aryl Groups at the Mutual Syn Positions. Organics, 4(2), 206-218. https://doi.org/10.3390/org4020017