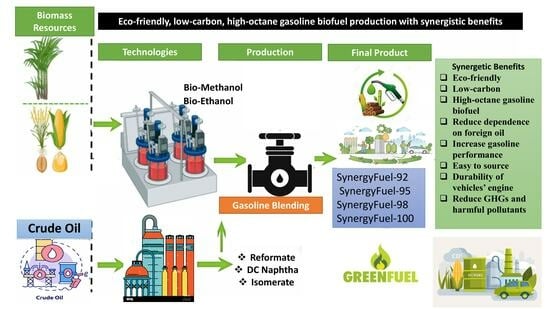
Sustainable Production of Eco-Friendly, Low-Carbon, High-Octane Gasoline Biofuels Through a Synergistic Approach for Cleaner Transportation
Abstract
1. Introduction
2. Research Methodology
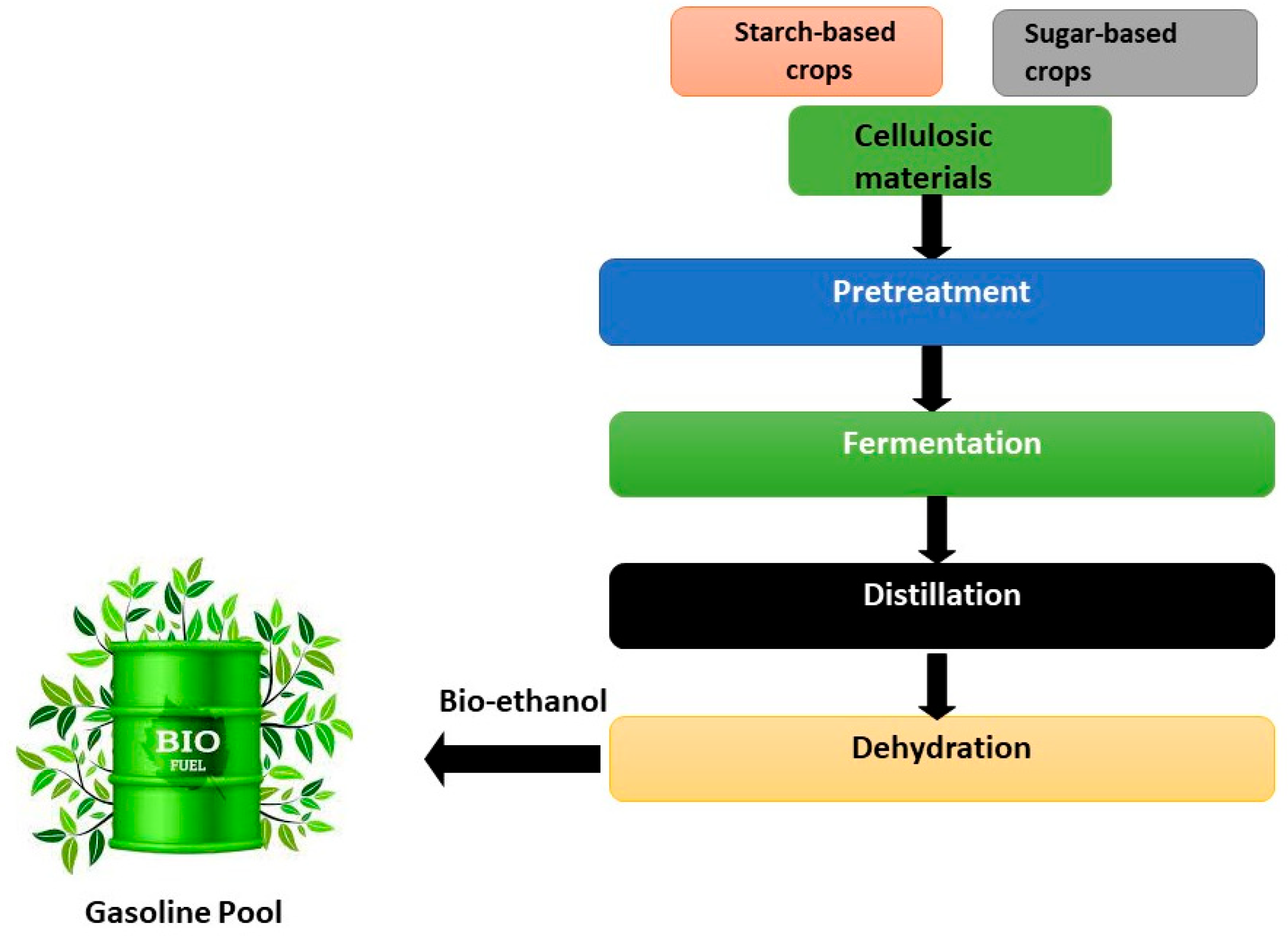
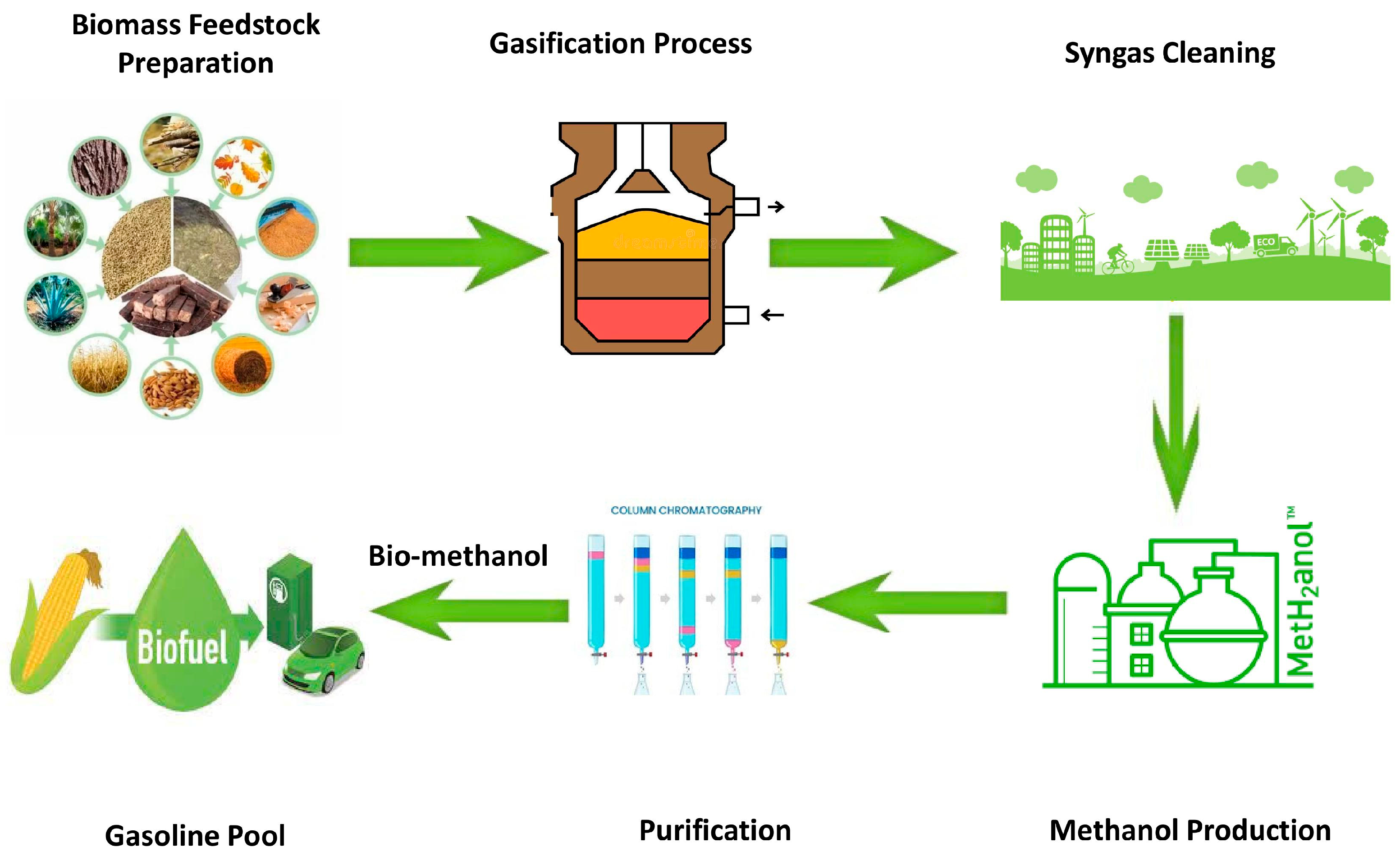
2.1. Materials
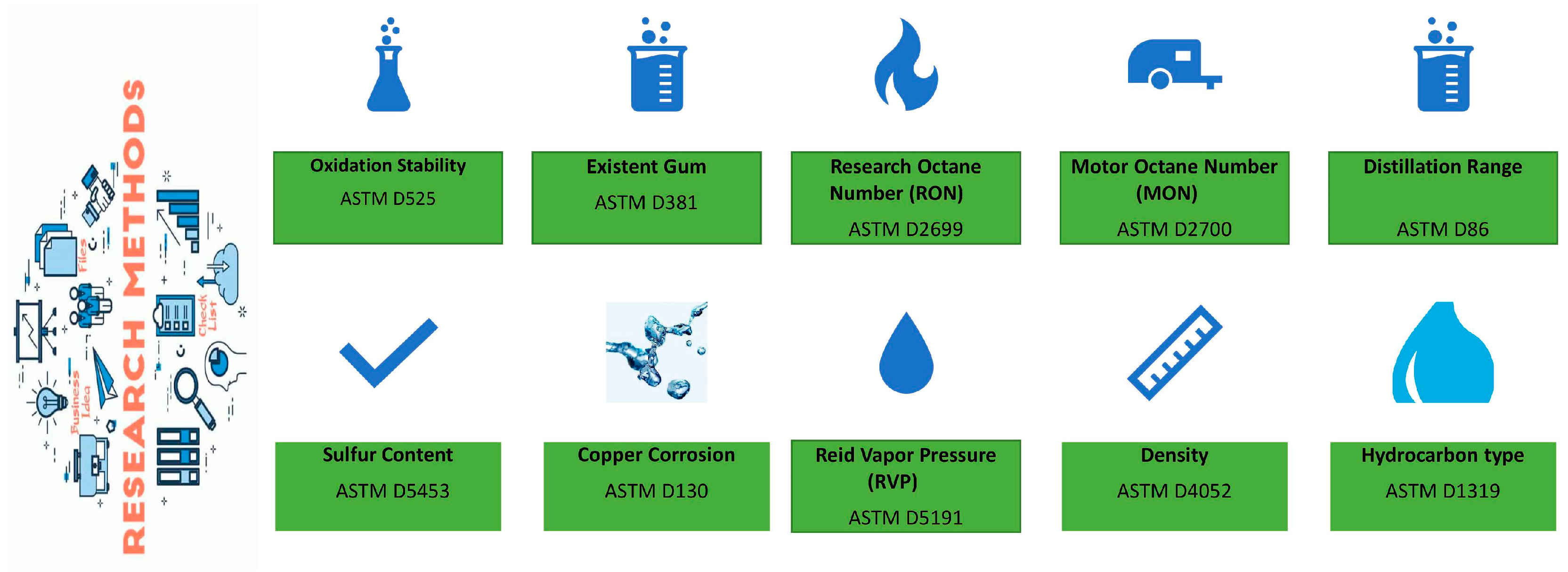
2.2. Methods
3. Results and Discussion
3.1. Market-Grade Gasoline Fuel
3.2. Eco-Friendly, Low-Carbon, and High-Octane Biofuel Gasoline Production
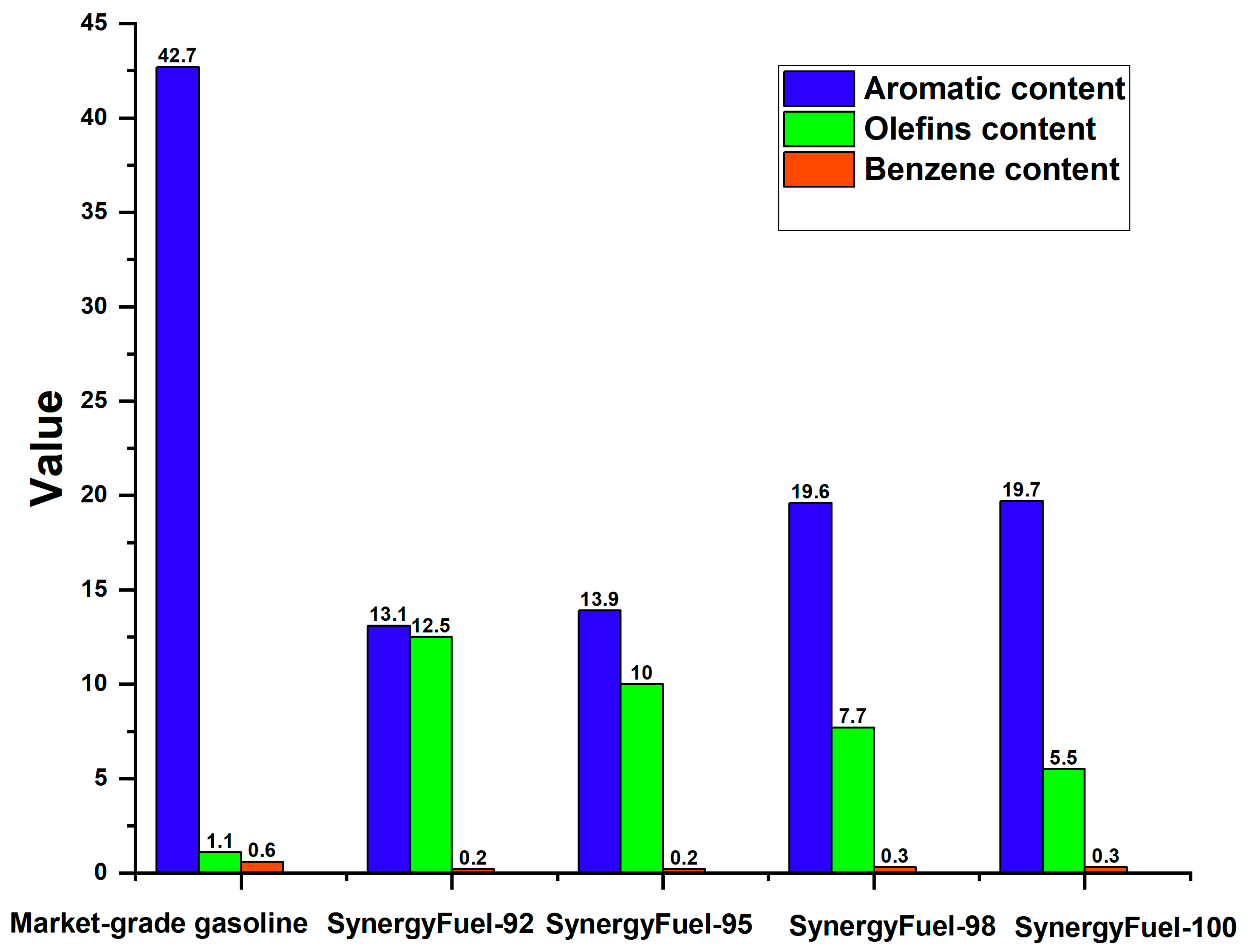
3.2.1. Gasoline Hydrocarbon Composition
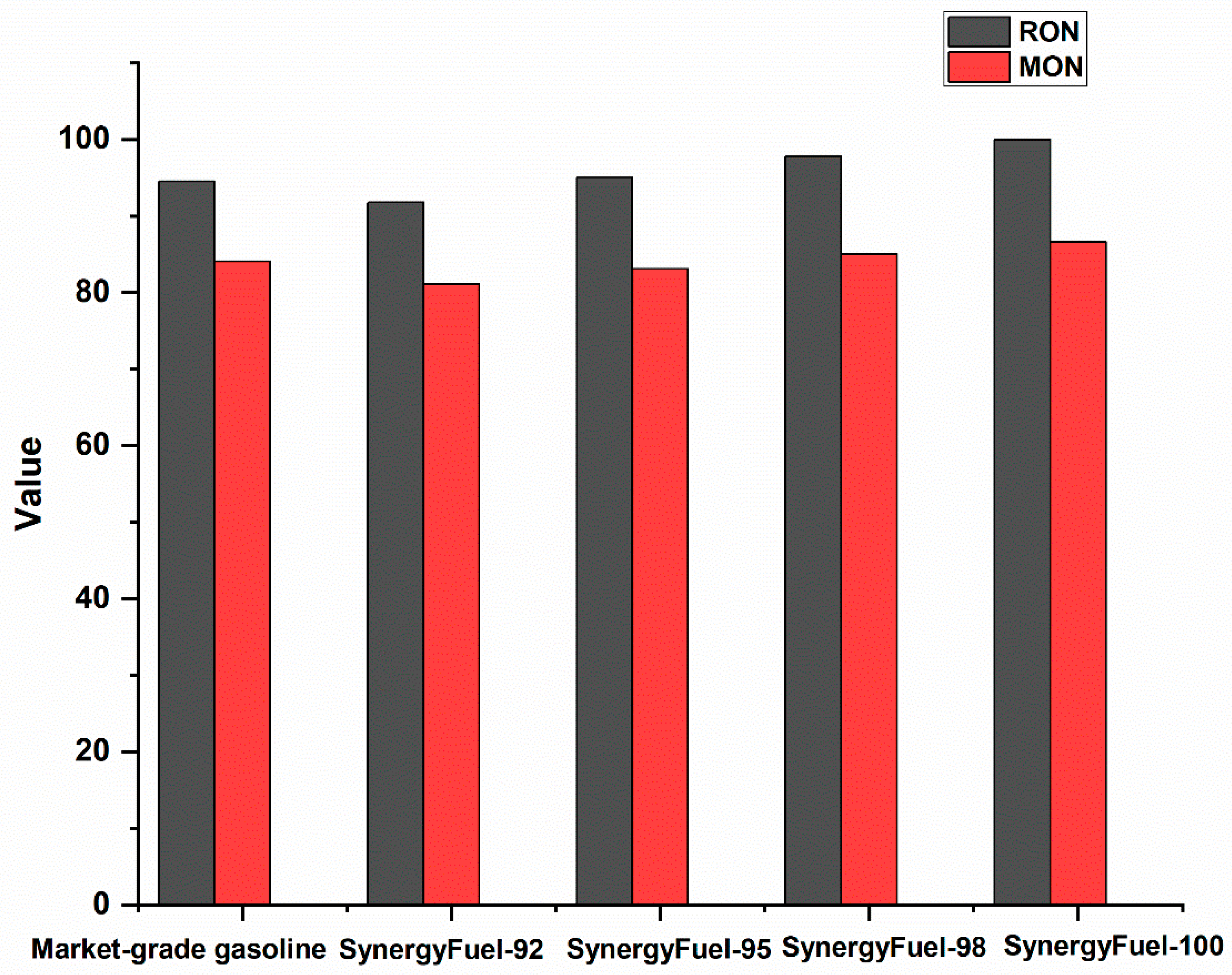
3.2.2. Gasoline Anti-Detonation Characteristics
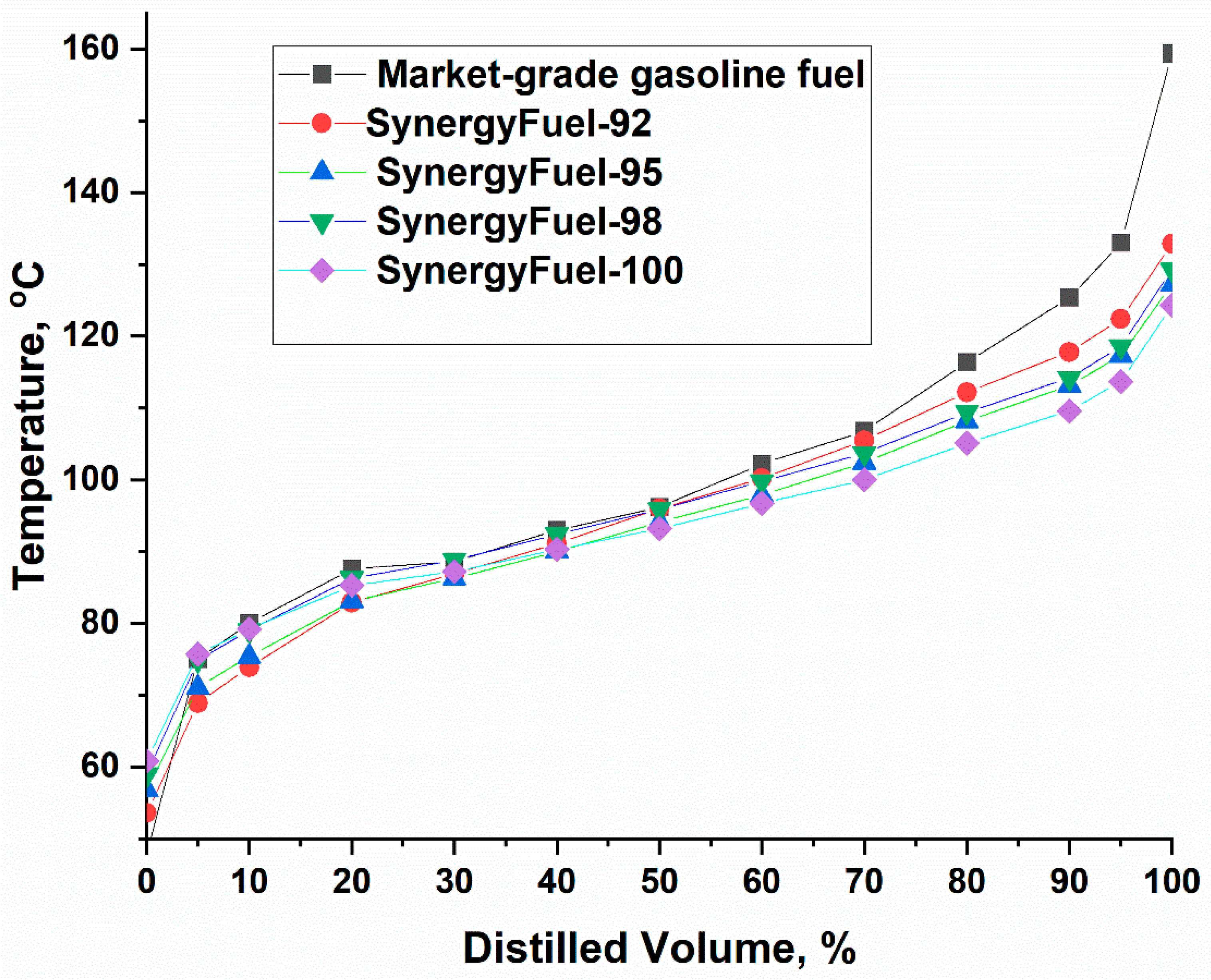
3.2.3. Gasoline Distillation Characteristics
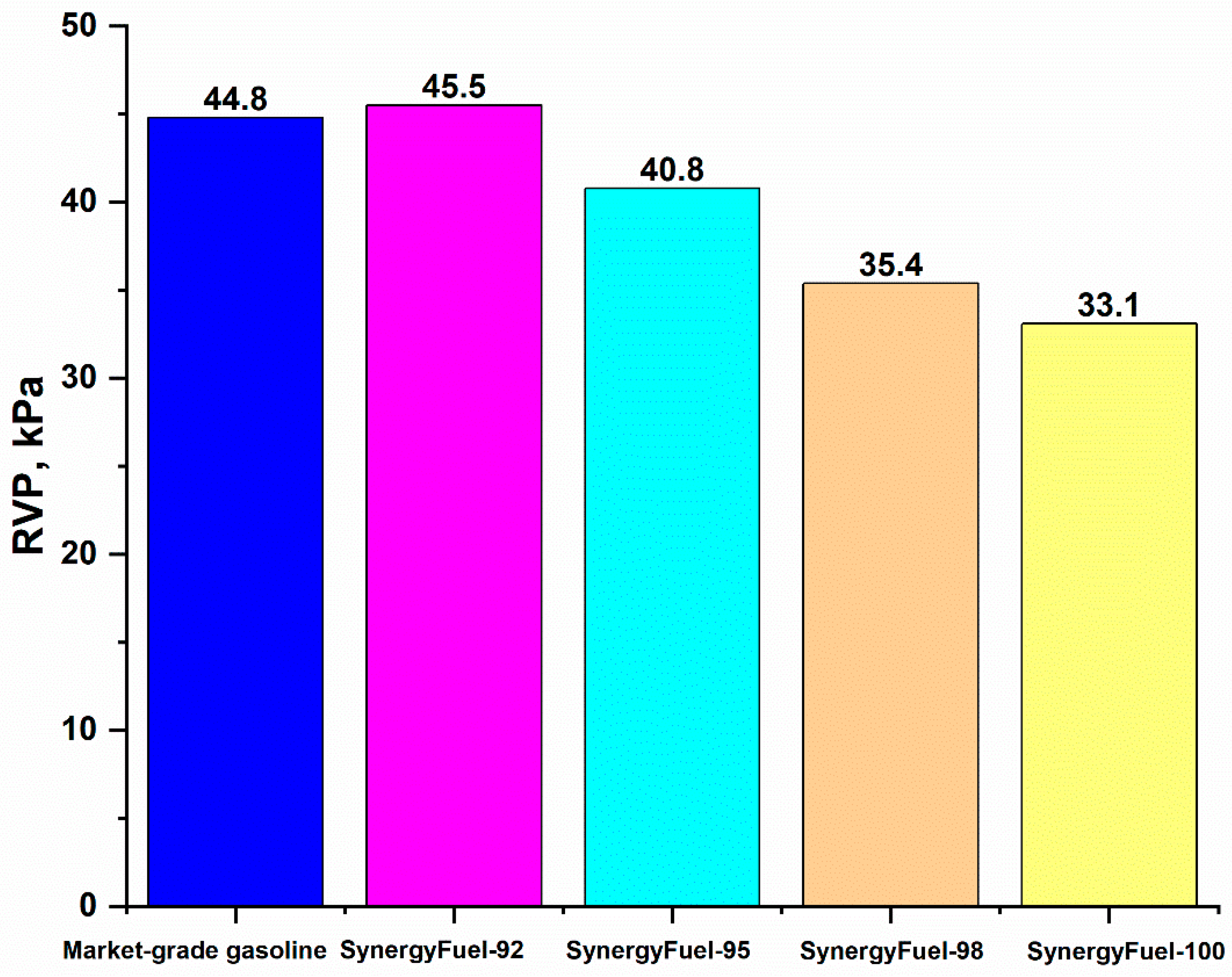
3.2.4. Gasoline Fuel RVP
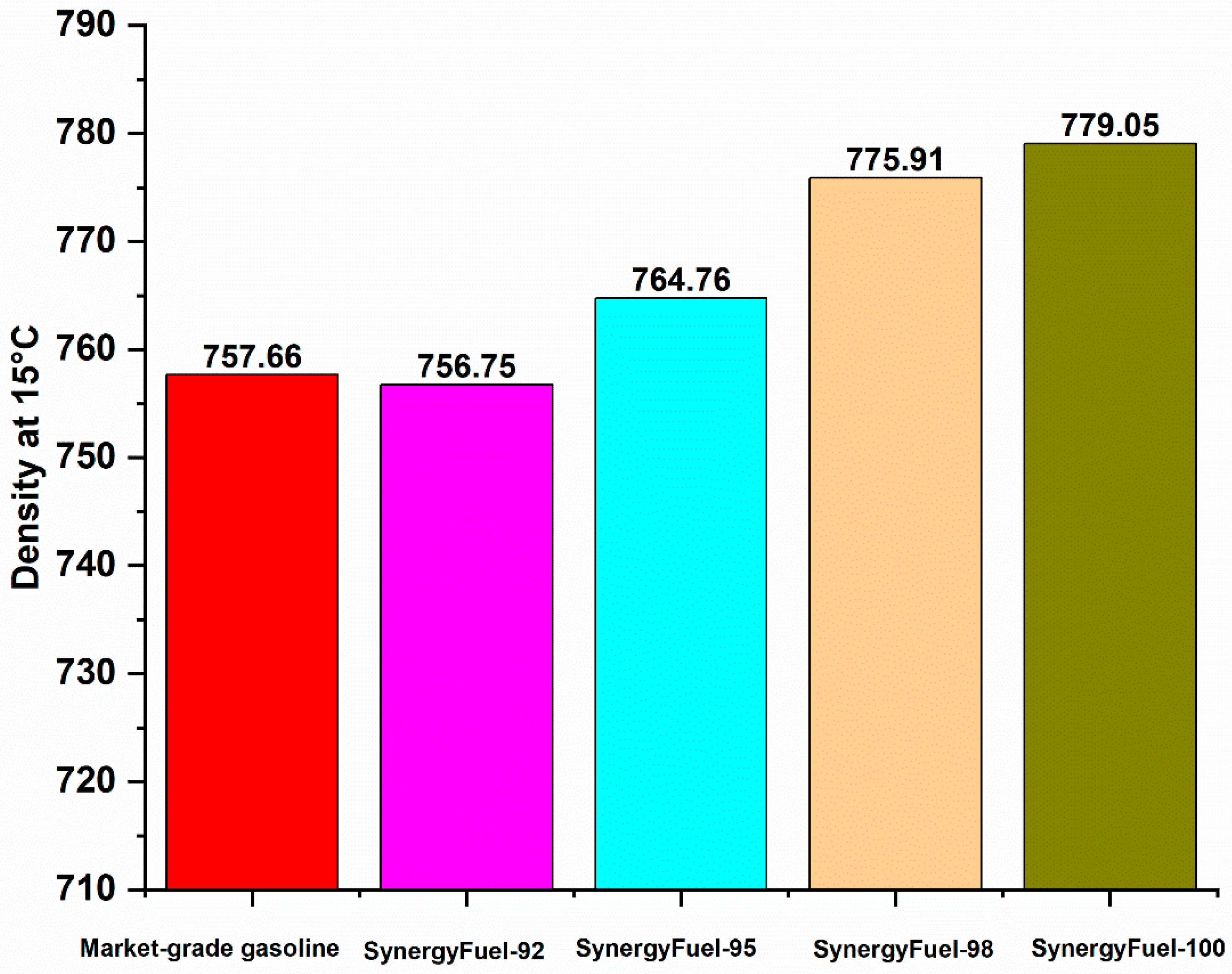
3.2.5. Gasoline Fuel Density
3.2.6. Gasoline Volatility Indexes
- T (10) = Temperatures (°C) at 10 % volume distilled;
- T (50) = Temperatures (°C) at 50% volume distilled;
- T (90) = Temperatures (°C) at 90% volume distilled;
- VP = RVP, kPa.
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Duan, X.; Chu, X.; Wang, R.; Chen, Z.; Zhou, F.; Abdellatief, T.M.M. The Performance and Emissions Characteristics of the Gasoline Spark Ignition Engine Fuelled with Green and Renewable Methanol and Hydrogen. Renew. Energy 2025, 240, 122184. [Google Scholar] [CrossRef]
- Almaktar, M.; Shaaban, M. Prospects of Renewable Energy as a Non-Rivalry Energy Alternative in Libya. Renew. Sustain. Energy Rev. 2021, 143, 110852. [Google Scholar] [CrossRef]
- Ansari, D.; Holz, F. Between Stranded Assets and Green Transformation: Fossil-Fuel-Producing Developing Countries towards 2055. World Dev. 2020, 130, 104947. [Google Scholar] [CrossRef]
- Dogru, T.; Bulut, U.; Kocak, E.; Isik, C.; Suess, C.; Sirakaya-Turk, E. The Nexus between Tourism, Economic Growth, Renewable Energy Consumption, and Carbon Dioxide Emissions: Contemporary Evidence from OECD Countries. Environ. Sci. Pollut. Res. 2020, 27, 40930–40948. [Google Scholar] [CrossRef]
- Sotirova, E.; Vasilev, S.; Stratiev, D.; Shishkova, I.; Sotirov, S.; Nikolova, R.; Veli, A.; Bureva, V.; Atanassov, K.; Georgieva, V.; et al. Comparison of the Methods for Predicting the Critical Temperature and Critical Pressure of Petroleum Fractions and Individual Hydrocarbons. Fuels 2025, 6, 36. [Google Scholar] [CrossRef]
- Gautam, P.; Kumar, S.; Lokhandwala, S. Chapter 11—Energy-Aware Intelligence in Megacities. In Computational Data and Bioengineering; Kumar, S., Kumar, R., Pandey, A.B., Eds.; Elsevier B.V.: Amsterdam, The Netherlands, 2019; pp. 211–238. ISBN 978-0-444-64083-3. [Google Scholar]
- Stratiev, D. Catalytic Cracking of Non-Hydrotreated, Hydrotreated and Sulfuric Acid-Treated Vacuum Gas Oils. Processes 2025, 13, 1351. [Google Scholar] [CrossRef]
- Stratiev, D. Hydrocracking of Various Vacuum Residues. Fuels 2025, 6, 35. [Google Scholar] [CrossRef]
- Stratiev, D.; Shishkova, I.; Argirov, G.; Dinkov, R.; Ivanov, M.; Sotirov, S.; Sotirova, E.; Bureva, V.; Nenov, S.; Atanassov, K.; et al. Roles of Catalysts and Feedstock in Optimizing the Performance of Heavy Fraction Conversion Processes: Fluid Catalytic Cracking and Ebullated Bed Vacuum Residue Hydrocracking. Catalysts 2024, 14, 616. [Google Scholar] [CrossRef]
- Rekhletskaya, E.S.; Ershov, M.A.; Savelenko, V.D.; Makhmudova, A.E.; Kapustin, V.M.; Abdellatief, T.M.M.; Potanin, D.A.; Smirnov, V.A.; Geng, T.; Abdelkareem, M.A.; et al. Unraveling the Superior Role of Characterizing Methyl Ester of Isohexene as an Innovative High-Octane Gasoline Mixing Component. Energy Fuels 2022, 36, 11829–11838. [Google Scholar] [CrossRef]
- Abdelghany, M.B.; Shafiqurrahman, A.; Dan, M.; Al-Durra, A.; El Moursi, M.S.; Ren, Z.; Gao, F. Advanced Relaxed Stochastic Control for Green Energy Management and Decarbonization in Large-Scale Heterogeneous Industrial Clusters. J. Clean. Prod. 2025, 501, 145210. [Google Scholar] [CrossRef]
- Abdelghany, M.B.; Al-Durra, A.; Zeineldin, H.; El Moursi, M.S.; Hu, J.; Gao, F. Optimizing Resilient Parallel Refueling Operations: Relaxed Stochastic Economic Mobility Scheduling for Fuel Cell Vehicles with Multiple Hydrogen Storage Systems. eTransportation 2025, 23, 100393. [Google Scholar] [CrossRef]
- Chaudhry, B.; Ahmad, M.; Munir, M.; Fawzy Ramadan, M.; Munir, M.; Ussemane Mussagy, C.; Faisal, S.; Abdellatief, T.M.M.; Mustafa, A. Unleashing the Power of Non-Edible Oil Seeds of Ipomoea Cairica for Cleaner and Sustainable Biodiesel Production Using Green Molybdenum Oxide (MoO3) Nano Catalyst. Sustain. Energy Technol. Assess. 2024, 65, 103781. [Google Scholar] [CrossRef]
- Khobragade, D.S. Biomass—An Environmental Concern. In Plant Biomass Derived Materials; Wiley-VCH: Weinheim, Germany, 2024; pp. 1–22. ISBN 9783527839032. [Google Scholar]
- Gücüyener, A. Energy Cooperation and Interdependence in the Central Asian and Caspian Region for Improving Energy Security. In Achieving Energy Security in Asia; World Scientific: Singapore, 2019; pp. 97–127. ISBN 978-981-12-0420-3. [Google Scholar]
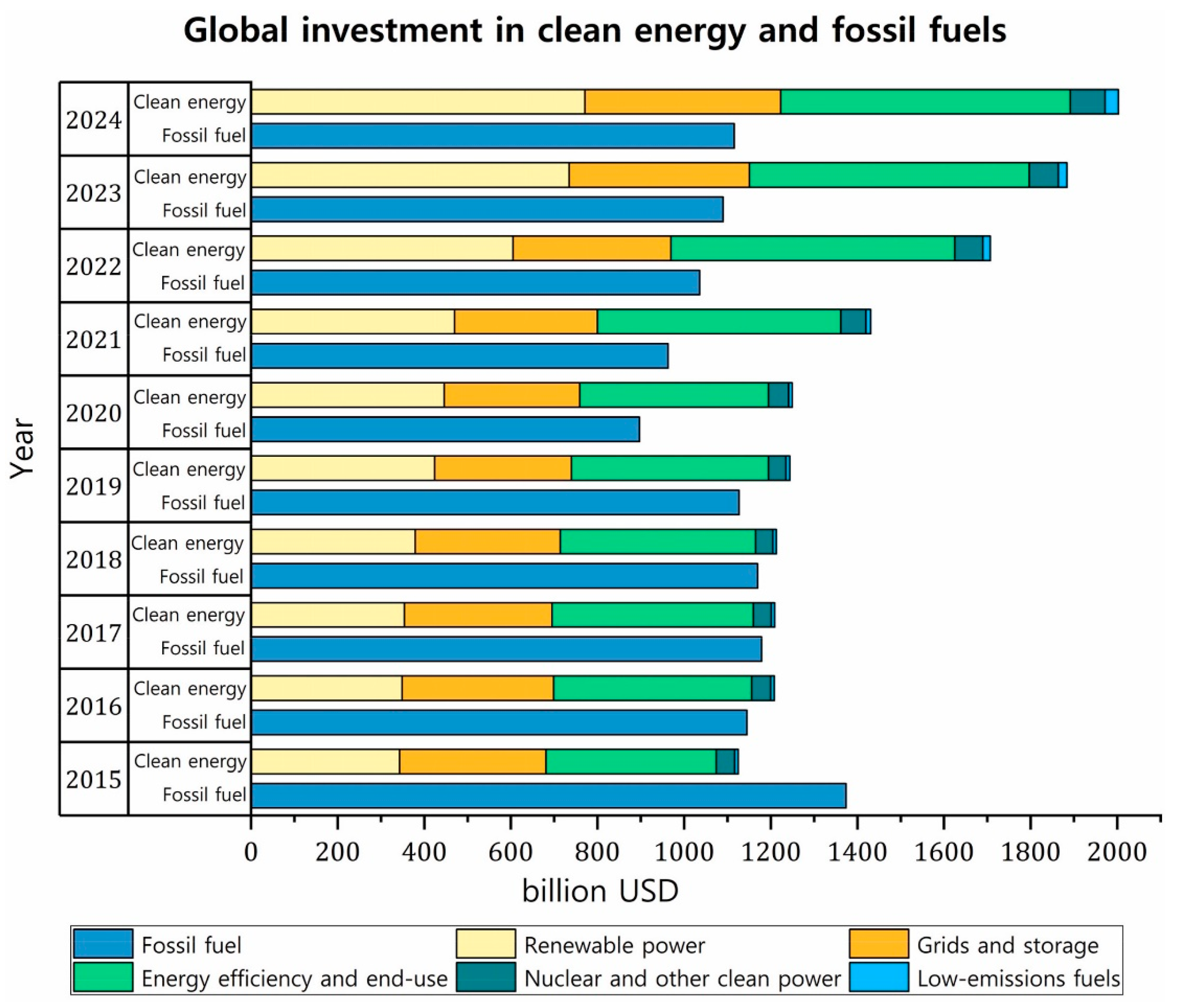
- International Energy Agency. World Energy Investment 2024: Overview and Key Findings. IEA 2024. Available online: https://www.iea.org/reports/world-energy-investment-2024/overview-and-key-findings (accessed on 9 June 2025).
- International Energy Agency. Global Investment in Clean Energy and Fossil Fuels, 2015–2024. Available online: https://www.iea.org/data-and-statistics/charts/global-investment-in-clean-energy-and-fossil-fuels-2015-2024 (accessed on 9 June 2025).
- Abdellatief, T.M.M.; Ershov, M.A.; Kapustin, V.M.; Ali Abdelkareem, M.; Kamil, M.; Olabi, A.G. Recent Trends for Introducing Promising Fuel Components to Enhance the Anti-Knock Quality of Gasoline: A Systematic Review. Fuel 2021, 291, 120112. [Google Scholar] [CrossRef]
- Ershov, M.A.; Grigorieva, E.V.; Abdellatief, T.M.M.; Chernysheva, E.A.; Makhin, D.Y.; Kapustin, V.M. A New Approach for Producing Mid-Ethanol Fuels E30 Based on Low-Octane Hydrocarbon Surrogate Blends. Fuel Process. Technol. 2021, 213, 106688. [Google Scholar] [CrossRef]
- Golikova, A.; Shasherina, A.; Anufrikov, Y.; Misikov, G.; Kuzmenko, P.; Smirnov, A.; Toikka, M.; Toikka, A. Excess Enthalpies Analysis of Biofuel Components: Sunflower Oil–Alcohols Systems. Int. J. Mol. Sci. 2024, 25, 3244. [Google Scholar] [CrossRef]
- Iliev, S. A Comparison of Ethanol, Methanol, and Butanol Blending with Gasoline and Its Effect on Engine Performance and Emissions Using Engine Simulation. Processes 2021, 9, 1322. [Google Scholar] [CrossRef]
- Turner, J.W.G.; Lewis, A.G.J.; Akehurst, S.; Brace, C.J.; Verhelst, S.; Vancoillie, J.; Sileghem, L.; Leach, F.C.P.; Edwards, P.P. Alcohol Fuels for Spark-Ignition Engines: Performance, Efficiency, and Emission Effects at Mid to High Blend Rates for Ternary Mixtures. Energies 2020, 13, 6390. [Google Scholar] [CrossRef]
- Bhatt, A.K.; Bhatia, R.K.; Thakur, S.; Rana, N.; Sharma, V.; Rathour, R.K. Fuel from Waste: A Review on Scientific Solution for Waste Management and Environment Conservation BT—Prospects of Alternative Transportation Fuels; Singh, A.P., Agarwal, R.A., Agarwal, A.K., Dhar, A., Shukla, M.K., Eds.; Springer Singapore: Singapore, 2018; pp. 205–233. ISBN 978-981-10-7518-6. [Google Scholar]
- Zacarías, A.; Grijalva, M.R.; Rubio, J.D.; Romage, G.; Mena, V.Y.; Hernández, R.; Carvajal, I.; Flores, A.; Guarneros, O.; Rodríguez, B.A. Improvement Efficiency and Emission Reduction in Used Cars for Developing Regions Using Gasoline–Bioethanol Blends. Energies 2025, 18, 638. [Google Scholar] [CrossRef]
- García Mariaca, A.; Villalba, J.; Morillo Castaño, R.; Bailera, M. Performance and Emissions of Spark-Ignition Internal Combustion Engine Operating with Bioethanol–Gasoline Blends at High Altitudes Under Low- and High-Speed Conditions. Energies 2025, 18, 1401. [Google Scholar] [CrossRef]
- Park, S.H.; Lee, C.S. Combustion Performance and Emission Reduction Characteristics of Automotive DME Engine System. Prog. Energy Combust. Sci. 2013, 39, 147–168. [Google Scholar] [CrossRef]
- Bongartz, D.; Doré, L.; Eichler, K.; Grube, T.; Heuser, B.; Hombach, L.E.; Robinius, M.; Pischinger, S.; Stolten, D.; Walther, G.; et al. Comparison of Light-Duty Transportation Fuels Produced from Renewable Hydrogen and Green Carbon Dioxide. Appl. Energy 2018, 231, 757–767. [Google Scholar] [CrossRef]
- Hansdah, D.; Murugan, S. Bioethanol Fumigation in a DI Diesel Engine. Fuel 2014, 130, 324–333. [Google Scholar] [CrossRef]
- Shu, M.; Liu, Z.; Wu, F.; Qiu, Y.; Pan, J. Experimental Study on the Combustion and Emission Characteristics of Methanol/Gasoline Fuels in Direct Injection Miller Cycle Gasoline Engines. Int. J. Automot. Technol. 2024, 25, 1517–1527. [Google Scholar] [CrossRef]
- Radzali, M.H.; Hakim Zulkifli, A.F.; Khalid, A.; Radzali, M.H.; Jacob, D.W. Effect of Methanol-Gasoline Blend and Ambient Pressure on Flame Propagation and Exhaust Emission of Spark Ignition (SI) Engine. Fuel Mix. Form. Combust. Process 2020, 2, 1–6. [Google Scholar]
- Abdellatief, T.M.M.; Ershov, M.A.; Makhmudova, A.E.; Kapustin, V.M.; Makhova, U.A.; Klimov, N.A.; Chernysheva, E.A.; Ali Abdelkareem, M.; Mustafa, A.; Olabi, A.G. Novel Variants Conceptional Technology to Produce Eco-Friendly Sustainable High Octane-Gasoline Biofuel Based on Renewable Gasoline Component. Fuel 2024, 366, 131400. [Google Scholar] [CrossRef]
- Kale, A.V.; Krishnasamy, A. Experimental Study on Combustion, Performance, and Emission Characteristics of a Homogeneous Charge Compression Ignition Engine Fuelled with Multiple Biofuel-Gasoline Blends. Energy 2024, 288, 129621. [Google Scholar] [CrossRef]
- Rahayu, S.M.N.; Hananto, A.L.; Herawan, S.G.; Asy’ari, M.Z.; Sule, A.; Idris, M.; Hermansyah, D.; Balogun, S.A.; Ali, E.A.B. A Review of Automotive Green Technology: Potential of Butanol as Biofuel in Gasoline Engine. Mechanical Engineer. Mech. Eng. Soc. Ind. 2022, 2, 82–97. [Google Scholar] [CrossRef]
- van Dyk, S.; Su, J.; Saddler, J. Recent Progress in the Production of Low Carbon-Intensive Drop-in Fuels—Standalone Production and Coprocessing IEA Bioenergy: Task 39 IEA Bioenergy; IEA Bioenergy: Paris, France, 2022; ISBN 9791280907035. [Google Scholar]
- Zhang, J.; Morsch, P.; Minwegen, H.; vom Lehn, F.; Wu, X.; Alexander Heufer, K.; Pitsch, H.; Cai, L. Insights into the Underlying Reaction Kinetics of Gasoline–Ethanol Interactions and Their Effects on the Auto-Ignition Characteristics of Gasoline/Ethanol Blends. Appl. Energy Combust. Sci. 2025, 22, 100333. [Google Scholar] [CrossRef]
- Ershov, M.A.; Potanin, D.A.; Grigorieva, E.V.; Abdellatief, T.M.M.; Kapustin, V.M. Discovery of a High-Octane Environmental Gasoline Based on the Gasoline Fischer–Tropsch Process. Energy Fuels 2020, 34, 4221–4229. [Google Scholar] [CrossRef]
- Sathyanarayanan, S.; Suresh, S.; Saravanan, C.G.; Vikneswaran, M.; Dhamodaran, G.; Sonthalia, A.; Josephin, J.S.F.; Varuvel, E.G. Experimental Investigation and Performance Prediction of Gasoline Engine Operating Parameters Fueled with Diisopropyl Ether-Gasoline Blends: Response Surface Methodology Based Optimization. J. Clean. Prod. 2022, 375, 133941. [Google Scholar] [CrossRef]
- Shirazi, S.A.; Foust, T.D.; Reardon, K.F. Identification of Promising Alternative Mono-Alcohol Fuel Blend Components for Spark Ignition Engines. Energies 2020, 13, 1955. [Google Scholar] [CrossRef]
- Zhang, Z.; Wen, M.; Cui, Y.; Ming, Z.; Wang, T.; Zhang, C.; Ampah, J.D.; Jin, C.; Huang, H.; Liu, H. Effects of Methanol Application on Carbon Emissions and Pollutant Emissions Using a Passenger Vehicle. Processes 2022, 10, 525. [Google Scholar] [CrossRef]
- ASTM D252; Standard Test Method for Oxidation Stability of Gasoline (Induction Period Method). American Society for Testing and Materials: West Conshohocken, PA, USA, 2019.
- ASTM D381; Standard Test Method for Gum Content in Fuels by Jet Evaporation. American Society for Testing and Materials: West Conshohocken, PA, USA, 2019.
- Sun, X.; Zhang, F.; Liu, J.; Duan, X. Prediction of Gasoline Research Octane Number Using Multiple Feature Machine Learning Models. Fuel 2023, 333, 126510. [Google Scholar] [CrossRef]
- Abdellatief, T.M.M. Enhancing the Properties of Egyption Gasoline through Modified Operations. Master’s Thesis, Minia University, Minia, Egypt, 2015. [Google Scholar]
- ASTM D2699; Standard Test Method for Research Octane Number of Spark-Ignition Engine Fuel. American Society for Testing and Materials: West Conshohocken, PA, USA, 2023.
- Abdellatief, T.; El-Bassiouny, A.-M.; Aboul-Fotouh, T. An Environmental Gasoline: Enhancing the Properties of the Gasoline through Modified Blending Operations; Lap Lambert Academic Publishing: Saarbrücken, Germany, 2015; ISBN 3659803324. [Google Scholar]
- ASTM D86; Standard Test Method for Distillation of Petroleum Products and Liquid Fuels at Atmospheric Pressure. American Society for Testing and Materials: West Conshohocken, PA, USA, 2023.
- ASTM D5453; Standard Test Method for Determination of Total Sulfur in Light Hydrocarbons, Spark Ignition Engine Fuel, Diesel Engine Fuel, and Engine Oil by Ultraviolet Fluorescence. American Society for Testing and Materials: West Conshohocken, PA, USA, 2024.
- ASTM D130; Standard Test Method for Corrosiveness to Copper from Petroleum Products by Copper Strip Test. American Society for Testing and Materials: West Conshohocken, PA, USA, 2019.
- Pfleger, G.S.; Teubler, R.; Schober, S. A Novel Gas Chromatographic Method for High-Resolution Analysis of Gasoline Fuels That Enables the Calculation of CHO Ratio, Higher and Lower Heating Value, Density and Energy Density. Fuel 2024, 376, 132704. [Google Scholar] [CrossRef]
- Abdellatief, T.M.M.; Ershov, M.A.; Kapustin, V.M.; Chernysheva, E.A.; Savelenko, V.D.; Makhmudova, A.E.; Potanin, D.A.; Salameh, T.; Abdelkareem, M.A.; Olabi, A.G. Innovative Conceptional Approach to Quantify the Potential Benefits of Gasoline-Methanol Blends and Their Conceptualization on Fuzzy Modeling. Int. J. Hydrogen Energy 2022, 47, 35096–35111. [Google Scholar] [CrossRef]
- ASTM D4052; Standard Test Method for Density, Relative Density, and API Gravity of Liquids by Digital Density Meter. American Society for Testing and Materials: West Conshohocken, PA, USA, 2022.
- ASTM D1319; Standard Test Method for Hydrocarbon Types in Liquid Petroleum Products by Fluorescent Indicator Adsorption. American Society for Testing and Materials: West Conshohocken, PA, USA, 2020.
- ASTM D4814; Standard Specification for Automotive Spark-Ignition Engine Fuel. ASTM International: West Conshohocken, PA, USA, 2021.
- EN 228; Automotive Fuels—Unleaded Petrol—Requirements and Test Methods. European Committee for Standardization (CEN): Brussels, Belgium, 2012.
- GB 17930-2016; Gasoline for Motor Vehicles. Standardization Administration of China (SAC): Beijing, China, 2016.
- Gao, L.; Geng, C.; Teng, B.; Xiang, H.; Wen, X.; Yang, Y.; Li, Y. Improvement of Octane Number in FCC Gasoline through the Extraction with Urea/Thiourea Complex Based on Property Analysis. Ind. Chem. Mater. 2024, 2, 613–621. [Google Scholar] [CrossRef]
- Săpunaru, O.V.; Sterpu, A.E.; Brînzei, M.; Pascu, S.; Koncsag, C.I. Etherification of Olefins from Catalytic Cracking Gasoline to Increase Its Octane Number. Chem. Eng. Process. Process Intensif. 2023, 188, 109374. [Google Scholar] [CrossRef]
- Hegedüs, B.; Palotás, Á.B.; Muránszky, G.; Dobó, Z. Investigation of Gasoline-like Transportation Fuel Obtained by Plastic Waste Pyrolysis and Distillation. J. Clean. Prod. 2024, 447, 141500. [Google Scholar] [CrossRef]
- Jiao, Y.; Yin, K.; Liu, T.; Meng, F.; Li, X.; Zhong, L.; Zhu, Z.; Cui, P.; Wang, Y. Process Design and Mechanism Analysis of Reactive Distillation Coupled with Extractive Distillation to Produce an Environmentally Friendly Gasoline Additive. J. Clean. Prod. 2022, 369, 133290. [Google Scholar] [CrossRef]
- Barbosa-Patrício, L.C.; Sales, R.F.; da Silva, N.C.; Fernandes da Silva, M.E.; Rodrigues e Brito, L.; Pimentel, M.F. An Approach Based on Virtual Samples for Gasoline Discrimination Using Physicochemical Properties or Distillation Curves. Chemom. Intell. Lab. Syst. 2022, 231, 104698. [Google Scholar] [CrossRef]
- Jiang, Y.; Phillips, S.D.; Singh, A.; Jones, S.B.; Gaspar, D.J. Potential Economic Values of Low-Vapor-Pressure Gasoline-Range Bio-Blendstocks: Property Estimation and Blending Optimization. Fuel 2021, 297, 120759. [Google Scholar] [CrossRef]
- Osman, S.; Sapunaru, O.V.; Sterpu, A.E.; Chis, T.V.; I.Koncsag, C. Impact of Adding Bioethanol and Dimethyl Carbonate on Gasoline Properties. Energies 2023, 16, 1940. [Google Scholar] [CrossRef]
| Analysis | DC Naphtha | Reformate | Isomerate | Methanol | Ethanol |
|---|---|---|---|---|---|
| Fuel density measured at 15 °C, (in kilograms per cubic meter) | 724.3 | 828 | 663.7 | 792 | 794 |
| Reid vapor pressure (RVP), kPa | 66.7 | 13.8 | 84 | 35 | 17 |
| Initial boiling point (IBP), °C | 37 | 73 | 36 | 65 | 78 |
| T10, (°C) | 74 | 106 | 44 | 65 | 78 |
| T30, (°C) | 106 | 118 | 46 | 65 | 78 |
| T50, (°C) | 127 | 130 | 50 | 65.5 | 78 |
| T70, (°C) | 147 | 143 | 57 | 65.5 | 78 |
| T90, (°C) | 168 | 165 | 75 | 66 | 78 |
| FBP, (°C) | 186 | 217 | 106 | 66 | 78 |
| Research octane number (RON) | 68 | 100 | 86.2 | 112 | 111 |
| Motor octane number (MON) | 64.8 | 83.5 | 83.4 | 91 | 92 |
| Olefins, (%) | 36.1 | 0.5 | 0.1 | 0 | 0 |
| Aromatic, (%) | 9.1 | 72.5 | 0.8 | 0 | 0 |
| Knock resistance | low | high | low | high | high |
| Analysis | Market-Grade Gasoline |
|---|---|
| Fuel density measured at 15 °C (in kilograms per cubic meter) | 757.66 |
| Sulfur content, ppm | 0.2 |
| RVP (kPa) | 44.8 |
| IBP (°C) | 48 |
| T10 (°C) | 80 |
| T30 (°C) | 88.6 |
| T50 (°C) | 96.2 |
| T70 (°C) | 106.8 |
| T90 (°C) | 125.4 |
| FBP (°C) | 159.4 |
| RON | 94.5 |
| MON | 84.1 |
| Olefins (%) | 1.1 |
| Benzene (%) | 0.6 |
| Aromatic (%) | 42.7 |
| Hydrocarbon Fraction | SynergyFuel-92 | SynergyFuel-95 | SynergyFuel-98 | SynergyFuel-100 |
|---|---|---|---|---|
| Reformate | 14 | 16 | 25 | 26 |
| Isomerate | 13 | 11 | 8 | 9 |
| DC Naphtha | 34 | 27 | 20 | 14 |
| Methanol | 15 | 15 | 15 | 15 |
| Ethanol | 24 | 31 | 32 | 36 |
| Property | Unit | SynergyFuel-92 | SynergyFuel-95 | SynergyFuel-98 | SynergyFuel-100 | USA (ASTM D4814) | Europe (EN 228) | China (GB 17930-2016) |
|---|---|---|---|---|---|---|---|---|
| Clarity | - | No visible impurities | No visible impurities | No visible impurities | No visible impurities | No visible impurities | No visible impurities | No visible impurities |
| RON | - | 91.8 | 95.1 | 97.8 | 100 | ≥91 (Regular), ≥95 (Premium) | ≥95 (Regular), ≥98 (Premium) | ≥92, 95, 98 |
| MON | - | 81.1 | 83.1 | 85 | 86.6 | ≥82 | ≥85 | ≥85 |
| Density at 15 °C | (kg/m3) | 756.75 | 764.76 | 775.91 | 779.05 | 720–775 | 720–775 | 720–775 |
| Reid Vapor Pressure | (kPa) | 45.5 | 40.8 | 35.4 | 33.1 | 48–103 (Seasonal Variation) | 45–90 | 40–88 |
| Sulfur Content | (ppm) | 3.2 | 3.7 | 3.7 | 4 | ≤10 | ≤10 | ≤10 |
| Benzene Content | (% v/v) | 0.2 | 0.2 | 0.3 | 0.3 | ≤1.0 | ≤1.0 | ≤1.0 |
| Aromatics | (% v/v) | 13.1 | 13.9 | 19.6 | 19.7 | ≤35 | ≤35 | ≤40 |
| Olefins | (% v/v) | 12.5 | 10 | 7.7 | 5.5 | ≤18 | ≤18 | ≤24 |
| Appearance | - | bright and clear | bright and clear | bright and clear | bright and clear | bright and clear | bright and clear | bright and clear |
| Initial Boiling Point | (°C) | 53.6 | 56.8 | 59 | 60.8 | ≤35 | ≤35 | ≤35 |
| Distillation 10% | (°C) | 73.9 | 75.4 | 79 | 79.2 | 50–70 | 50–70 | 50–70 |
| Distillation 50% (°C) | (°C) | 96 | 94.1 | 95.9 | 93.2 | 77–121 | 70–120 | 70–120 |
| Distillation 90% | (°C) | 117.8 | 113.1 | 114.2 | 109.6 | 150–190 | ≤180 | ≤190 |
| Final Boiling Point | (°C) | 132.9 | 127.2 | 129.3 | 124.3 | ≤225 | ≤210 | ≤215 |
| Residue | (% v/v) | 1.2 | 1.1 | 1.1 | 1.1 | ≤2 | ≤2 | ≤2 |
| Existent Gum | (mg/100 mL) | 1.8 | 1.5 | 1.3 | 1.1 | ≤5 | ≤5 | ≤5 |
| Oxidation Stability | (min) | >415 | >415 | >415 | >415 | ≥360 | ≥360 | ≥360 |
| Copper Corrosion, (3 h at 50 °C) | - | Class 1 | Class 1 | Class 1 | Class 1 | Class 1 | Class 1 | Class 1 |
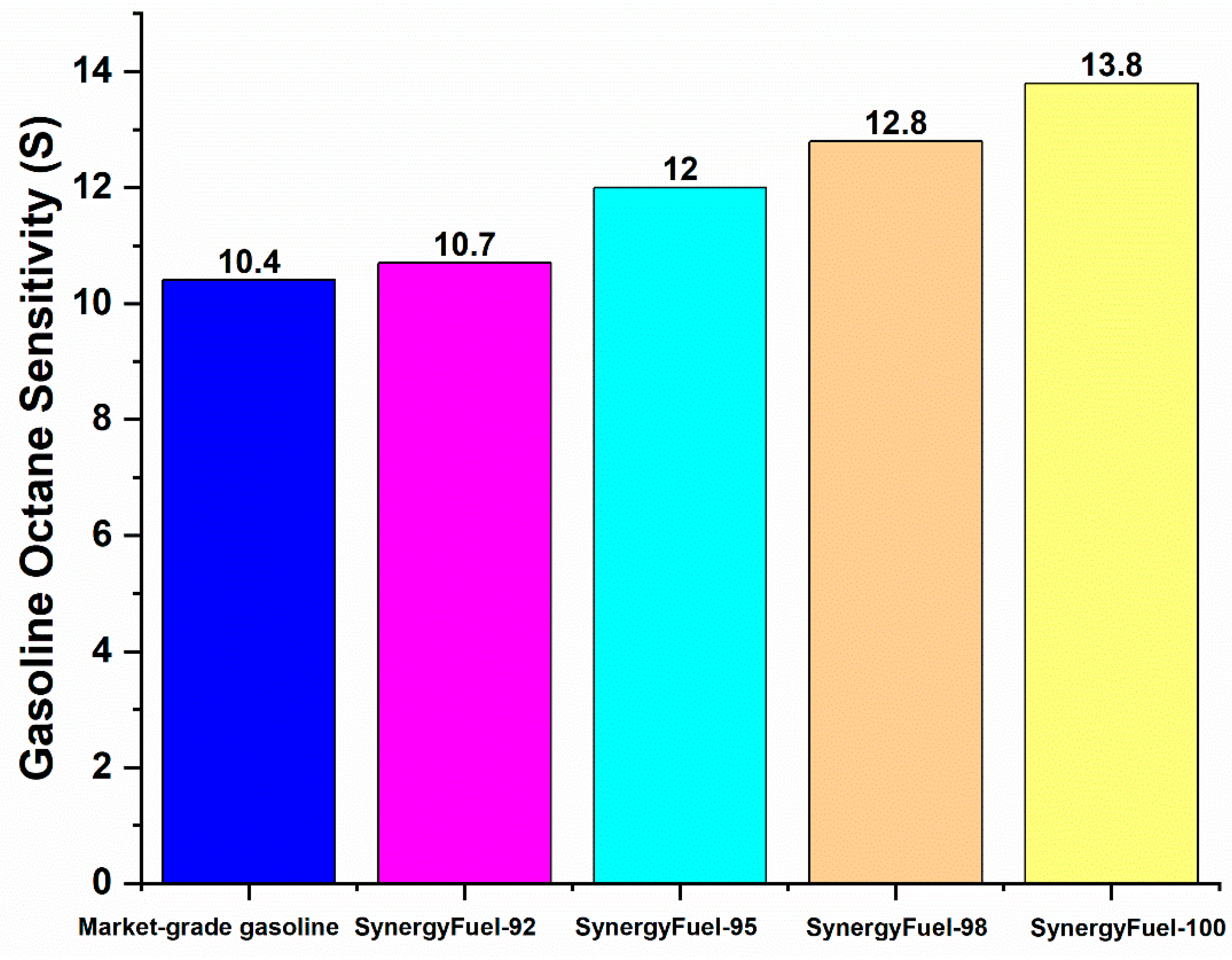
| Fuel Type | RON | MON | Gasoline Octane Sensitivity (S) | Antiknock Index (AKI) |
|---|---|---|---|---|
| Market-grade gasoline | 94.5 | 84.1 | 10.4 | 89.3 |
| SynergyFuel-92 | 91.8 | 81.1 | 10.7 | 86.45 |
| SynergyFuel-95 | 95.1 | 83.1 | 12 | 89.1 |
| SynergyFuel-98 | 97.8 | 85 | 12.8 | 91.4 |
| SynergyFuel-100 | 100 | 86.6 | 13.8 | 93.3 |
| Fuel Type | RVP | T10, (°C) | T50, (°C) | T70, (°C) | T90, (°C) | DI | VLI | T (V/L=20), °C |
|---|---|---|---|---|---|---|---|---|
| Market-grade gasoline fuel | 44.8 | 80.0 | 96.2 | 106.8 | 125.4 | 534 | 1195.6 | 55.64 |
| SynergyFuel-92 | 45.5 | 73.9 | 96 | 105.5 | 117.8 | 516.65 | 1193.5 | 55.253 |
| SynergyFuel-95 | 40.8 | 75.4 | 94.1 | 102.4 | 113.1 | 508.5 | 1124.8 | 56.511 |
| SynergyFuel-98 | 35.4 | 79.0 | 95.9 | 103.7 | 114.2 | 520.4 | 1079.9 | 58.671 |
| SynergyFuel-100 | 33.1 | 79.2 | 93.2 | 100.0 | 109.6 | 508 | 1031 | 58.975 |
| ASTM Standard values | - | - | - | - | - | STM-D4814 (375 to 610, °C) | ASTM D4814 (800 to 1250) | ASTM-D4814 (35 to 60 °C) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdellatief, T.M.M.; Mustafa, A.; Handawy, M.K.M.; Abdelghany, M.B.; Duan, X. Sustainable Production of Eco-Friendly, Low-Carbon, High-Octane Gasoline Biofuels Through a Synergistic Approach for Cleaner Transportation. Fuels 2025, 6, 49. https://doi.org/10.3390/fuels6030049
Abdellatief TMM, Mustafa A, Handawy MKM, Abdelghany MB, Duan X. Sustainable Production of Eco-Friendly, Low-Carbon, High-Octane Gasoline Biofuels Through a Synergistic Approach for Cleaner Transportation. Fuels. 2025; 6(3):49. https://doi.org/10.3390/fuels6030049
Chicago/Turabian StyleAbdellatief, Tamer M. M., Ahmad Mustafa, Mohamed Koraiem M. Handawy, Muhammad Bakr Abdelghany, and Xiongbo Duan. 2025. "Sustainable Production of Eco-Friendly, Low-Carbon, High-Octane Gasoline Biofuels Through a Synergistic Approach for Cleaner Transportation" Fuels 6, no. 3: 49. https://doi.org/10.3390/fuels6030049
APA StyleAbdellatief, T. M. M., Mustafa, A., Handawy, M. K. M., Abdelghany, M. B., & Duan, X. (2025). Sustainable Production of Eco-Friendly, Low-Carbon, High-Octane Gasoline Biofuels Through a Synergistic Approach for Cleaner Transportation. Fuels, 6(3), 49. https://doi.org/10.3390/fuels6030049