1. Introduction
Due to economic and industrial expansion, the global demand for energy continues to rise. This increase has led to several impacts, particularly environmental issues, as most energy production still relies on fossil fuels. This reliance results in the release of carbon dioxide into the atmosphere, nitrogen oxides that damage the ozone layer, and sulfur dioxide that causes acid rain. These emissions contribute to climate change, affecting ecosystems and human health through the severity of global warming. Consequently, this situation has driven the promotion of biomass as a significant renewable energy source and an alternative to fossil fuels. There is an effort to use biomass alongside fossil fuel in small-scale settings or industrial furnaces, particularly in power plants where biomass is co-fired with coal [
1,
2,
3,
4].
The use of biomass as a renewable energy source has garnered attention because it is a globally available resource and does not add additional carbon dioxide to the atmosphere. During their growth process, plants can absorb up to 93% of the carbon dioxide released from biomass combustion [
5]. Moreover, co-firing biomass with coal offers several advantages. It promotes the use of agricultural waste materials available in each region, reduces waste, and adds value to these residual materials, thus stimulating the local economy. This approach decreases coal imports in some countries, enhancing energy security, reducing dependence on imports, and mitigating global energy market volatility. Utilizing local fuels also lowers transportation costs and increases local employment opportunities. Blending biomass with coal helps reduce pollution at the source by lowering the overall nitrogen content of the fuel, which directly decreases the production of nitrogen dioxide, a gas that impacts the climate and public health, during combustion. Additionally, since biomass contains significantly less sulfur than coal, it substantially reduces the formation of sulfur dioxide, a primary cause of acid rain. Lowering these emissions improves air quality and mitigates long-term environmental impacts.
Currently, four common systems are used for biomass fuel energy production: direct-fired combustion, gasification, pyrolysis, and co-firing. Each method has distinct advantages and disadvantages. The direct-fired combustion system has relatively low efficiency in converting heat to electricity, ranging from 20 to 25%. Additionally, it produces relatively high pollution levels due to incomplete combustion. However, this system is simple, requires minimal maintenance, and is stable and highly reliable. The gasification system processes biomass fuel by converting it into a gaseous form. This method has a combustion efficiency of 30–40%, which can reach up to 80% when combined with other processes. The resulting pollution is relatively low, with the main emission being higher levels of carbon monoxide. However, the system is somewhat unstable, requiring significant maintenance and precise control of fuel size; if the fuel is too small, air cannot flow properly, and if it is too large, it may not fully combust. The pyrolysis system involves the thermal decomposition of biomass under low-oxygen conditions. It is similar to gasification but produces three types of fuel in different states: char, bio-oil, and biogas. The advantage is that it yields three forms of fuel of higher quality than raw biomass. However, the process is complex, requiring a good understanding from the operators, and the fuels produced by pyrolysis need further refinement before use. The final system is co-firing, where biomass fuel is mixed with other fossil fuels, such as coal. This is an appropriate option because coal is currently burned in a pulverized form, with fuel particles smaller than 1 mm, allowing for better homogeneity in mixing. Additionally, it helps maintain flame stability by using swirl-induced air injection [
6,
7,
8,
9,
10,
11,
12,
13,
14,
15,
16]. This method also allows for better control of thermal load compared to conventional technologies.
Studies on pulverized co-firing highlight its high potential and feasibility because it can be used alongside existing coal, a solid fuel with similar combustion characteristics. This compatibility minimizes the need for extensive modifications to the combustion chamber and allows the use of basic equipment designed for coal combustion without significant infrastructure investment. Therefore, this method is an efficient alternative that can be quickly implemented in the industry.
Although co-firing offers many benefits by combining biomass with coal, it still faces several challenges, such as improving combustion efficiency, controlling emissions, and maintaining high stability and performance during combustion. Since biomass and coal have different physical and chemical properties, co-firing requires the specific analysis and design of combustion conditions to effectively address these differences. Testing co-firing in furnaces requires substantial resources, such as large-scale facilities, because solid fuel combustion involves larger machinery compared to other fuel types. Additionally, the high cost of operators and the extended duration of tests make it challenging to explore all variables related to combustion comprehensively. To address these issues, mathematical modeling (simulation) has become a popular method for reducing resource use in testing. Simulations offer the advantage of a detailed representation of phenomena occurring in the combustion chamber, allowing for more in-depth analysis compared to tests limited by equipment constraints.
This study uses numerical simulations to analyze and predict the behavior of co-firing between coal and biomass, including emissions. Mathematical modeling allows for a detailed examination of combustion at the microscopic level and enables the adjustment of various parameters to find optimal conditions for co-firing. Additionally, simulations reduce costs and risks associated with experiments. Practical studies of co-firing often face challenges such as high costs, difficulty in controlling experimental conditions, and the complexity of the combustion process. Therefore, simulation is an effective and cost-efficient method for analyzing and optimizing co-firing processes. It allows for the precise testing of factors such as temperature, pressure, airflow, and fuel composition, and helps develop strategies to improve combustion efficiency and reduce emissions. However, creating accurate models requires substantial data to ensure precision.
Research in biomass and coal co-firing systems has revealed various insights into combustion characteristics and emission controls. Several studies have focused on analyzing biomass combustion at the microscopic particle level to investigate the associated combustion characteristics and kinetic parameters [
17,
18,
19,
20].
Ballester et al. (2005) [
21] conducted combustion experiments in a pilot-scale pulverized fuel furnace using bituminous coal, lignite coal, and oak wood. This study investigated the effects of fuel differences on combustion characteristics, such as flame shape, temperature distribution, and combustion gas composition, including O
2, CO, NO
x, and unburned carbon. Findings indicated that higher volatile content, particularly in lignite and biomass, led to more intense combustion and longer flame lengths compared to coal.
Ndibe et al. (2015) [
22] analyzed the combustion of 100% pulverized coal, 100% torrefied biomass, and a 1:1 mixture of torrefied biomass and coal in a 500 kW pulverized fuel furnace. With secondary air heated to 195 °C, the study concluded that torrefied biomass addition significantly reduced NO
x and SO
x emissions.
Traditional combustion systems designed for coal face challenges in burning 100% biomass due to differing internal properties. Biomass requires more time to evaporate its moisture and has a lower heating value of volatiles compared to coal, causing flame stability issues. Additionally, biomass combustion occurs more slowly due to larger particle sizes and slower reaction rates, limiting the feasibility of completely replacing coal with biomass in industrial settings.
Wang et al. (2015) [
23] used a drop-tube furnace to investigate coal and biomass co-firing. Their results demonstrated satisfactory performance and NO
x emissions at a co-firing ratio of 0.4 biomass to coal. Aziz et al. (2016) [
24] employed computational fluid dynamics (CFD) to study the combustion behavior of pulverized coal mixed with biomass in power plants, finding that up to 15% biomass could be added without adversely affecting temperature distribution within the combustion chamber.
CFD has been extensively used in researching pulverized firing systems. Ma et al. (2007) [
25] conducted simulations of biomass combustion in an existing pulverized coal furnace, finding close agreement between simulation results and experimental data. Yin et al. (2012), Yin et al. (2012), and Li et al. (2013) [
26] further explored the combustion characteristics of pulverized coal and biomass, revealing differences in flame characteristics and the effects of drag force and oxygen concentration on the devolatilization process and flame characteristics.
Elfasakhany et al. (2013) [
27] and Elorf and Sarh (2019) [
28] studied the combustion behavior of finely ground biomass using experimental and CFD modeling approaches. Findings indicated that higher volatile release leads to increased hydrocarbons and carbon monoxide concentrations. Adjusting combustion variables can improve burner performance to achieve a similar efficiency to coal combustion.
The combustion of biomass fuel in pulverized form has high potential for replacing existing or co-fire with existing coal fuels. In traditional burner designs, fuel is introduced into the combustion chamber by injecting air through a swirler, which helps disperse the fuel and enhance combustion [
29,
30]. However, biomass differs from coal in terms of ignition and combustion characteristics due to its fixed carbon content. Therefore, additional adjustments to the combustion variables are necessary to improve the performance of burners and achieve similar efficiency, as with coal.
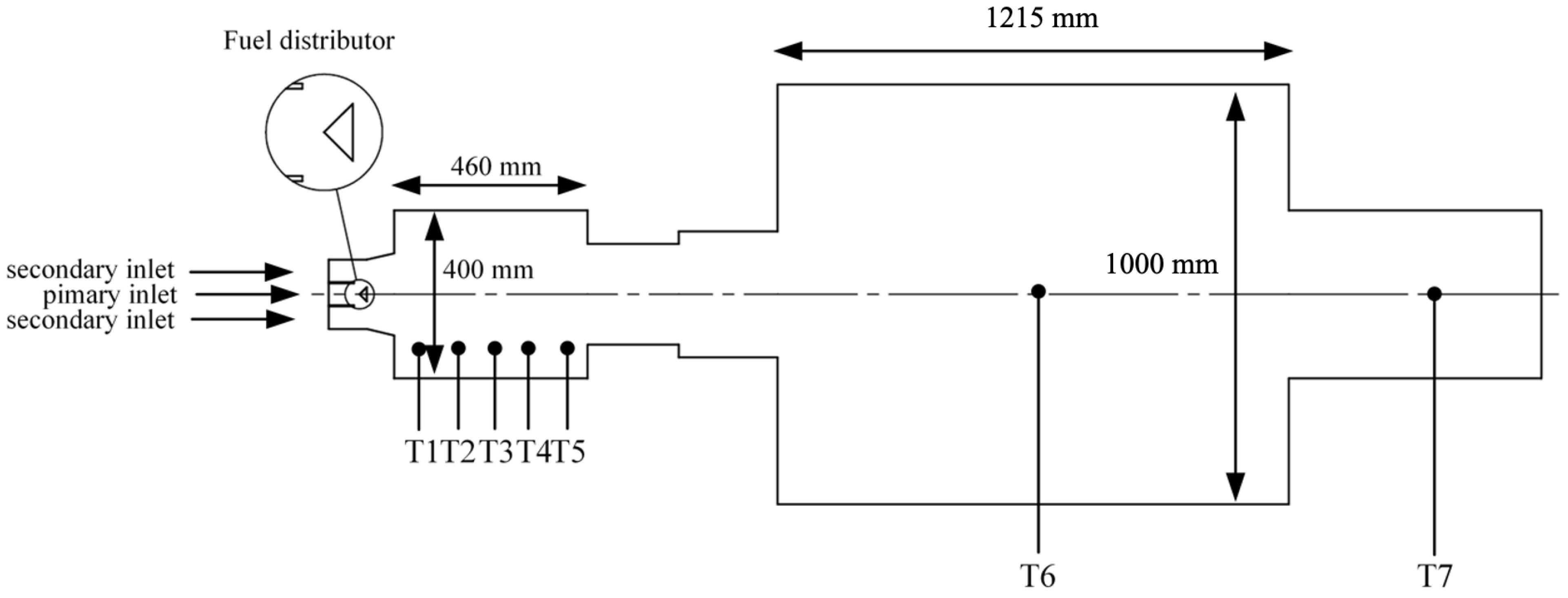
Previous research has explored co-firing biomass with coal at low proportions, typically not exceeding 25% by mass, with the aim of avoiding adverse effects on temperature distribution and combustion efficiency. However, these studies are insufficient for a future scenario in which we aim to use more renewable fuel sources to promote clean energy use. Therefore, it is essential to thoroughly understand the co-combustion behavior of coal and biomass. In this research, we utilized computational modeling to simulate the co-firing process within a furnace. This model uses experimental data and relevant databases to create realistic and accurate combustion conditions. The simulation allows us to quickly and efficiently test and optimize various parameters without the need for extensive physical experiments. Consequently, the research team designed a study on co-firing coal and biomass by conducting tests in a 500 kW heat input pulverized fuel furnace with a two-chamber design. Data were collected on fuel usage, air consumption, combustion temperatures in different areas of the furnace, and exhaust gas composition. These data were used to develop a model to study the combustion behavior and emissions when varying the ratios of coal and biomass.
3. Numerical Model
The Fluent 2020 R1 software was used to perform three-dimensional finite volume simulations using computational fluid dynamics (CFD), a sophisticated tool for analyzing fluid flow, heat transfer, and related phenomena through numerical solution of governing equations. To accurately model combustion processes, the discrete phase model (DPM) was employed. This model effectively tracks particle trajectories and simulates their interactions with the gas phase. Furthermore, chemical reactions involved in combustion were simulated using the species transport model, which incorporates an eddy dissipation-concept (EDM). This sub-model is crucial for capturing the complex interactions between turbulence and chemical reactions, ensuring a realistic simulation of the combustion process.
For pressure–velocity coupling, which is a crucial aspect of fluid flow simulations, a coupled algorithm was employed. This approach enhances the stability and accuracy of the simulation by solving the pressure and velocity fields simultaneously. To further improve the precision of the simulations, a second-order upwind scheme was used for spatial discretization. This scheme helps in reducing numerical diffusion and improving the accuracy of the gradient calculations.
Moreover, the pressure solver in Fluent was set to use the PRESTO! (Pressure Staggering Option) algorithm, which is particularly effective for handling high-speed flows and flows with strong pressure gradients. Convergence criteria were rigorously set to ensure the reliability of the simulation results. Specifically, residuals were required to fall below 10−3 for most equations, ensuring that the numerical solution had stabilized. For the energy equation, as well as for pollutants and radiation, stricter convergence criteria of 10−5 were applied, reflecting the importance of accurately capturing these aspects of the combustion process.
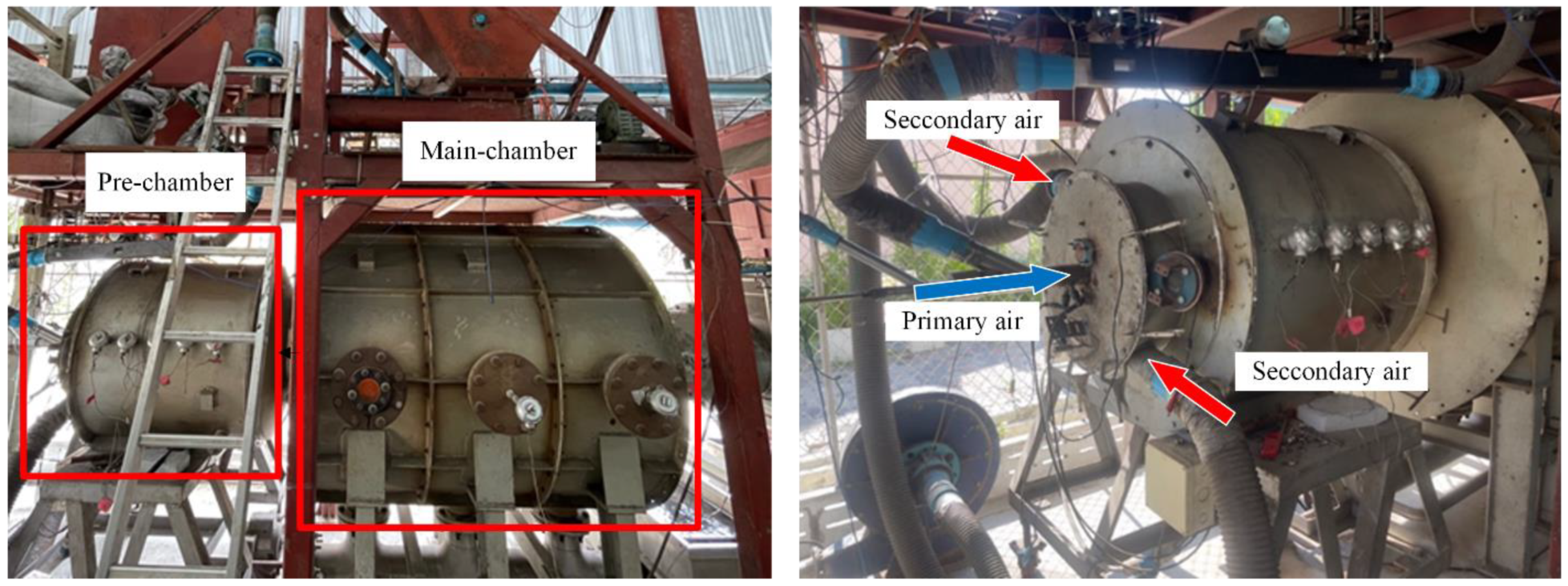
3.1. Grid and Mesh Sensitivity
Figure 4 depicts the three-dimensional domain of the flow and showcases the polyhedral elements employed in the mesh. To ensure accurate simulation results, a mesh sensitivity analysis was conducted to optimize element sizes. The domain was divided into two distinct parts based on their characteristics: the pre-chamber, extending up to a radius of 0.7 m from the inlet, utilized smaller 10 mm elements. This choice was driven by the turbulent and reactive nature of this critical region, requiring finer resolution for precise modeling of fluid dynamics and combustion processes. In contrast, the main chamber employed larger 20 mm elements to balance computational efficiency while maintaining accuracy. Overall, the simulation utilized approximately 957,000 elements, strategically distributed to capture the complex interactions within the furnace and provide reliable insights into its operational dynamics.
3.2. Mathematics Models
3.2.1. Governing Equation
The equations of the continuous phase are governed by the conservation laws for mass, momentum, energy, and species concentration. For steady turbulent flow, these equations, when time-averaged for the gas phase, describe incompressible and turbulent flow behavior that can be expressed as [
31]
where
is the fluid phase density,
is the Cartesian velocity,
is the dependent variable,
is the diffusion coefficient, and
is source term.
3.2.2. Turbulent Model
Turbulent fluid flow is typically modeled by solving the steady-state Reynolds-averaged Navier–Stokes (RANS) equations, as described by Deniz et al. (2015) [
32]. This approach involves using a turbulent viscosity-based turbulence model, specifically the realizable k-ε model, which is enhanced by standard wall functions to accurately capture near-wall turbulence. Laphirattanakul et al. (2020) [
33] found this model to be particularly suitable for complex flow scenarios, such as recirculating and swirling flows, providing reliable and accurate predictions for these challenging conditions.
3.2.3. Particles Transport Model
In this modeling approach, the two-phase flow was evaluated using the Eulerian–Lagrangian method, as detailed by Li et al. (2012) [
3]. The gas phase equations are solved within the Eulerian domain, while particle motion is treated within a Lagrangian framework, as described by Tabet et al. (2015) [
34]. The discrete phase model (DPM) is employed to track the motion of fuel particles. This model determines the trajectories of the fuel particles by considering various forces acting on them, primarily gravitational and drag forces [
3]. These forces significantly influence particle motion, leading to complex interaction patterns within the flow. The comprehensive force balance on each particle as it moves through the domain is mathematically represented in Equation (3). This method allows for a detailed analysis of the dynamic behavior of particles in the flow, providing insights into their interactions and the overall flow characteristics.
where
is the particle mass,
are forces acting on the particle,
is the translation velocity vector of the particle center of mass,
is the dynamics viscosity,
is the particle density,
is the drag coefficient,
is the relative Reynold number, and
is the gravity vector [
34].
3.2.4. Devolatilization Model
After the drying process, devolatilization occurs at around 200–300 °C. The constant rate model, as used by Backreedy et al. (2004) [
35], is applied to predict the volatile yield rate during this phase. This model assumes that volatiles are released at a constant rate, which is mathematically represented by Equation (5). By using this model, the number of volatiles generated during devolatilization can be accurately predicted, providing essential insights into the behavior of the material under thermal decomposition conditions
where
is the fraction of volatile initially present in the particle,
is the initial particle mass, and
is the rate constant (default value is 50 s
−1, 20 s
−1 for coal and biomass, respectively).
3.2.5. Combustion Model
The combustion model consists of two essential processes: volatile combustion and char oxidation. During the volatilization process, the volatile components within the fuel particle are released into the surrounding environment. These volatile gases mix with air and are combusted. For this work, the eddy dissipation model (EDM) was utilized to predict homogeneous reactions, accounting for chemical turbulence interactions, as described by Suksam et al. (2019) [
36] and Laphirattanakul et al. (2020) [
33]. Additionally, a two-step global reaction mechanism was adopted for gas phase combustion.
The char combustion process begins once the volatile components of the particles are fully released. This reaction occurs at the particle’s surface, consuming its combustible fraction until it is entirely depleted, as detailed by Backreedy et al. (2004) [
35]. The process was evaluated using the diffusion-limited surface reaction rate model, according to Laphirattanakul et al. (2020) [
33]. In this model, char is assumed to oxidize to carbon monoxide through a one-step heterogeneous surface reaction.
3.2.6. Radiation Mode
A radiation model was used to evaluate radiative heat transfer in the combustion chamber. The Discrete Ordinate Method (DOM), as described by Fernando (2005) [
37], was employed alongside the absorption coefficient weight-sum-of-gray-gas model (WSGGM) to calculate the radiative heat transfer of the gas mixture within the domain.
3.2.7. NOx Model
The nitric oxide (NO
x) model consists of NO
x formation from thermal NO
x, prompt NO
x, and fuel NO
x. Thermal NO
x is formed by the oxidation of nitrogen in the air during combustion at relatively high temperatures. The formation of thermal NO
x is determined by the extended Zeldovich mechanism [
3]. Fuel NO
x is formed by a reaction with the nitrogen content in the fuel. The nitrogen in the fuel is usually released as HCN and NH
3, accompanied by a volatile release that is eventually either oxidized to NO or reduced to N
2. In this work, it is assumed that the nitrogen contained in the char is directly converted to NO, since the conversion mechanisms are complex [
3]. Lastly, prompt NO
x, which is formed between OH radicals and nitrogen molecules near the flame, was also considered.
3.2.8. SOx Model
The calculation of SO
2 formation begins from the fundamental theory of mass conservation, where the sulfur component in the fuel is entirely converted to SO
2. The effect of residence time in SO
x mechanisms, a Lagrangian reference frame concept, is included through the convection terms in the governing equations written in the Eulerian reference frame [
38].
4. Experiment Results
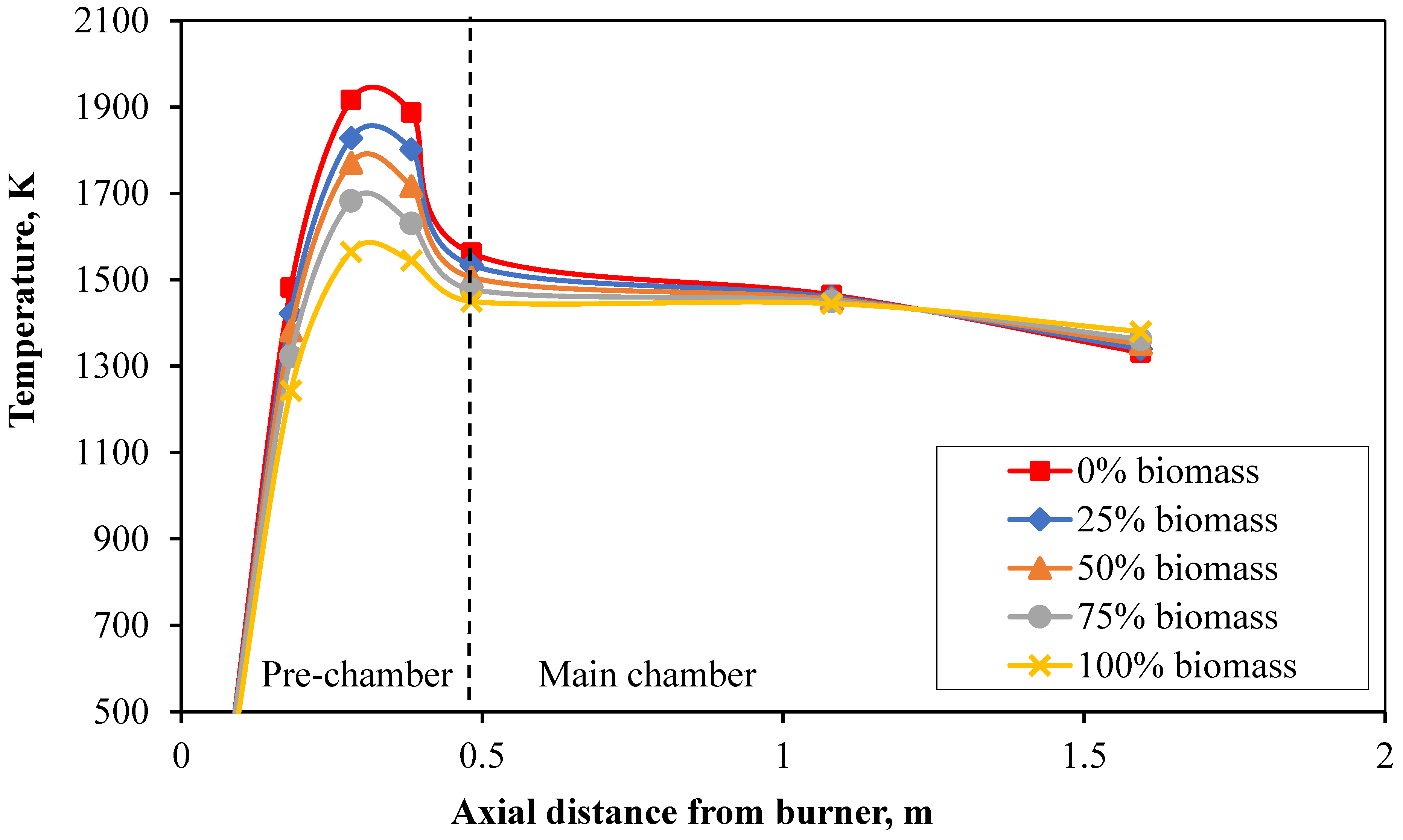
From the combustion testing of coal and biomass in a 500 kW pulverized fuel burner with two combustion chambers, the measurement data from the experiments, as shown in
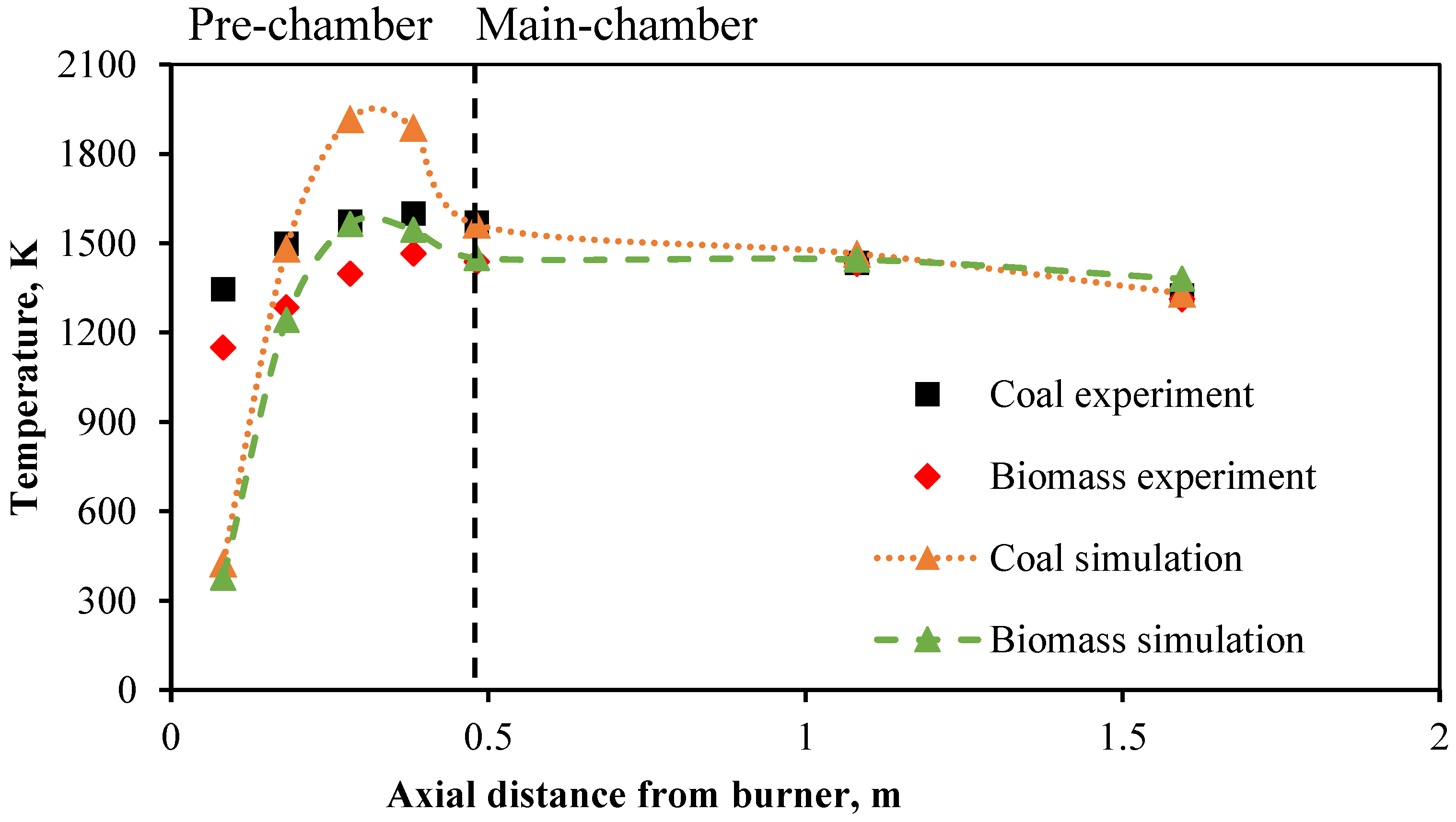
Table 2, indicate that the temperature profiles inside the combustion chamber, illustrated in
Figure 5, exhibit similar behavior.
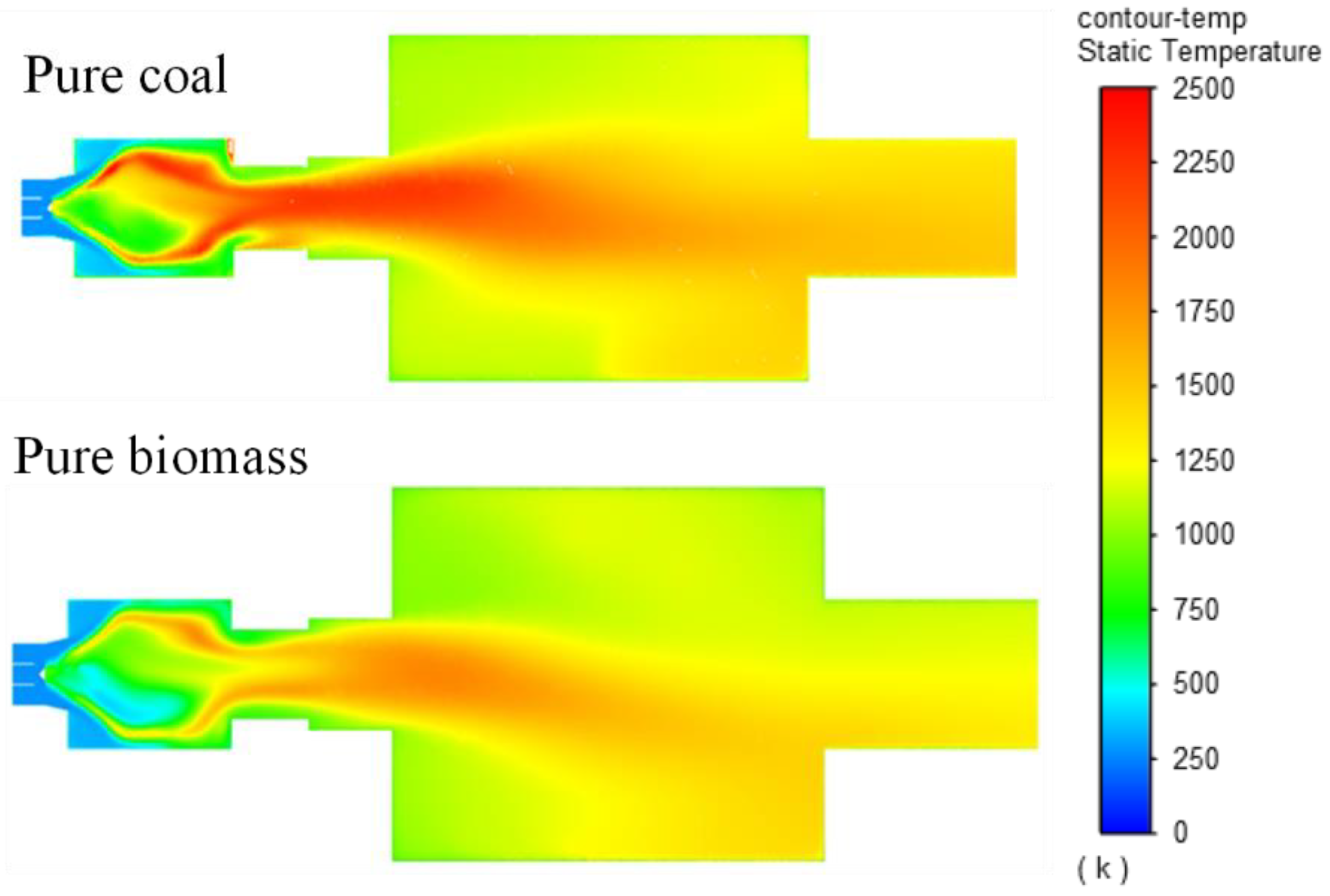
The temperature increases notably in the pre-chamber area, with pulverized coal heating up faster, reaching a maximum temperature of 1600 K compared to biomass at 1465 K upon entering the main chamber. Both temperatures decrease slightly as they exit the combustion chamber, reaching final temperatures of 1323 K for coal and 1312 K for biomass. This shows that their temperatures are closely aligned, unlike the initial stages where there was a significant difference. This is because biomass has slower combustion due to larger fuel particle size and lower devolatilization rate. However, by the end of the combustion process, sufficient time allows the temperatures to converge. The average combustion temperatures are 1478 K for pure coal and 1353 K for pure biomass. In summary, coal burns at an average temperature 9.1% higher than biomass.
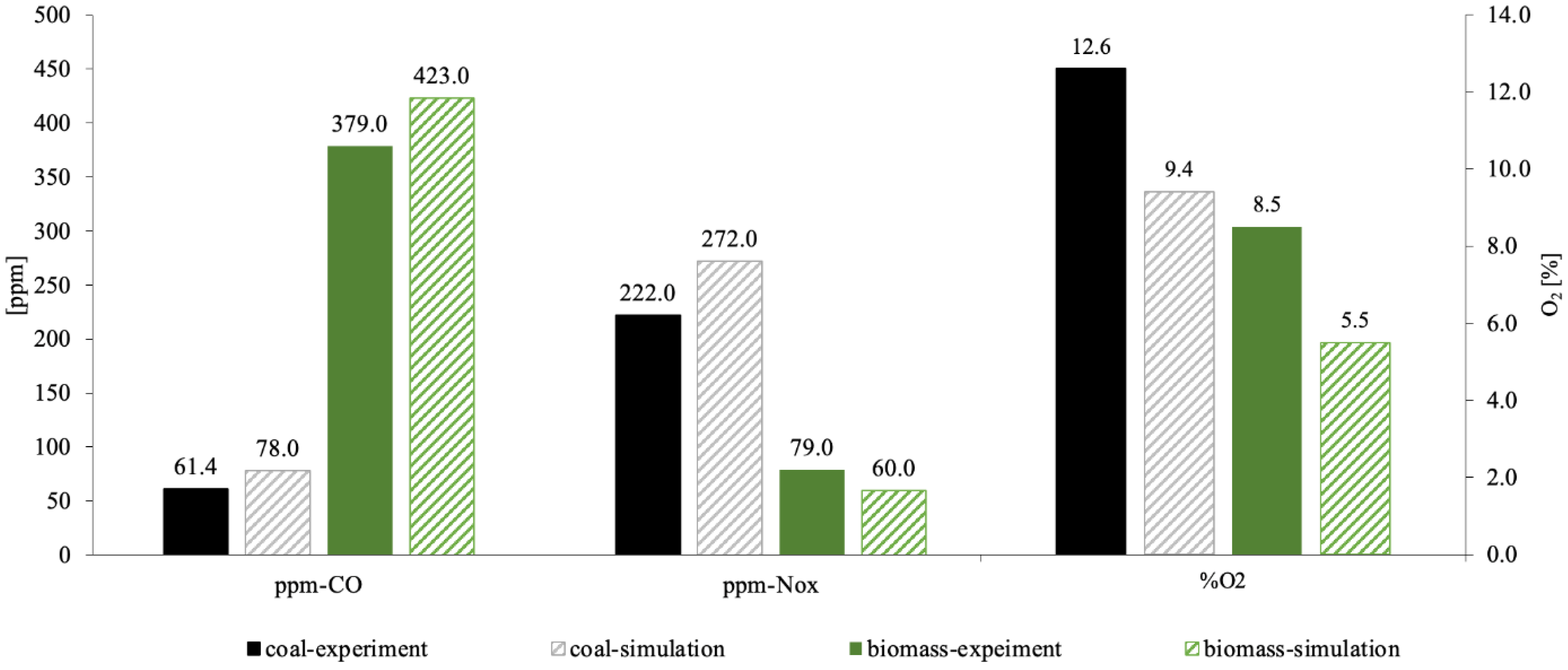
The experiment involved measuring the concentration of exhaust components, as shown in
Table 2 and
Figure 6. Combustion tests revealed that the carbon dioxide concentration at the outlet was 61.4 ppm for coal, compared to 379.0 ppm for pure biomass, an increase of more than 5.2 times, as illustrated in
Figure 6. Additionally, it was found that NO
x emissions were 221.9 ppm from burning coal and 79 ppm from biomass, indicating an increase of more than 1.8 times. Oxygen is critically important in combustion processes. In the initial testing, the air-to-fuel ratio was set at an equivalence ratio of 1.1. As the combustion process progressed, the oxygen concentration at the outlet of the combustion furnace was 8.5%vol for biomass and 12.6%vol for coal, which is less by 32%. The reasons for this behavior will be discussed in the
Section 7.
9. Conclusions
The combustion of pulverized coal and biomass co-firing at 500 kW thermal throughput with 10% excess air revealed several key effects of increasing the thermal fraction of biomass in fuel. Through a combination of modeling and experimental testing, it was determined that increasing the proportion of thermal shearing of biomass in the fuel influences various aspects of combustion behavior, as summarized below.
Model Validation and Combustion Behavior: The 3D CFD model of co-firing pulverized coal and biomass aligns well with the experimental data, demonstrating accurate predictions of combustion behavior and exhaust gas composition. Some discrepancies in temperature distribution were noted, but overall results were satisfactory.
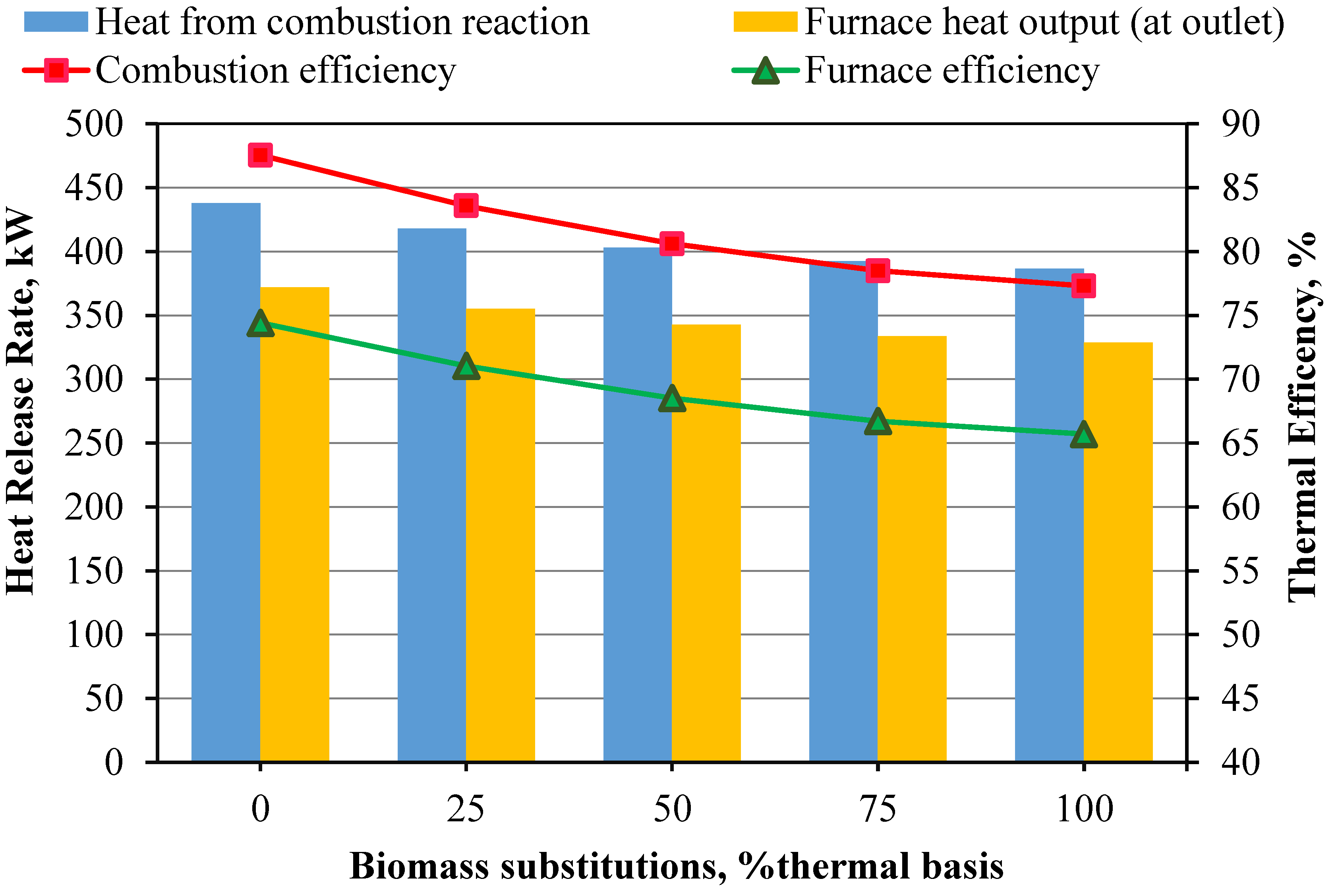
Temperature and Emissions Trends: Increasing the biomass proportion from 0% to 100% significantly lowers the average combustion temperature from 1438 K to 1289 K. This reduction is due to biomass’s higher moisture content and lower heating value. Concurrently, NOx emissions decrease by up to 77.9% and SO2 emissions drop to zero with higher biomass content.
Combustion Completeness and Efficiency: The completeness of combustion declines as biomass content increases, evidenced by higher CO and CO2 emissions. The combustion efficiency decreases from 87.7% with coal to 77.3% with biomass, due to larger biomass particle size and incomplete combustion.
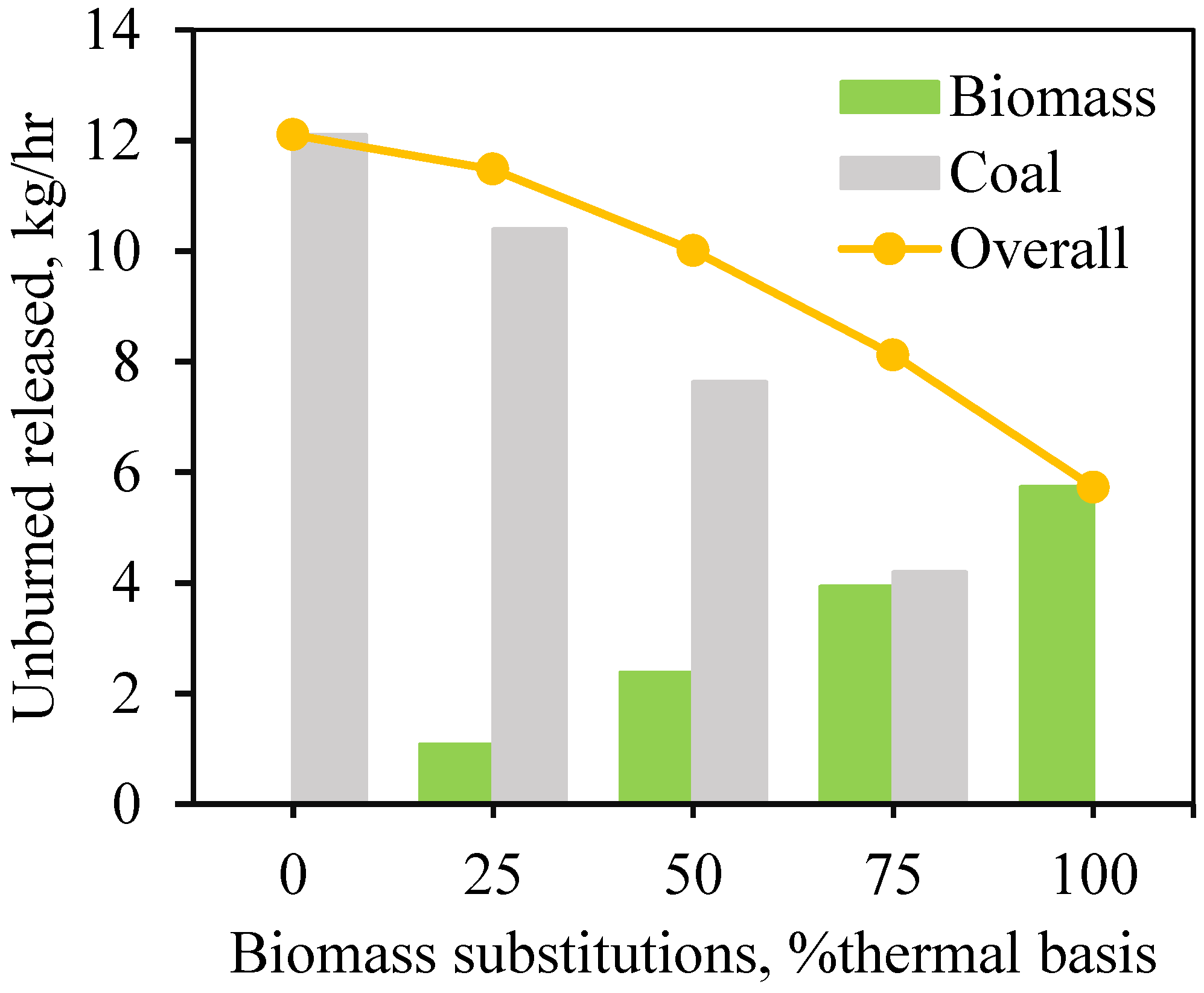
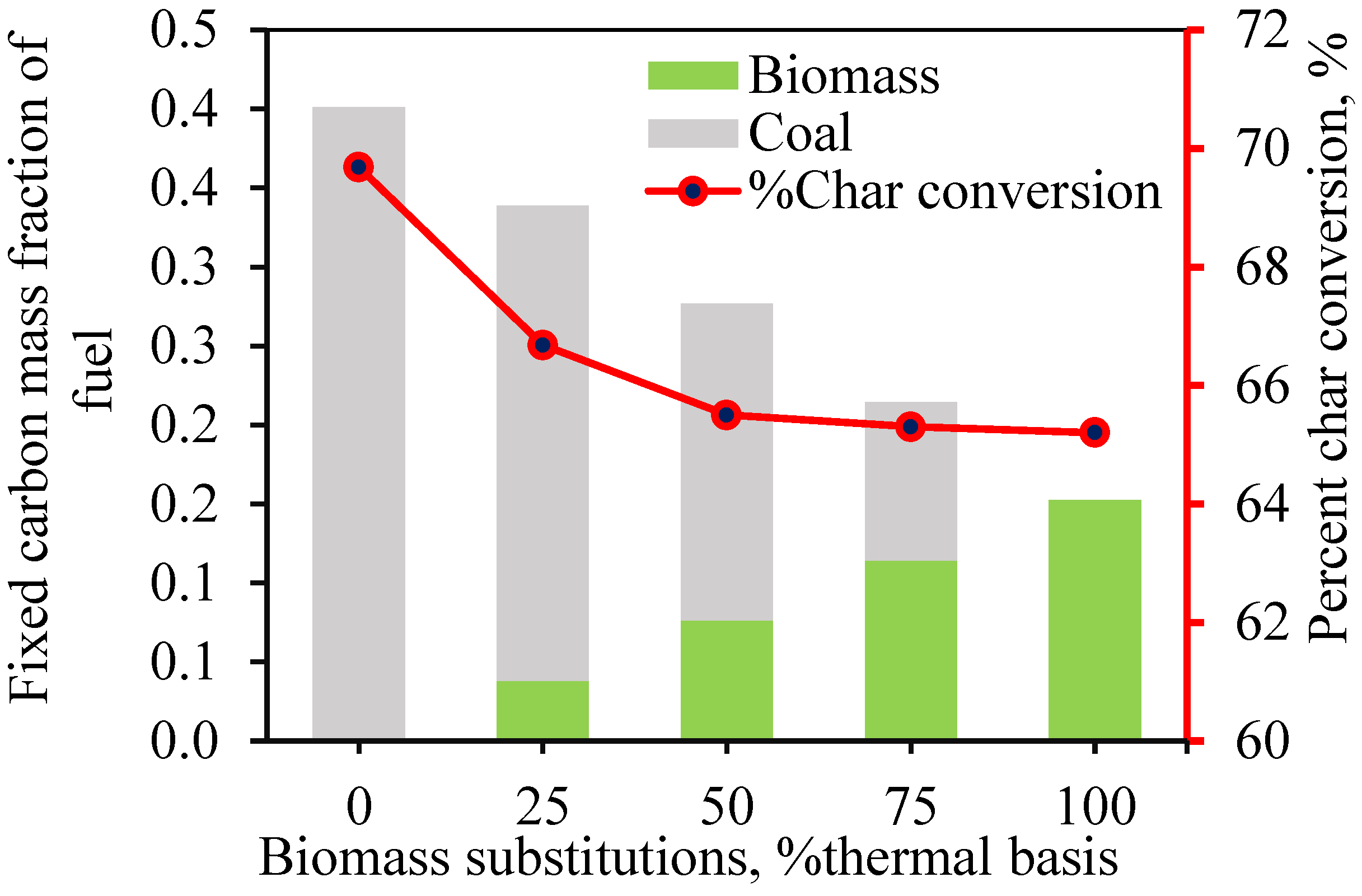
Oxygen and Carbon Dynamics: The oxygen content in exhaust gases decreases from 9.5% to 5.5% as biomass proportion increases due to higher CO levels from incomplete combustion. Biomass releases less unburned carbon compared to coal, but coal has a higher char conversion rate (69.7% vs. 65.2%).
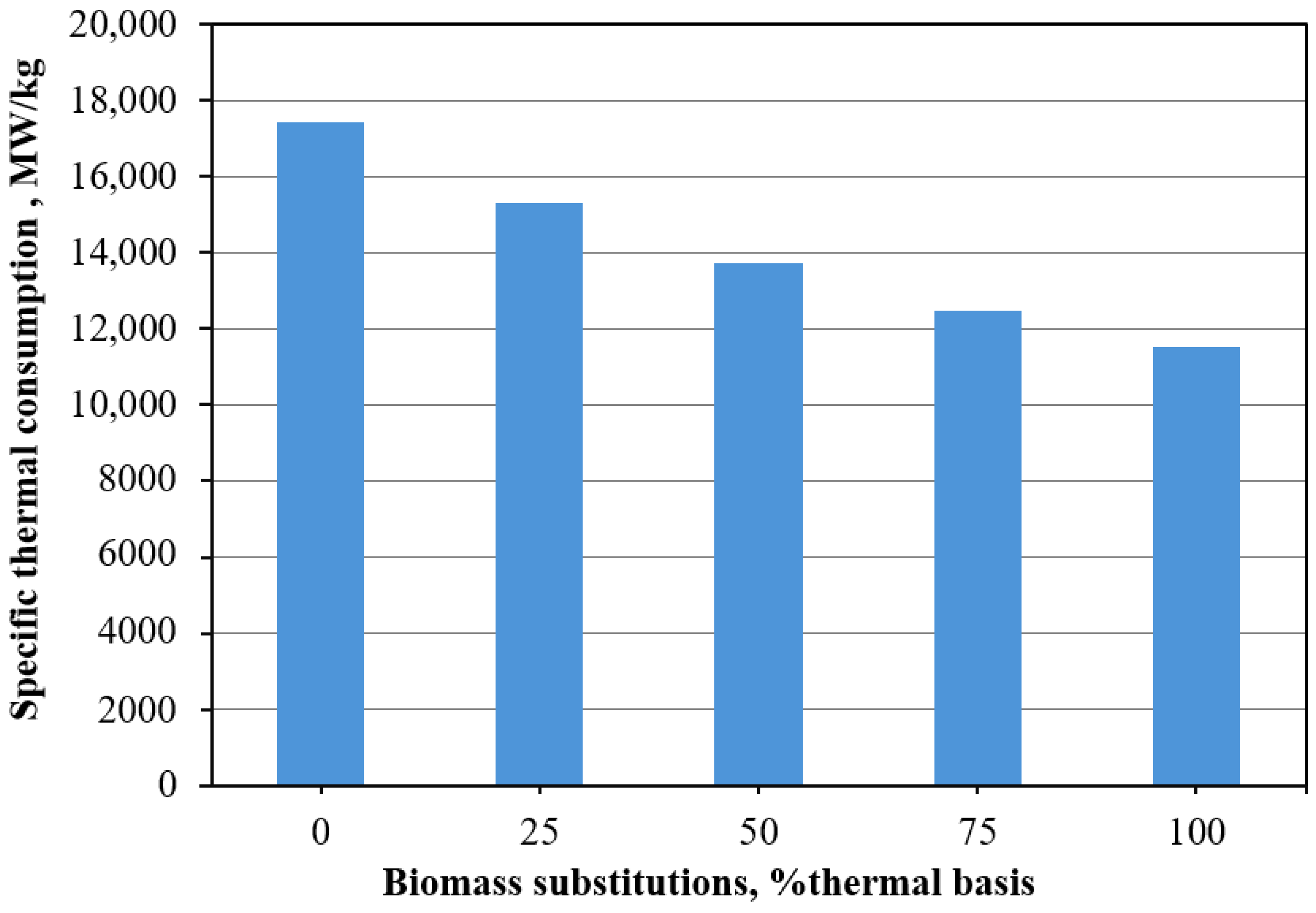
Environmental and Cost Implications: Biomass combustion does not increase atmospheric CO2, potentially reducing CO2 emissions by up to 148.8 gCO2/MWhth compared to coal. Although biomass combustion is 11.8% less efficient, the increased fuel cost can be offset by carbon credits. It is noteworthy that Thailand has not yet started implementing a carbon tax, but there are plans to begin in 2025 with a proposed rate of 200 baht per ton of carbon dioxide emissions. The Thai Ministry of Finance has mandated a study within the year to develop a carbon tax policy that fits the national context and considers approaches from other countries as well. The introduction of this carbon tax aims to encourage businesses to accelerate the use of clean energy and environmentally friendly technologies, helping to reduce the costs associated with importing fuels and supporting the country’s environmental goals. If implemented, this push could significantly enhance the competitiveness of biomass as a fuel alternative, benefiting from lower fuel costs compared to fossil fuels.
In the study, it was found that biomass can be co-fired with coal as an option to reduce energy usage from fossil fuels. Biomass has several advantages over other clean energy alternatives, such as reducing agricultural waste that would otherwise need to be disposed of by landfilling, which would generate methane, a greenhouse gas, or by open burning, which would cause air pollution and particulate emissions. Biomass offers a more stable energy supply compared to solar and wind energy, which depend on weather conditions, has lower costs and greater readiness than hydrogen technology, and does not involve the radiation risks associated with nuclear energy.
With all of the above-mentioned conclusions, it is clear this research has high potential for application in other countries, especially those with biomass resources and coal infrastructure. The methods can be adapted to various types of biomasses, according to regional characteristics, through adjustments in model parameters such as calorific values, fuel composition, particle size, and moisture content. It serves as a tool to meet greenhouse gas (GHG) emission reduction targets under the Paris Agreement. Co-firing biomass can be performed without significant modifications to existing coal-burning equipment, making it suitable for countries with financial and technical resource limitations and allowing for rapid implementation. The research also presents a CFD model that is transferable and easily adapted to local contexts, reducing the cost of physical trials. It provides a pathway for scaling from experimentation to industrial application, where countries reliant on coal burning can reduce pollution and comply with environmental regulations effectively, making biomass co-firing a key technology for sustainable energy production globally. To ensure the credibility of this research and its readiness for industrial application, it is necessary to undertake further studies to resolve potential issues, enhance efficiency, and confirm the scalability of biomass co-firing in actual industrial settings. Full-scale industrial trials will verify the scalability and identify potential operational challenges. Studies on ash behavior and slagging will help address fouling issues and optimize combustion conditions. Moisture management will enhance combustion efficiency through biomass preparation processes such as drying or pelletization. Logistics and supply chain analysis are crucial for evaluating the economic feasibility of biomass supply. Health, safety, and environmental impact assessments will help manage particle emissions and ensure workplace safety. Economic feasibility studies will provide financial models and return on investment data for stakeholders. Studies on system flexibility will ensure efficient operation under varying conditions. Life Cycle Assessment (LCA) will evaluate the environmental impact, support sustainability, and integration with new technologies like carbon capture and storage (CCS) will improve system efficiency. Long-term reliability studies will guide maintenance scheduling and material selection, while policy and regulatory alignment will ensure compliance with environmental standards and take advantage of financial incentives, creating a sustainable and widely applicable operational framework for biomass co-firing technology in the industry.