Numerical Analysis on Impact of Membrane Thickness and Temperature on Characteristics of Biogas Dry Reforming Membrane Reactor Using Pd/Cu Membrane and Ni/Cr Catalyst
Abstract
:1. Introduction
2. Numerical Simulation Procedure of BDR Membrane Reactor Using Pd/Cu Membrane and Ni/Cr Catalyst
2.1. A Mathematical Formation
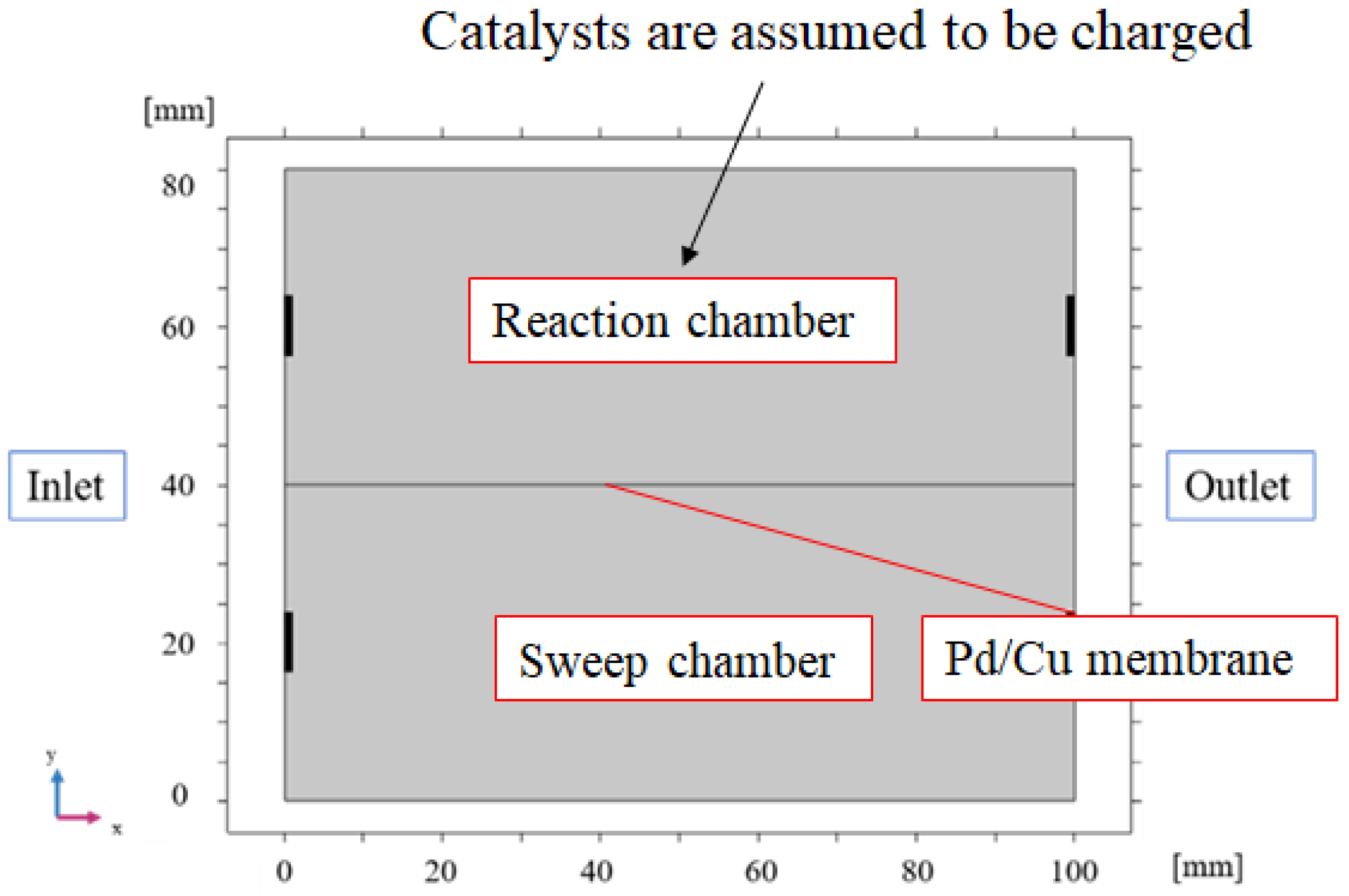
2.2. 2D Numerical Simulation Model of BDR Membrane Reactor
2.3. Kinetic Model of BDR
2.4. Parameters and Conditions of Numerical Simulation
- (i)
- The catalyst was assumed to be a porous material. The porosity, permeability, constant-pressure specific heat, thermal conductivity, and isotropy were set to be constant.
- (ii)
- The wall temperature was set to be isothermal.
- (iii)
- The gas was set to be a Newton fluid and treated to be an ideal gas.
- (iv)
- The wall of the reactor, except for the inlet and outlet, was set to be no-slip.
- (v)
- The pressure of the outlet was set to be atmospheric pressure (gauge pressure: 0 Pa).
- (vi)
- The temperature of the inflow gas was the same as the initial reaction temperature.
- (vii)
- The produced carbon was assumed to be a gas.
2.5. Evaluation Factor for Reaction Characteristics in the Present Study
3. Results and Discussion
3.1. Comparison of the Distriburtion of Pressure Along X-Direction Among Different Initial Reaction Temperatures and Thicknesses of Pd/Cu Membranes
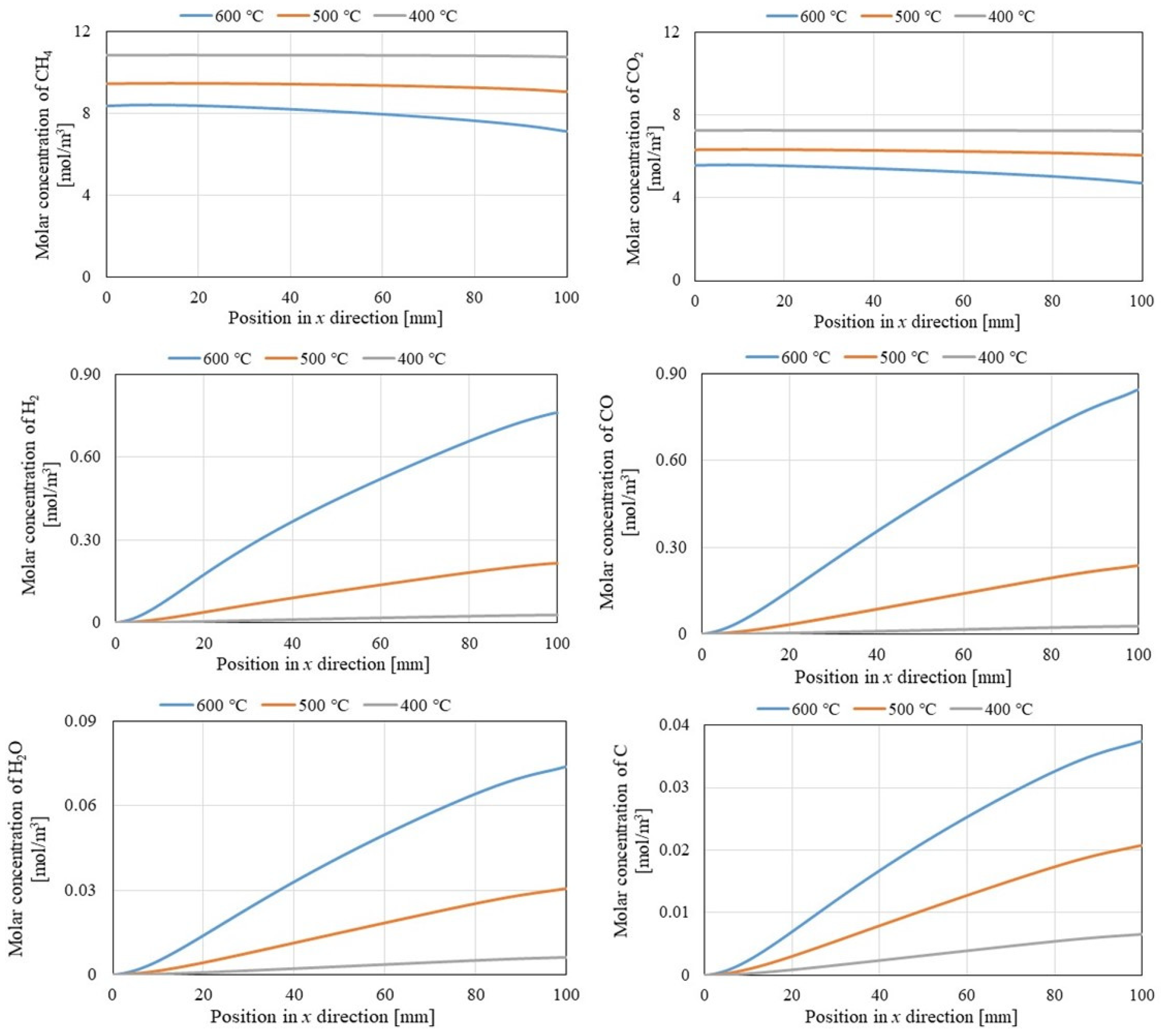
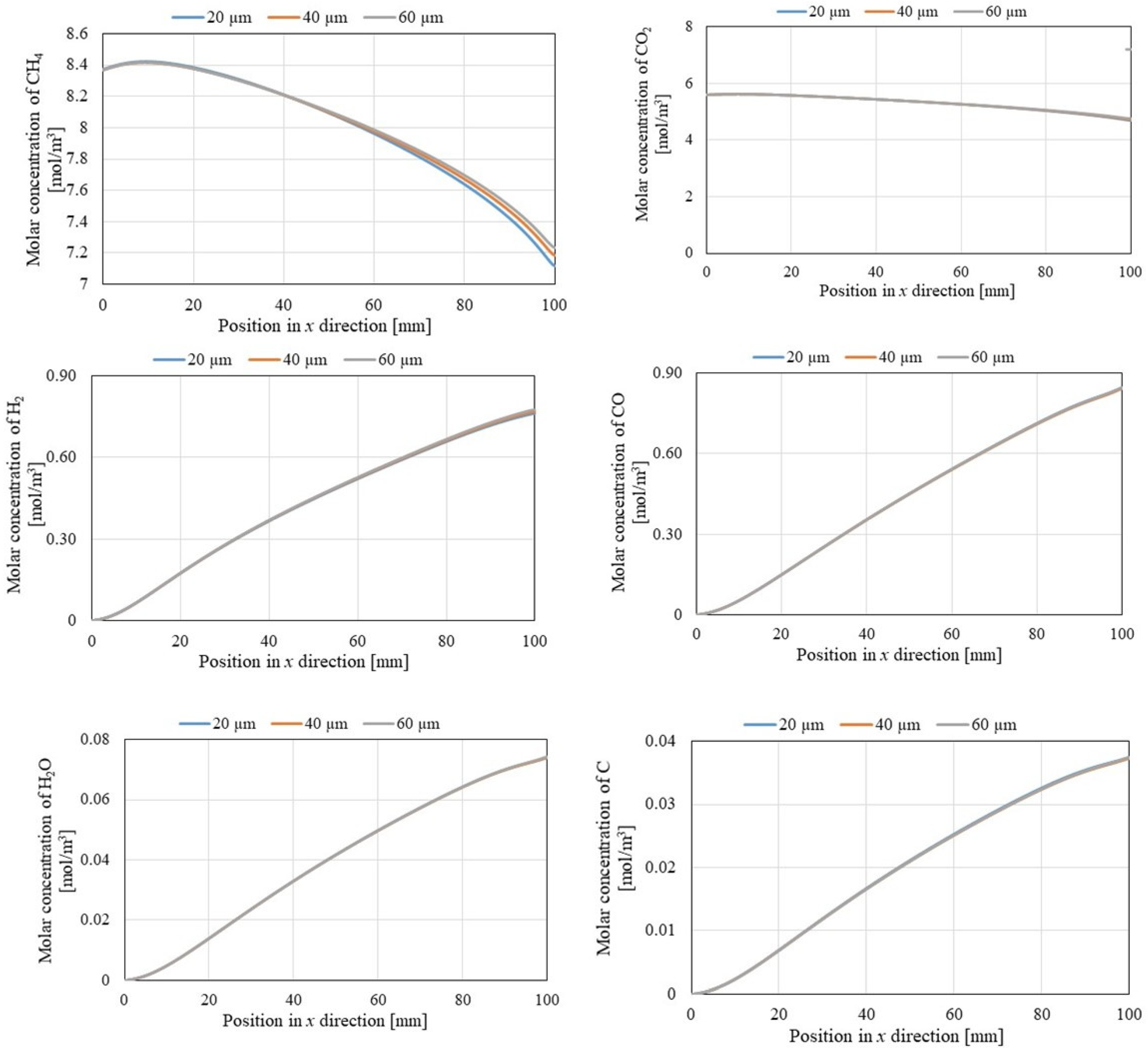
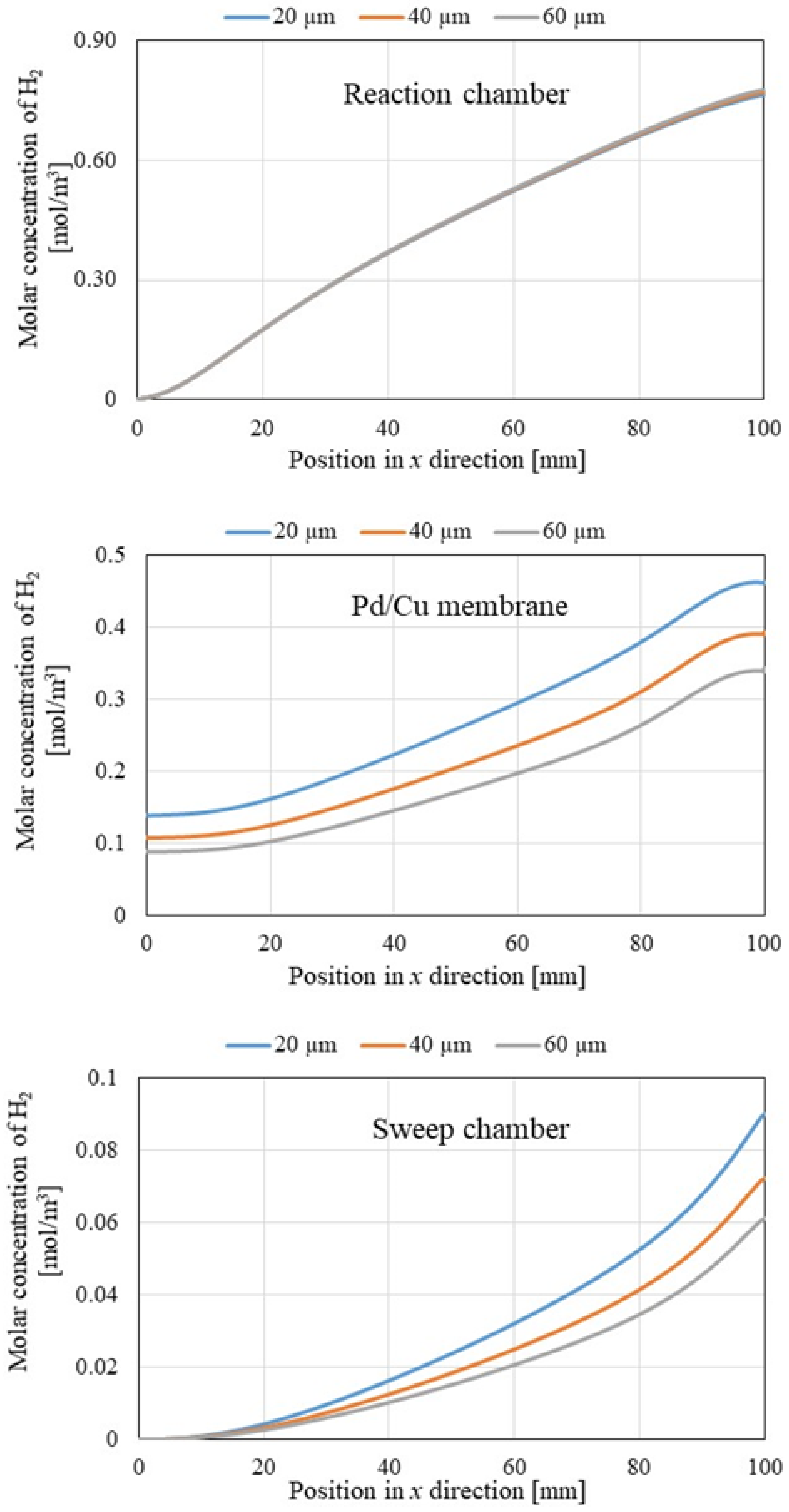
3.2. Comparison of Gas Concentrations Along X-Direction Among Different Initial Reaction Temperatures and Thicknesses of Pd/Cu Membranes
3.3. Investigation on Reaction Characteristics by Evaluation Factors
4. Conclusions
- (i)
- The pressure in the reaction chamber and Pd/Cu membrane decreased along the x-direction and they rose with the increase in the initial reaction temperature.
- (ii)
- The pressure in the Pd/Cu membrane rose with the decrease in the thickness of the Pd/Cu membrane, though the influence was very small.
- (iii)
- It can be revealed that the molar concentrations of CH4 and CO2 decreased with the rise in the initial reaction temperature, while the molar concentrations of H2, CO, H2O, and C increased with the increase in the initial reaction temperature. Moreover, the formed H2O and C demonstrate that Equations (3)–(6) occurred.
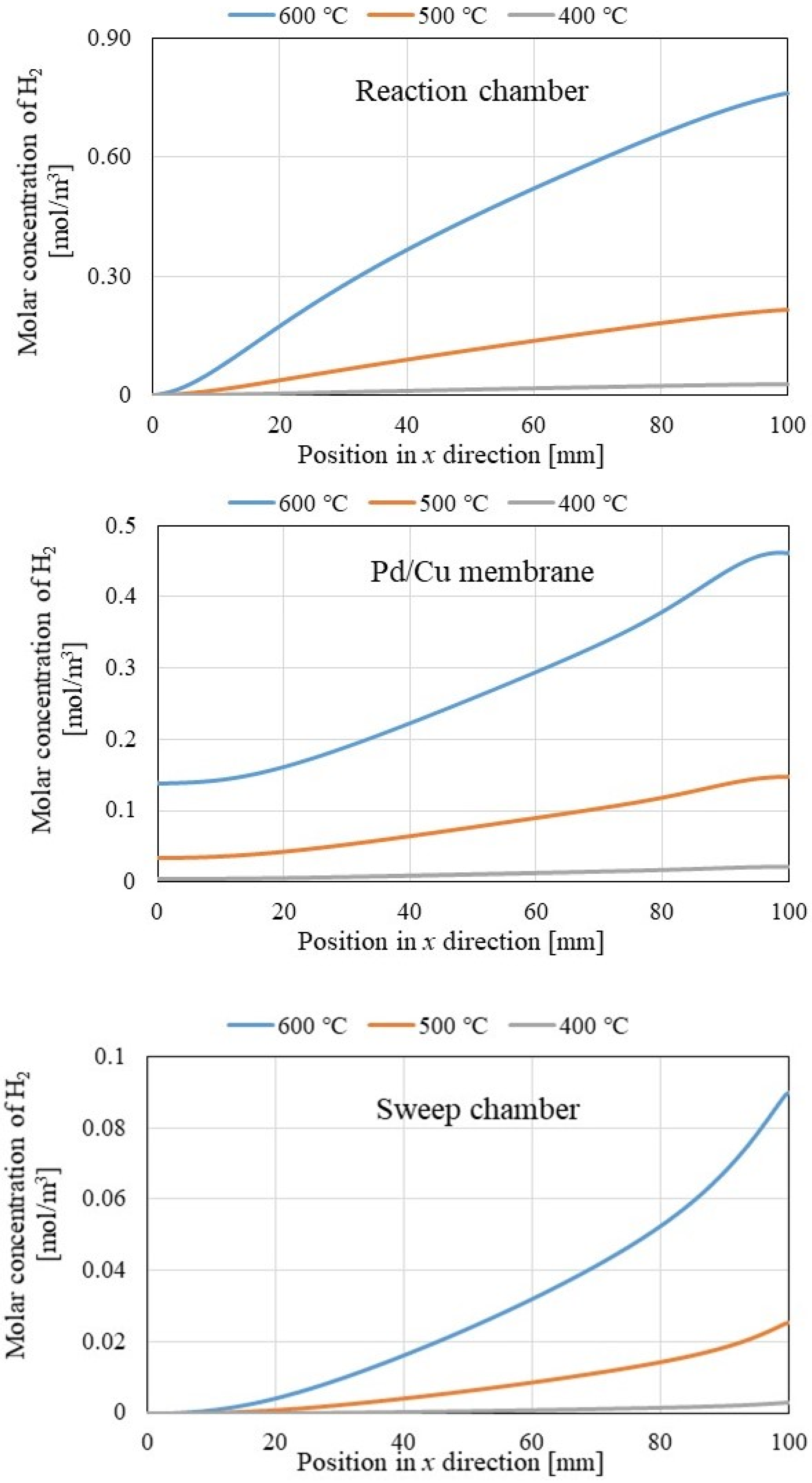
- (iv)
- The molar concentration of H2 rose along the x-direction of the reaction chamber, Pd/Cu membrane, and sweep chamber. Since the H2 production reactions, i.e., Equations (1), (2) and (5), occur along the x-direction, the molar concentration of H2 increasing along the x-direction was expected.
- (v)
- The molar concentrations of H2 in the reaction chamber, Pd/Cu membrane, and sweep chamber increased with the increase in the initial reaction temperature.
- (vi)
- The molar concentrations of H2 in the Pd/Cu membrane and sweep chamber were higher when the molar concentration of H2 in the reaction chamber was higher.
- (vii)
- It is clear that the molar concentrations of H2 in the Pd/Cu membrane and sweep chamber rose with the decrease in the thickness of the Pd/Cu membrane.
- (viii)
- The CH4 conversion, CO2 conversion, and H2 yield rose with the increase in the initial reaction temperature and the reduction in the thickness of the Pd/Cu membrane.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Pei, A.; Xie, R.; Zhang, Y.; Feng, Y.; Wang, W.; Zhang, S.; Huang, Z.; Zhu, L.; Chai, G.; Yang, Z.; et al. Effective electronic tuning of Pt single atoms via heterogeneous atomic coordination of (Co, Ni)(OH)2 for efficient hydrogen evolution. Energy Environ. Sci. 2023, 16, 1035–1048. [Google Scholar] [CrossRef]
- Pei, A.; Zhu, L.; Huang, Z.; Ye, J.; Chang, Y.C.; Osman, S.M.; Pao, C.W.; Gao, Q.; Chen, B.H.; Luque, R. Nickel hydroxide-supported Ru single atoms and Pd nanoclusters for enhanced electrocatalytic hydrogen evolution and ethanol oxidation. Adv. Funct. Mater. 2022, 32, 2208587. [Google Scholar] [CrossRef]
- Wu, F.; Zeng, L.; Pei, A.; Feng, Y.; Zhu, L. N, P co-doped graphene-supported monometallic nanoparticles for highly efficient hydrogen evolution by acid electrolysis of water. J. Mater. Chem. A 2024, 17, 10300–10306. [Google Scholar] [CrossRef]
- Kalai, D.Y.; Stangeland, K.; Jin, R.; Tucho, W.M.; Yu, Z. Biogas dry reforming for syngas production on La promoted hydrotalcitederived Ni catalyst. Int. J. Hydrogen Energy 2018, 43, 19438–19450. [Google Scholar] [CrossRef]
- World Bioenergy Association. Available online: https://worldbioenergy.org/global-bioenergy-statistics (accessed on 12 December 2024).
- The Japan Gas Association. Available online: https://www.gas.or.jp/gas-life/biogas/ (accessed on 12 December 2024).
- Nishimura, A.; Hayashi, Y.; Ito, S.; Kolhe, M.L. Performance analysis of hydrogen production for a solid oxide fuel cell system using a biogas dry reforming membrane reactor with Ni and Ni/Cr catalysts. Fuels 2023, 4, 295–313. [Google Scholar] [CrossRef]
- Dogan, M.Y.; Arbag, H.; Tasdemir, M.; Yasyerli, N.; Yasyerli, S. Effect of ceria content in Ni-Ce-Al catalyst on catalytic performance and carbon/coke formation in dry reforming of CH4. Int. J. Hydrogen Energy 2023, 48, 23013–23030. [Google Scholar] [CrossRef]
- Kiani, P.; Meshksar, M.; Rahimpour, M.R. Biogas reforming over La-promoted Ni/SBA-16 catalyst for syngas production: Catalytic structure and process activity investigation. Int. J. Hydrogen Energy 2023, 48, 6262–6274. [Google Scholar] [CrossRef]
- Carrasco-Ruiz, S.; Zhang, Q.; Gandara-Loe, J.; Pastor-Perez, L.; Odriozola, J.A.; Reina, T.R.; Bobadilla, L.F. H2-rich syngas production from biogas reforming: Overcoming coking and sintering using bimetallic Ni-based catalysts. Int. J. Hydrogen Energy 2023, 48, 27907–27917. [Google Scholar] [CrossRef]
- Ponugoti, P.V.; Pathmanathan, P.; Rapolu, J.; Gomathi, A.; Janardhanan, V.M. On the stability of Ni/γ-Al2O3 catalyst and the effect of H2O and O2 during biogas reforming. Appl. Catal. A Gen. 2023, 651, 119033. [Google Scholar] [CrossRef]
- Martin-Espejo, J.L.; Merkouri, L.P.; Gandara-Loe, J.; Odriozola, J.A.; Reina, T.R.; Pastor-Perez, L. Nickel-based cerium zirconate inorganic complex structures for CO2 valorisation via dry reforming of methane. J. Environ. Sci. 2024, 140, 12–23. [Google Scholar] [CrossRef]
- Cao, A.N.T.; Nguyen, H.H.; Pham, T.P.T.; Pham, L.K.H.; Phuong, D.H.L.; Nguyen, N.A.; Vo, D.V.N.; Pham, P.T.H. Insight into the role of material basicity in the coke formation and performance of Ni/Al2O3 catalyst for the simulated-biogas dry reforming. J. Energy Inst. 2023, 108, 101252. [Google Scholar] [CrossRef]
- Veiga, S.; Romero, M.; Faccio, R.; Segobia, D.; Apesteguia, C.; Perez, A.L.; Brondino, C.D.; Bussi, J. Biogas dry reforming over Ni-La-Ti catalysts for synthesis gas production: Effects of preparation method and biogas composition. Fuel 2023, 346, 128300. [Google Scholar] [CrossRef]
- Cherbanski, R.; Kotkowski, T.; Molga, E. Thermogravimetric analysis of coking during dry reforming of methane. Int. J. Hydrogen Energy 2023, 48, 7346–7360. [Google Scholar] [CrossRef]
- Nishimura, A.; Yamada, S.; Ichii, R.; Ichikawa, M.; Hayakawa, T.; Kolhe, M.L. Hydrogen yield enhancement in biogas dry reforming with a Ni/Cr catalyst: A numerical study. Energies 2024, 17, 5421. [Google Scholar] [CrossRef]
- Jokar, S.M.; Farokhnia, A.; Tavakolian, M.; Pejman, M.; Parvasi, P.; Javanmardi, J.; Zare, F.; Clara Gonqalves, M.; Basile, A. The recent areas of applicability of palladium based membrane technologies for hydrogen production from methane and natural gas: A review. Int. J. Hydrogen Energy 2023, 48, 6451–6476. [Google Scholar] [CrossRef]
- Bang, G.; Moon, D.K.; Kang, J.H.; Han, Y.J.; Kim, K.M.; Lee, C.H. High-purity hydrogen production via a water-gas-shift reaction in a palladium-copper catalytic membrane reactor integrated with pressure swing adsorption. Chem. Eng. J. 2021, 411, 128473. [Google Scholar] [CrossRef]
- Lee, S.; Lim, H. The power of molten salt in methane dry reforming: Conceptual design with a CFD study. Chem. Eng. Process.-Process Intersif. 2021, 159, 108230. [Google Scholar] [CrossRef]
- Lee, S.; Lim, H. The effect of changing the number of membranes in methane carbon dioxide reforming: A CFD study. Int. J. Eng. Chem. 2020, 87, 110–119. [Google Scholar] [CrossRef]
- Ghasemazadeh, K.; Ghahremani, M.; Amiri, T.Y.; Basile, A. Performance evaluation of Pd-Ag membrane reactor in glyceol steam reforming process: Development of the CFD model. Int. J. Hydrogen Energy 2019, 44, 1000–1009. [Google Scholar] [CrossRef]
- Lee, B.; Lee, S.; Lim, K. Numerical modeling studies for a methane dry reforming in a membrane reactor. J. Nat. Gas. Sci. Eng. 2016, 34, 1251–1261. [Google Scholar] [CrossRef]
- Nishimura, A.; Mishima, D.; Ito, S.; Konbu, T.; Hu, E. Impact of separator thickness on relationship between temperature distribution and mass & current density distribution in single HT-PEMFC. Therm. Sci. Eng. 2023, 6, 4424. [Google Scholar] [CrossRef]
- Bian, Z.; Xia, H.; Wang, Z.; Jian, B.; Yu, Y.; Yu, K.; Zhong, W.; Kawi, S. CFD simulation of a hydrogen-permeable membrane reactor for CO2 reforming of CH4: The interplay of the reaction and hydrogen permeation. Energy Fuels 2020, 34, 12366–12378. [Google Scholar] [CrossRef]
- Anzelmo, B.; Wilcox, J.; Liguori, S. Hydrogen production via natural steam reforming in a Pd-Au membrane reactor. Comparison between methane and natural gas steam reforming reactions. J. Membr. Sci. 2018, 568, 113–120. [Google Scholar] [CrossRef]
- Bosko, M.L.; Munera, J.F.; Lombardo, E.A.; Cornaglia, L.M. Dry reforming of methane in membrane reactors using Pd and Pd-Ag composite membranes on a NaA zeolite modified porous stainless steel support. J. Membr. Sci. 2010, 364, 17–26. [Google Scholar] [CrossRef]
- Nishimura, A.; Ito, S.; Ichikawa, M.; Kolhe, M.L. Impact of thickness of Pd/Cu membrane on performance of biogas dry reforming membrane reactor utilizing Ni/Cr catalyst. Fuels 2024, 5, 439–457. [Google Scholar] [CrossRef]
- Amini, A.; Sedaghat, M.H.; Jamshidi, S.; Shariati, A.; Rahimpour, M.R. A Comprehensive CFD simulation of an industrial-scale side-fired steam methane reformer to enhance hydrogen production. Chem. Eng. Process. 2023, 184, 109269. [Google Scholar] [CrossRef]
- Bengerbaa, Y.; Virginieb, M.; Dumas, C. Computational fluid dynamics study of the dry reforming of methane over Ni/Al2O3 catalyst in membrane reactor—Coke deposition. Kinet. Catal. 2017, 58, 345–355. [Google Scholar] [CrossRef]
- Roa, F.; Way, J.D. Influence of alloy composition and membrane fabrication on the pressure dependence of the hydrogen flux of palladium-copper membranes. Ind. Eng. Chem. Res. 2003, 42, 5827–5835. [Google Scholar] [CrossRef]
- Kaviani, M.; Rezaei, M.; Alavi, S.M.; Akbari, E. Biogas dry reforming over nickel-silica sandwiched core-shell catalyst with various shell thickness. Fuel 2024, 355, 129533. [Google Scholar] [CrossRef]
- Georgiadis, A.G.; Siakavelas, G.I.; Tsiotsias, A.I.; Charisous, N.D.; Ehrhardt, B.; Wang, W.; Sebastian, V.; Hinder, S.J.; Baker, M.A.; Mascotto, S.; et al. Biogas dry reforming over Ni/LnOx-type catalysts (Ln = La, Ce, Sm or Pr). Int. J. Hydrogen Energy 2023, 48, 19953–19971. [Google Scholar] [CrossRef]
- Kumar, R.; Kumar, A.; Pal, A. Overview of hydrogen production from biogas reforming: Technological advancement. Int. J. Hydrogen Energy 2022, 47, 34831–34855. [Google Scholar] [CrossRef]
- Rosha, P.; Rosha, A.K.; Ibrahim, H.; Kumar, S. Recent advances in biogas upgrading to value added products: A review. Int. J. Hydrogen Energy 2021, 46, 21318–21337. [Google Scholar] [CrossRef]



| Initial Reaction Temperature [°C] | 400, 500, 600 |
| Pressure of gas in reactor [Pa] | 1.013 × 105 |
| Flow rate of CH4 at inlet [kg/s] (CH4:CO2 = 1.5:1) | 5.78 × 10−11 |
| Flow rate of CO2 at inlet [kg/s] (CH4:CO2 = 1.5:1) | 1.23 × 10−11 |
| Pressure at the outlet of reactor [Pa] | 1.013 × 105 |
| Density of Ni/Cr catalyst [kg/m3] | 1045, 1042, 1040 (@400 °C, 500 °C, 600 °C) |
| Porosity of Ni/Cr catalyst (εp) [-] | 0.95 |
| Permeability of Ni/Cr catalyst [m2] | 1.7 × 10−8, 1.6 × 10−8, 1.5 × 10−9 |
| Specific heat at constant pressure of Ni/Cr catalyst [J/(kg·K)] | 327, 333, 340 |
| Thermal conductivity of Ni/Cr catalyst [W/(m·K)] | 197, 194, 192 |
| Thickness of Pd/Cu membrane of 20 μm | |||||
| Initial Reaction Temperature [°C] | CH4 Conversion [%] | CO2 Conversion [%] | H2 Yield [%] | H2 Selectivity [%] | CO Selectivity [%] |
| 600 | 15.0 | 15.9 | 5.09 | 49.1 | 50.9 |
| 500 | 4.03 | 3.73 | 1.14 | 46.4 | 53.6 |
| 400 | 1.10 | 0.49 | 0.15 | 52.2 | 47.8 |
| Initial Temperature of 600 °C | |||||
| Thickness of Pd/Cu Membrane [μm] | CH4 Conversion [%] | CO2 Conversion [%] | H2 Yield [%] | H2 Selectivity [%] | CO Selectivity [%] |
| 20 | 15.0 | 15.9 | 5.09 | 49.1 | 50.9 |
| 40 | 14.2 | 15.4 | 5.02 | 49.2 | 50.8 |
| 60 | 13.5 | 15.0 | 5.00 | 49.2 | 50.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nishimura, A.; Ichii, R.; Yamada, S.; Ichikawa, M.; Hayakawa, T.; Hu, E. Numerical Analysis on Impact of Membrane Thickness and Temperature on Characteristics of Biogas Dry Reforming Membrane Reactor Using Pd/Cu Membrane and Ni/Cr Catalyst. Fuels 2025, 6, 25. https://doi.org/10.3390/fuels6020025
Nishimura A, Ichii R, Yamada S, Ichikawa M, Hayakawa T, Hu E. Numerical Analysis on Impact of Membrane Thickness and Temperature on Characteristics of Biogas Dry Reforming Membrane Reactor Using Pd/Cu Membrane and Ni/Cr Catalyst. Fuels. 2025; 6(2):25. https://doi.org/10.3390/fuels6020025
Chicago/Turabian StyleNishimura, Akira, Ryoma Ichii, Souta Yamada, Mizuki Ichikawa, Taisei Hayakawa, and Eric Hu. 2025. "Numerical Analysis on Impact of Membrane Thickness and Temperature on Characteristics of Biogas Dry Reforming Membrane Reactor Using Pd/Cu Membrane and Ni/Cr Catalyst" Fuels 6, no. 2: 25. https://doi.org/10.3390/fuels6020025
APA StyleNishimura, A., Ichii, R., Yamada, S., Ichikawa, M., Hayakawa, T., & Hu, E. (2025). Numerical Analysis on Impact of Membrane Thickness and Temperature on Characteristics of Biogas Dry Reforming Membrane Reactor Using Pd/Cu Membrane and Ni/Cr Catalyst. Fuels, 6(2), 25. https://doi.org/10.3390/fuels6020025







