Study on the Effect of Sodium Dodecyl Benzene Sulfonate on Coal Moisture Imbibition and Gas Adsorption
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Program
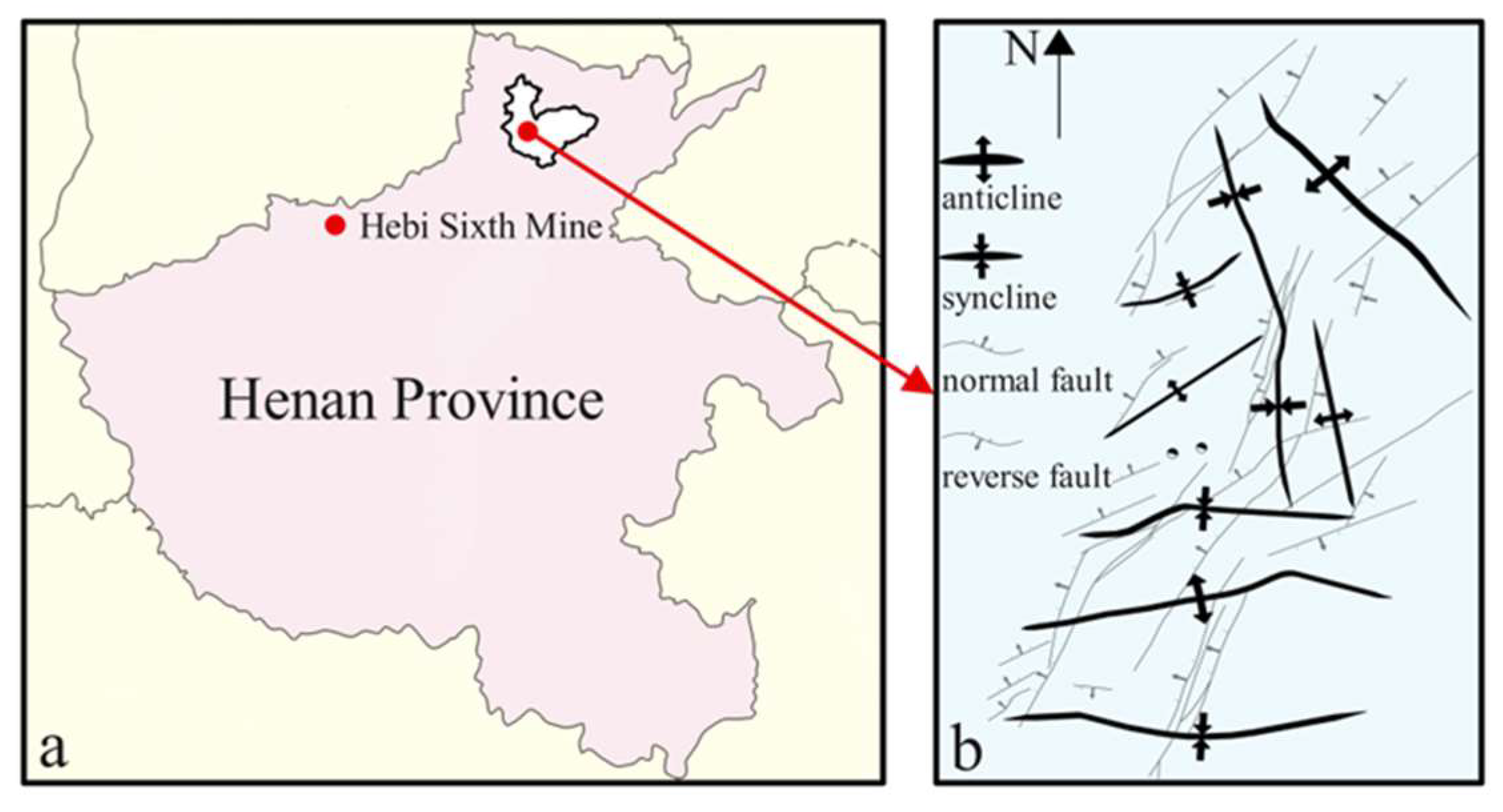
2.2. Coal Sample Preparation
2.3. Imbibition Experimental Method
- (1)
- Reagent preparation. Relevant studies show that the critical micelle concentration of SDBS is about 0.05–0.08 wt% [23]. Based on the core theory of Surfactant Science, this study needs to prepare 200 mL SDBS solutions with concentrations of 0.025 wt%, 0.050 wt%, 0.075 wt%, 0.100 wt%, and 0.200 wt%, respectively, and to prepare 200 mL of distilled water at the same time.
- (2)
- Coal sample processing. The pressed briquette sample is dried in a drying oven, and a Φ 50 mm metal filter screen is placed at the bottom of the dried briquette sample. A section of heat-shrinkable film is cut and sleeved on the coal sample, which is heated by a hot air gun to make its shrinkage closely fit the coal sample surface.
- (3)
- Spontaneous imbibition. Fill the drying dish with SDBS solutions of different concentrations, place the coal sample with one end of the metal filter facing downwards, and stand it vertically in the solution. The SDBS solution can only contact the coal sample through the metal filter screen, and its contact area is the end face of the briquette sample.
- (4)
- Moisture content monitoring. During the spontaneous imbibition of SDBS solution in coal samples, the coal samples are weighed at regular intervals. By comparing the quality of dry coal samples and the quality of coal samples with different imbibition times, the change in moisture content of coal samples during spontaneous imbibition is determined.
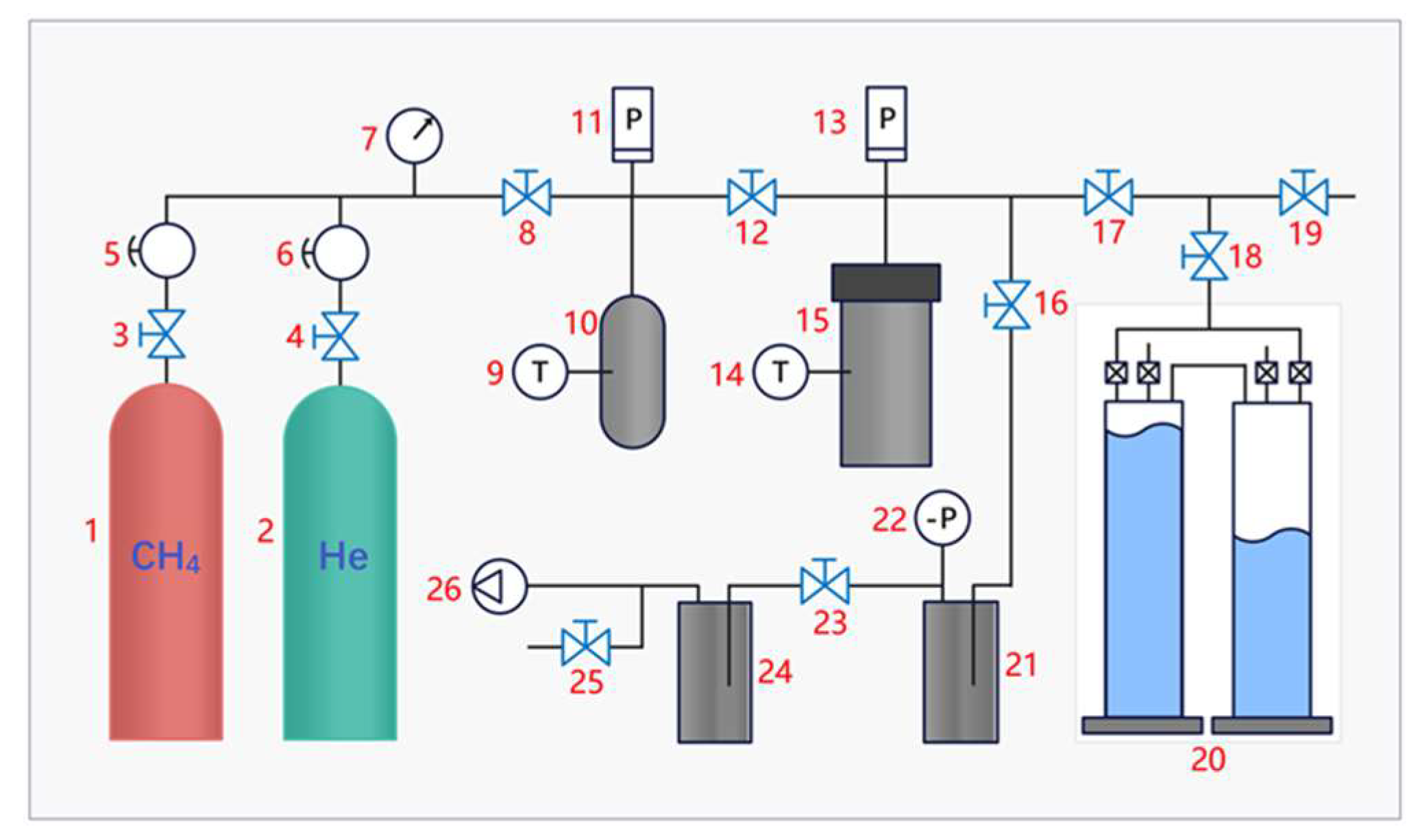
2.4. Gas Adsorption Experimental Device
- (1)
- Vacuum degassing system. The system mainly consists of a vacuum pump, a vacuum sensor, a vacuum buffer container, a drying container, and connected pipelines. Its primary function is to perform vacuum degassing of the pre-experiment inflation tank, coal sample tank, and experiment pipelines.
- (2)
- Gas supply system. The system mainly consists of high-pressure methane cylinders, high-pressure helium cylinders, pressure-reducing valves, pressure gauges, and connected pipelines. It can provide methane and helium gas supplies at different pressures according to the needs of the experiment.
- (3)
- Adsorption equilibrium system. The system is mainly composed of a reference cylinder, a special coal sample tank, valves, and connected pipelines, which can fill the coal sample tank with quantitative methane gas to make the middling coal sample in the coal sample tank absorb and balance under a certain gas pressure.
- (4)
- Desorption metering system. The system mainly consists of desorption valves, free gas automatic collectors, fully automatic gas meters, and connected pipelines, which can achieve uninterrupted collection and measurement of desorbed gas throughout the entire process.
- (5)
- Data collection and analysis system. The system is mainly composed of a pressure sensor (measurement accuracy is ±0.01 MPa), pressure acquisition module, temperature sensor (measurement accuracy is ±0.5 °C), temperature acquisition module, data processing computer, and related lines. Its primary function is to collect and record the signals fed back by the sensors at various parts of the device in real time during the test.
2.5. Gas Adsorption Experimental Steps
- (1)
- Data recording.
- (2)
- Device airtightness experimentation.
- (3)
- Volume calibration.
- (4)
- Vacuum degassing.
- (5)
- Inflatable adsorption equilibrium.
3. Results
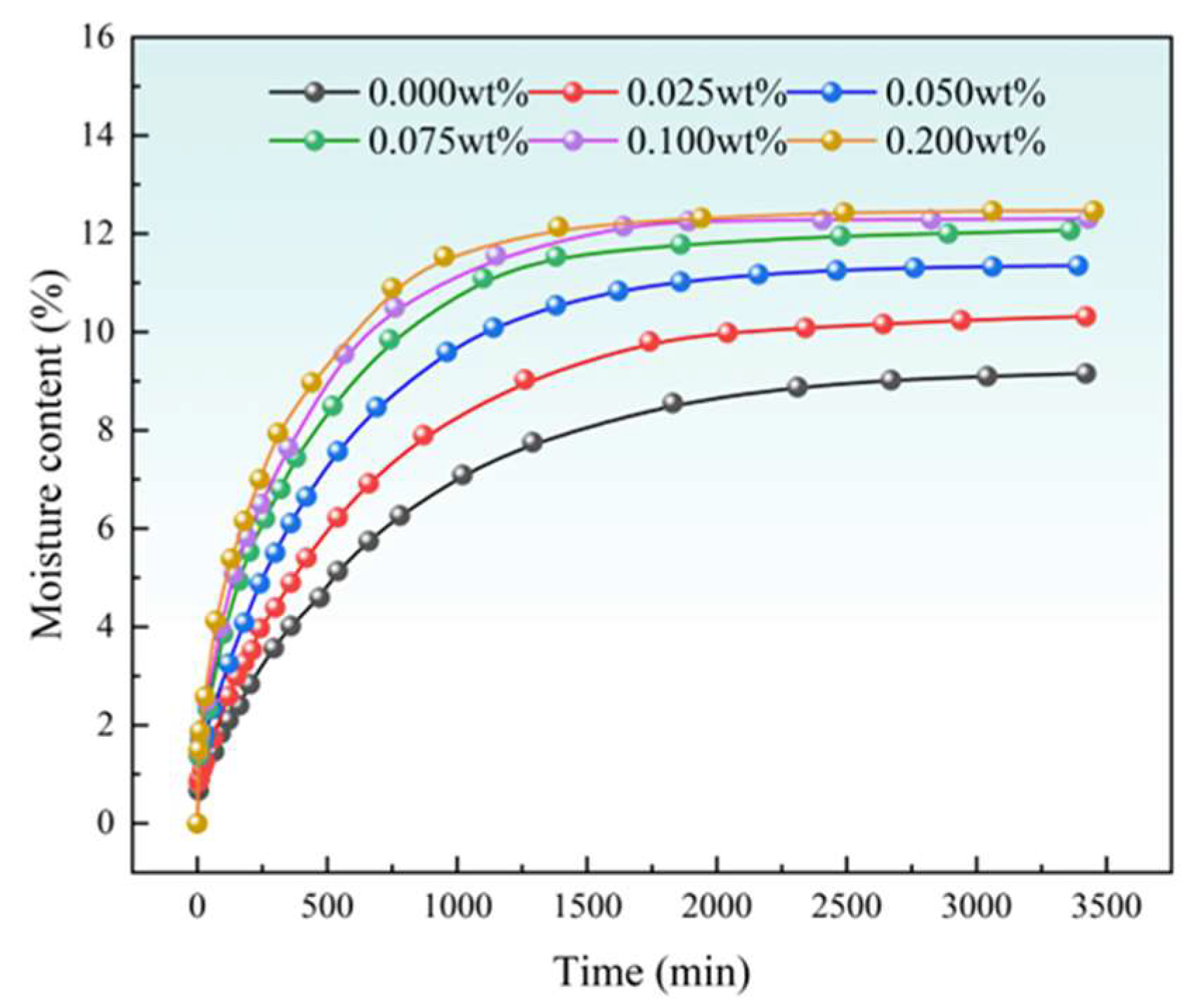
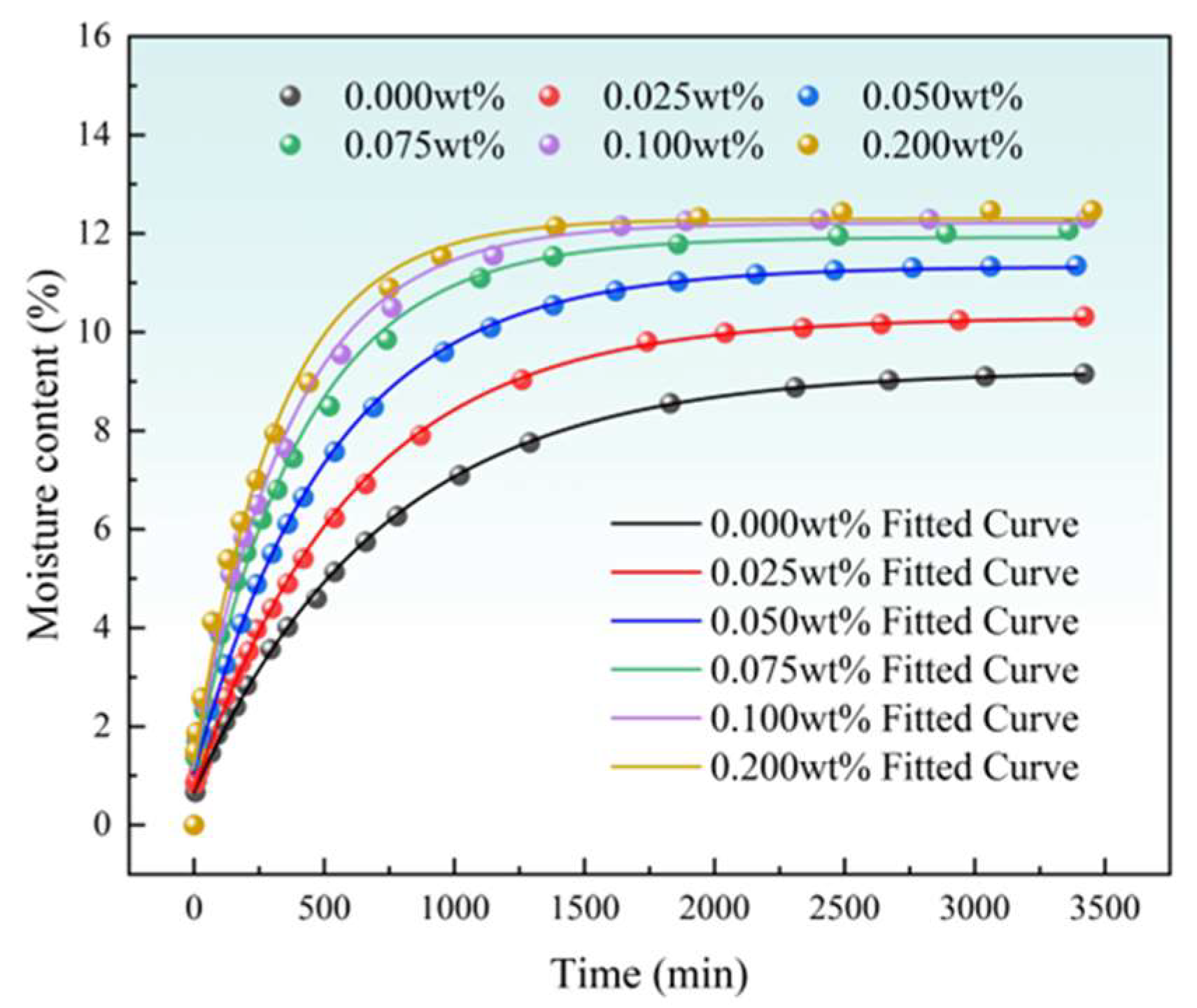
3.1. Imbibition Experimental Results
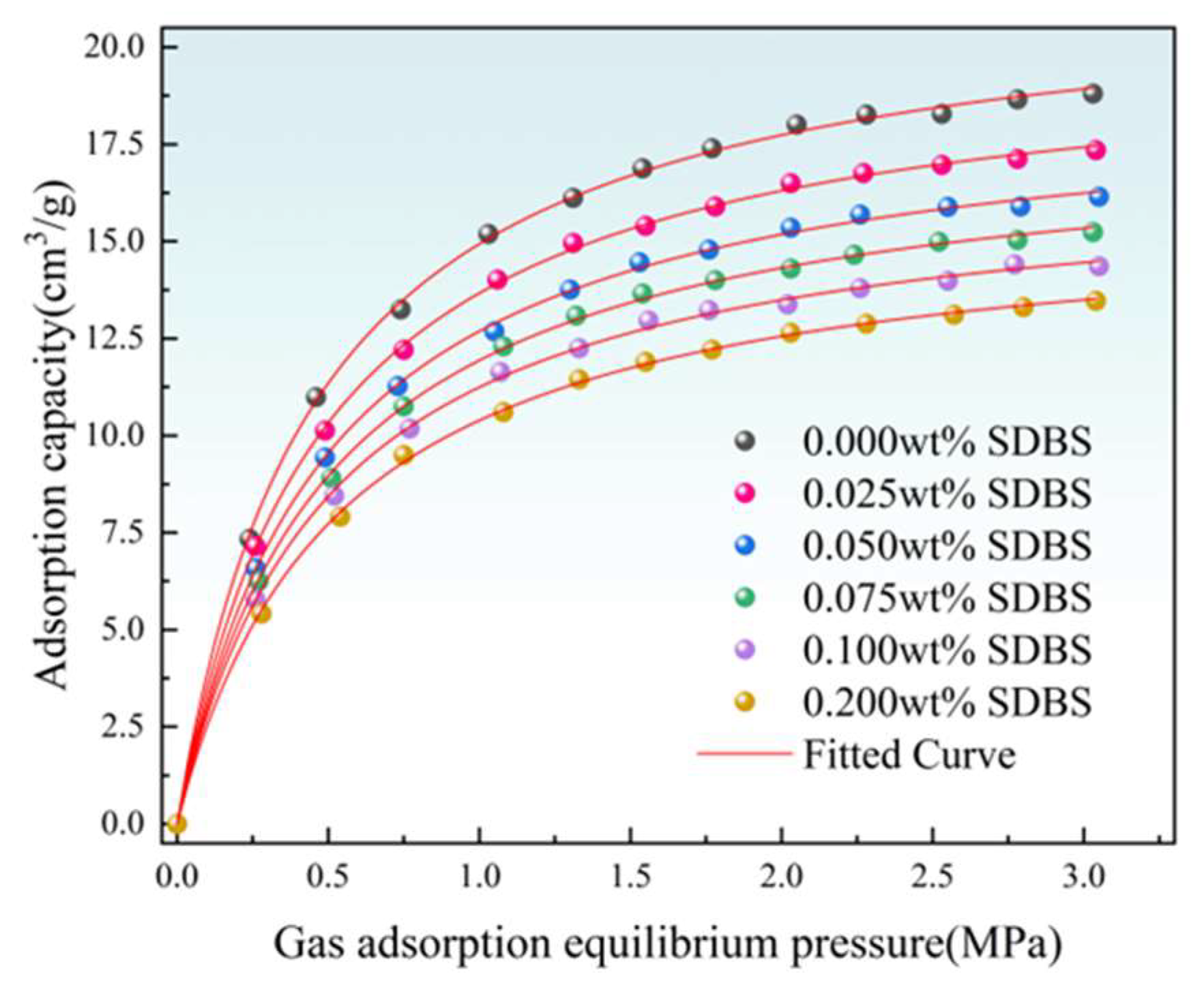
3.2. Adsorption Experimental Results
4. Discussion
5. Conclusions
- (1)
- SDBS, as an anionic surfactant, plays two leading roles in enhancing the effectiveness of coalbed methane treatment after its application in hydraulic measures. One approach is to enhance the efficiency of spontaneous water absorption and saturation moisture content in coal. The other involves SDBS molecules entering the coal, which can weaken its ability to adsorb gas to a certain extent.
- (2)
- Under the experimental conditions of this study, 0.050–0.075 wt% is the key concentration range for the practical application of SDBS. When the concentration of the SDBS solution is lower than 0.050 wt%, with the increase in the concentration of the SDBS solution, the spontaneous imbibition capacity of coal increases significantly, and the adsorption capacity of coal to gas decreases significantly. When the concentration of the SDBS solution is higher than 0.075 wt%, the spontaneous imbibition water capacity and gas adsorption capacity of coal hardly change significantly with the increase in solution concentration.
- (3)
- Considering the effectiveness, economical nature, and environmental sustainability of SDBS enhanced water conservancy measures for controlling coalbed methane, it is recommended that the solution concentration of SDBS used as a surfactant should be 0.050 wt%.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Xie, H.; Gao, F.; Ju, Y. Research and development of rock mechanics in deep ground engineering. Chin. J. Rock Mech. Eng. 2015, 34, 2161–2178. [Google Scholar] [CrossRef]
- Yuan, L.; Wang, E.; Ma, Y.; Liu, Y.; Li, X. Research progress of coal and rock dynamic disasters and scientific and technological problems in China. J. China Coal Soc. 2023, 48, 1825–1845. [Google Scholar] [CrossRef]
- Tan, Y.; Zhang, X.; Xiao, Z.; Fan, D.; Yin, Y.; Chen, Y.; Liu, X. Main control factors of rock burst and its disaster evolution mechanism. J. China Coal Soc. 2024, 49, 367–379. [Google Scholar] [CrossRef]
- Zhou, L.; Yan, X.; Liu, H.; Xiong, X.; Wang, F.; Li, S.; Zhang, J.; Liu, C.; Hu, X.; Yuan, P.; et al. Development mechanism and practical significance of deep coalbed methane in-filtration and displacement. J. China Coal Soc. 2025, 50, 3534–3551. [Google Scholar] [CrossRef]
- Wang, B.; Xu, F.; Liu, W.; Shao, S.; Wang, N.; Wen, J.; Cheng, G.; Qu, Z.; Xie, Y.; Han, J.; et al. Key technologies for surface control of gas dynamic disasters in coal mines and their application. Coal Geol. Explor. 2025, 53, 30–45. [Google Scholar] [CrossRef]
- Liu, J.; Xia, H.; Yang, H.; Wang, Z. Comprehensive gas prevention and control technology of fully mechanized gateway driving face in seam with coal and gas outburst. Coal Sci. Technol. 2012, 40, 67–70. [Google Scholar] [CrossRef]
- Chen, X.; Wang, Z.; Cheng, Y.; Wu, G. Application of outburst elimination technology with hydraulic pressing to seam gateway driving in shuijingtou mine. Coal Sci. Technol. 2012, 40, 49–52. [Google Scholar] [CrossRef]
- Sun, S.; Li, W.; Zhang, J.; Chen, D.; Zhao, J.; Zheng, K.; Long, W.; Wang, C.; Jia, B.; Du, T.; et al. Research progress and development trend of staged hydraulic fracturing technology in long-borehole underground coal mine. Coal Geol. Explor. 2022, 50, 1–15. [Google Scholar] [CrossRef]
- Wang, L.; Xu, Y.; Bhattacharyya, S.; Peng, X. Coalbed methane stimulation by hydraulic punching with air cannon. Energy Sources Part A Recovery Util. Environ. Eff. 2021, 5, 9318–9332. [Google Scholar] [CrossRef]
- Liu, S.; Yao, C.; Gao, D.; Wang, X. Influence factors on the energy regulation law of a coal seam after hydraulic slotting. Processes 2024, 12, 2062. [Google Scholar] [CrossRef]
- Chen, X.; Du, Y.; Li, L. Study on comprehensive outburst elimination effect of hydraulic measures applied to coal mass. Coal Sci. Technol. 2017, 45, 43–49. [Google Scholar] [CrossRef]
- Kai, Z.; Bin, L.I. Mechanism and experiment study on surfactant solution to block methane. Procedia Eng. 2012, 45, 298–304. [Google Scholar] [CrossRef]
- Das, S.; Katiyar, A.; Rohilla, N.; Bonnecaze, R.T.; Nguyen, Q. A methodology for chemical formulation for wettability alteration induced water imbibition in carbonate reservoirs. J. Pet. Sci. Eng. 2021, 198, 108–136. [Google Scholar] [CrossRef]
- Gong, L.; Liao, G.; Luan, H.; Chen, Q.; Nie, X.; Liu, D.; Feng, Y. Oil solubilization in sodium dodecylbenzenesulfonate micelles: New insights into surfactant enhanced oil recovery. J. Colloid Interface Sci. 2020, 569, 219–228. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Sheng, X.; Xing, W.; Dong, G.; Liu, Y.; Zhang, C.; Chen, X.; Zhou, N.; Qin, Z. Molecular dynamic simulation on the absorbing process of isolating and coating of α-olefin drag reducing polymer. Chin. J. Chem. Phys. 2010, 23, 630–636. [Google Scholar] [CrossRef]
- Meng, Y.; Xia, Y.; Niu, J.; Meng, H.; Kan, L. Study of the wetting mechanism of SDBS solution on Zhaozhuang coal surface. J. China Univ. Min. Technol. 2021, 50, 381–388. [Google Scholar] [CrossRef]
- Li, S.; Guo, D.; Bai, Y.; Yan, M.; Lin, H.; Shi, Y. Effect of SDBS of different mass fractions on coal’s wettability by molecular simulation. China Saf. Sci. J. 2020, 30, 21–27. [Google Scholar] [CrossRef]
- Xie, Z.; Li, X. Research on complex dust suppressants for transport roadways in open mines. J. Univ. Sci. Technol. Beijing 2012, 34, 1240–1244. [Google Scholar] [CrossRef]
- Luo, R.; Lin, M.; Luo, Y.; Dong, J. Preparation and properties of a new type of coal dust suppressant. J. China Coal Soc. 2016, 41, 454–459. [Google Scholar] [CrossRef]
- Cao, S.; Yang, Z.; He, Y.; Zhai, M. Preparation and performance of foam dust suppression agent for underground coal dust. Coal Eng. 2017, 49, 87–90. [Google Scholar] [CrossRef]
- Dou, G.; Xu, C. Comparison of effects of sodium carboxymethylcellulose and superabsorbent polymer on coal dust wettability by surfactants. J. Dispers. Sci. Technol. 2017, 38, 1542–1546. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, D.; Wei, C.; Sun, J. Compound optimization of surfactant in shift alternate stage of coal seam water injection. China Coal 2020, 46, 87–92. [Google Scholar] [CrossRef]
- Han, X.; Gao, Z.; Bai, L. Determination of Critical Micelle Concentration of Aqueous Sodium Dodecyl Benzenesulfonate (SDBS)Solution by Absorption Voltammetry. J. Ningxia Univ. (Nat. Sci. Ed. Chin. Engl.) 2005, 4, 356–359. [Google Scholar] [CrossRef]
- Sutton, R.P. Fundamental PVT Calculations for Associated and Gas/Condensate Natural-Gas Systems. SPE Reserv. Eval. Eng. 2007, 10, 270–284. [Google Scholar] [CrossRef]
- Dranchuk, P.M.; Abou-Kassem, H. Calculation of Z Factors For Natural Gases Using Equations of State. J. Can. Pet. Technol. 1975, 14, 34–36. [Google Scholar] [CrossRef]


| TRD (g/cm3) | ARD (g/cm3) | Porosity (%) | Aad (%) | Mad (%) | Vad (%) |
|---|---|---|---|---|---|
| 1.5303 | 1.4579 | 4.7 | 10.88 | 1.51 | 8.535 |
| Calibration Frequency | Volume of Reference Cylinder (cm3) | Volume of Coal Sample Tank (cm3) |
|---|---|---|
| 1 | 108.39 | 366.36 |
| 2 | 109.06 | 368.74 |
| 3 | 108.97 | 367.96 |
| Average value | 108.81 | 367.69 |
| Coal Sample Number | Coal Sample Weight (g) | Coal Sample Calibration Volume (cm3) | Coal Sample Calibration Density (cm3/g) |
|---|---|---|---|
| 1# | 271.26 | 176.54 | 1.5365 |
| 2# | 272.88 | 177.36 | 1.5385 |
| 3# | 272.89 | 177.48 | 1.5375 |
| 4# | 273.67 | 178.22 | 1.5355 |
| 5# | 274.40 | 179.48 | 1.5288 |
| 6# | 275.31 | 178.13 | 1.5455 |
| SDBS Solution Concentration (wt%) | a (cm3/g) | b (MPa−1) |
|---|---|---|
| 0.000 | 21.8442 | 2.1598 |
| 0.025 | 20.2061 | 2.0891 |
| 0.050 | 18.8937 | 2.0442 |
| 0.075 | 17.8843 | 2.0063 |
| 0.100 | 16.8646 | 1.9990 |
| 0.200 | 15.8651 | 1.8967 |
| SDBS Solution Concentration | Fitting Formula for Changes in Moisture Content | R2 |
|---|---|---|
| 0.000 wt% | y = −8.56 × exp(−x/724.88) + 9.22 | 0.99678 |
| 0.025 wt% | y = −9.59 × exp(−x/613.68) + 10.31 | 0.99741 |
| 0.050 wt% | y = −10.27 × exp(−x/528.83) + 11.33 | 0.99597 |
| 0.075 wt% | y = −10.79 × exp(−x/415.98) + 11.93 | 0.99200 |
| 0.100 wt% | y = −11.00 × exp(−x/371.69) + 12.21 | 0.99139 |
| 0.200 wt% | y = −11.01 × exp(−x/324.01) + 12.31 | 0.98899 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, K.; Chen, Y.; Wang, Z.; Wang, L.; Chen, D.; Ma, S.; Li, S. Study on the Effect of Sodium Dodecyl Benzene Sulfonate on Coal Moisture Imbibition and Gas Adsorption. Fuels 2025, 6, 80. https://doi.org/10.3390/fuels6040080
Li K, Chen Y, Wang Z, Wang L, Chen D, Ma S, Li S. Study on the Effect of Sodium Dodecyl Benzene Sulfonate on Coal Moisture Imbibition and Gas Adsorption. Fuels. 2025; 6(4):80. https://doi.org/10.3390/fuels6040080
Chicago/Turabian StyleLi, Kaizhi, Yanqi Chen, Zhaofeng Wang, Liguo Wang, Demin Chen, Shujun Ma, and Shijie Li. 2025. "Study on the Effect of Sodium Dodecyl Benzene Sulfonate on Coal Moisture Imbibition and Gas Adsorption" Fuels 6, no. 4: 80. https://doi.org/10.3390/fuels6040080
APA StyleLi, K., Chen, Y., Wang, Z., Wang, L., Chen, D., Ma, S., & Li, S. (2025). Study on the Effect of Sodium Dodecyl Benzene Sulfonate on Coal Moisture Imbibition and Gas Adsorption. Fuels, 6(4), 80. https://doi.org/10.3390/fuels6040080






