Development and Characterization of κ-Carrageenan and Boron Nitride Nanoparticle Membranes for Improved Ionic Conductivity in Fuel Cells
Abstract
1. Introduction
2. Materials and Methods
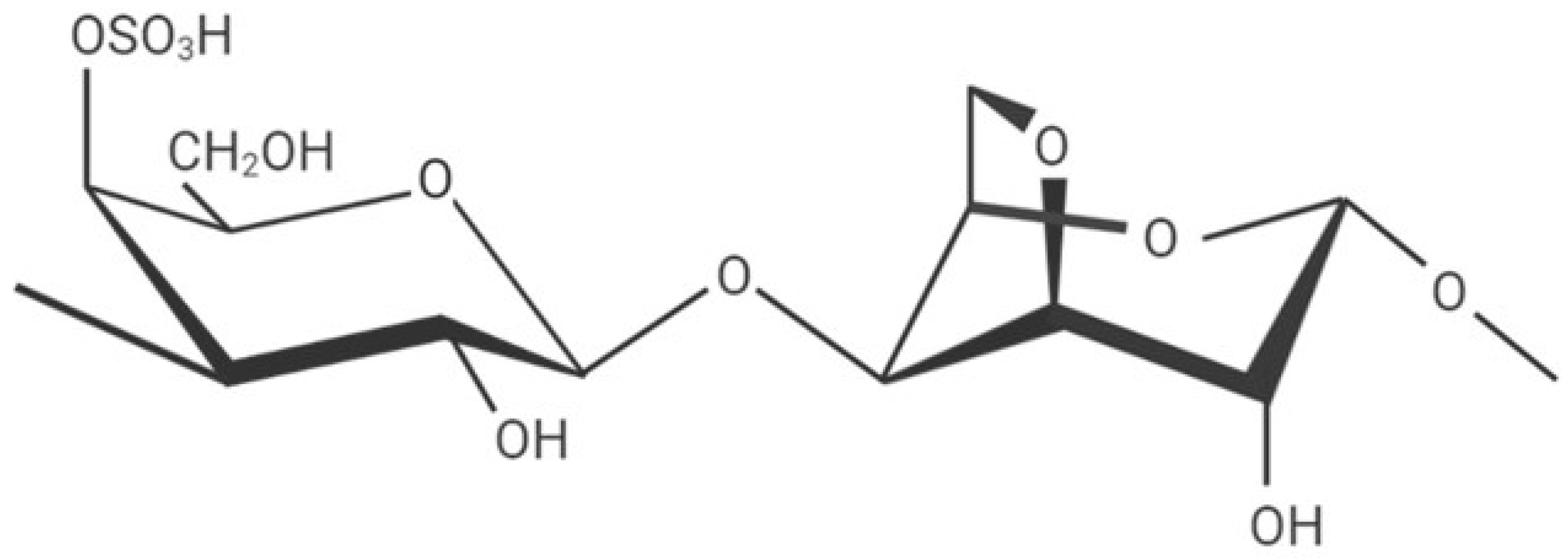
2.1. Synthesis of Samples
2.2. Differential Scanning Calorimetry (DSC)
2.3. Thermogravimetric Analysis (TGA)
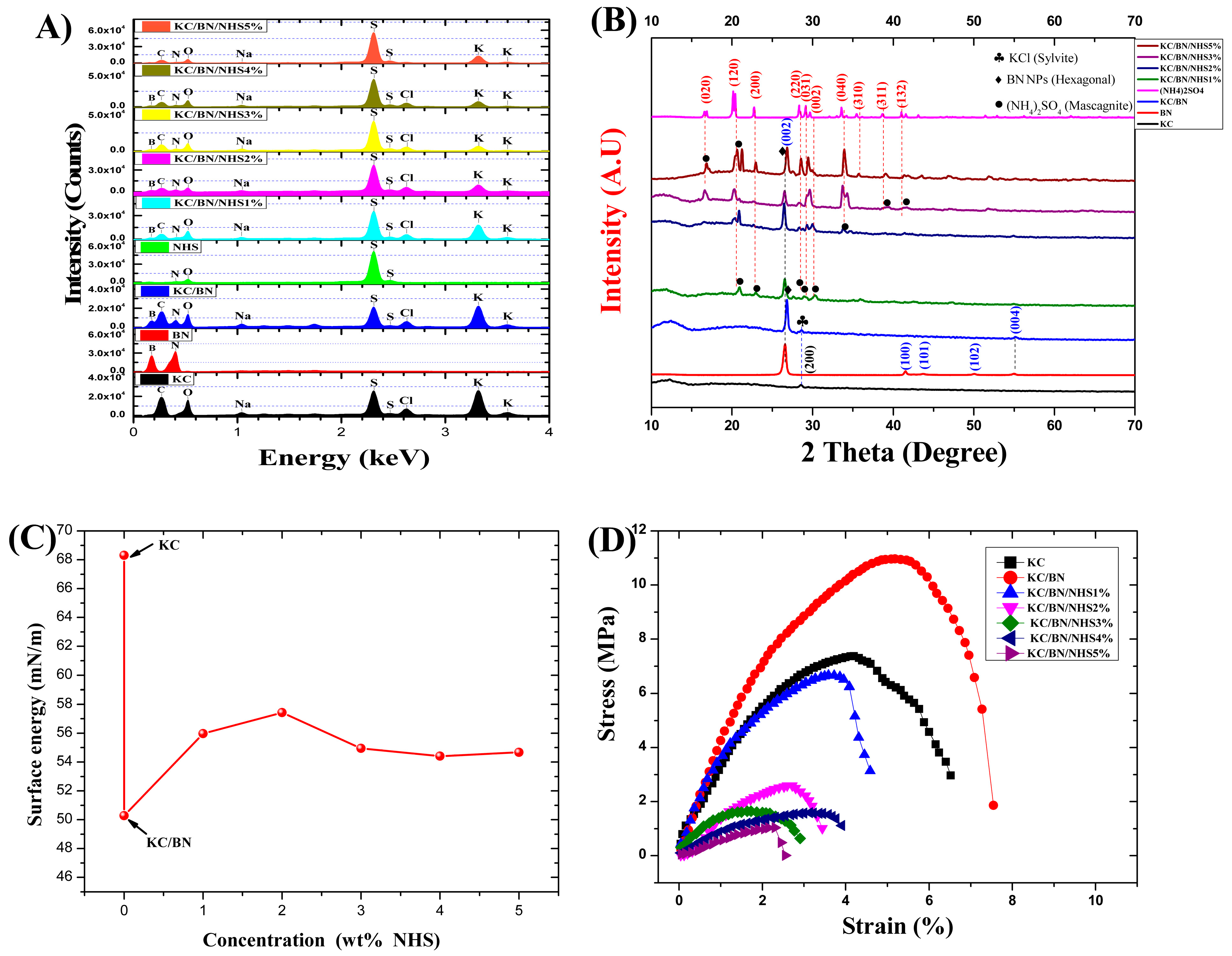
2.4. X-Ray Diffraction (XRD)
2.5. Fourier Transform Infrared Spectroscopy (FTIR)
2.6. Impedance Spectroscopy (IS)
2.7. Scanning Electron Microscopy (SEM) with Energy-Dispersive X-Ray Spectroscopy (EDS)
2.8. Mechanical Properties
2.9. Hydrophilicity
3. Results and Discussion
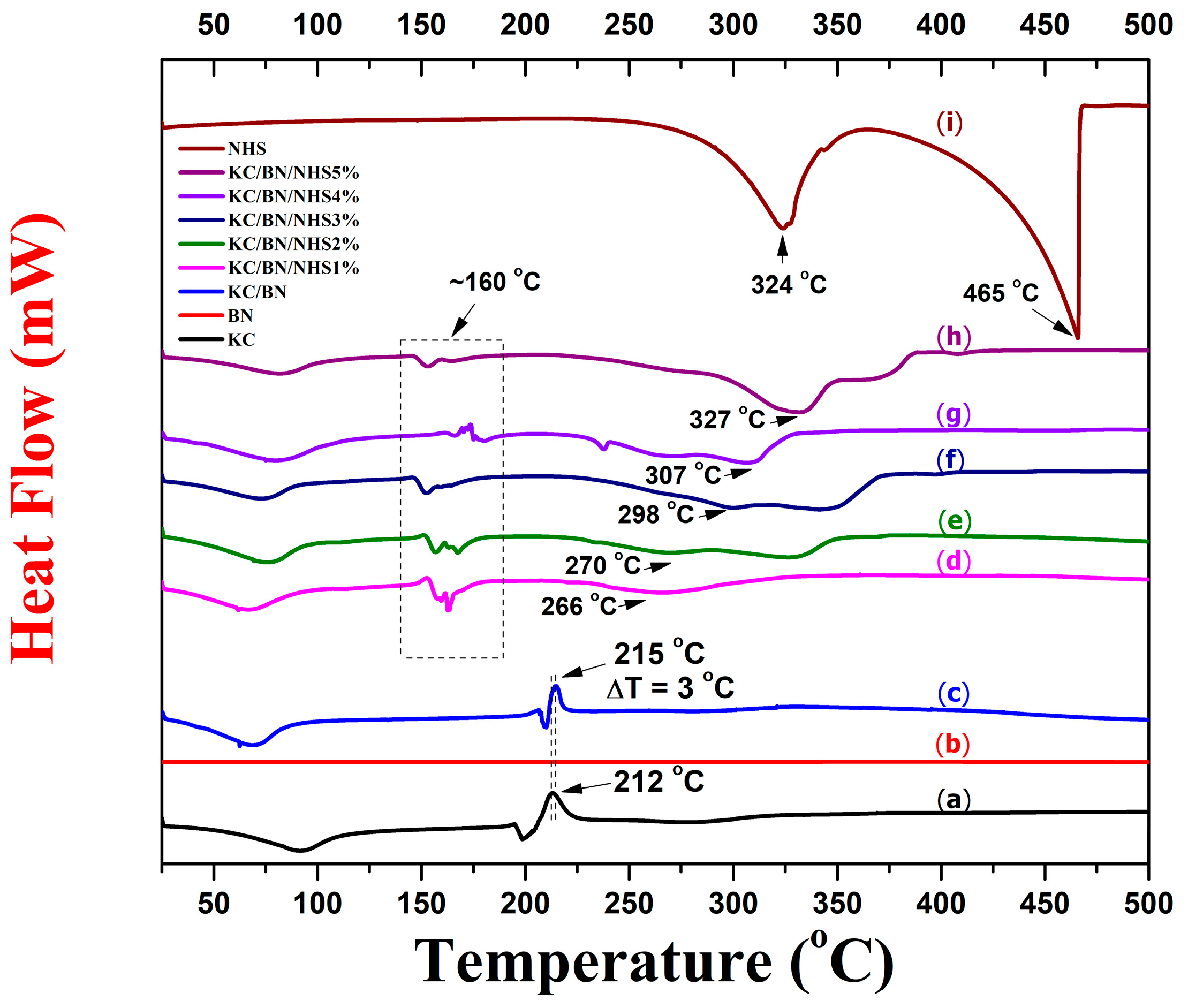
3.1. Thermal Stability
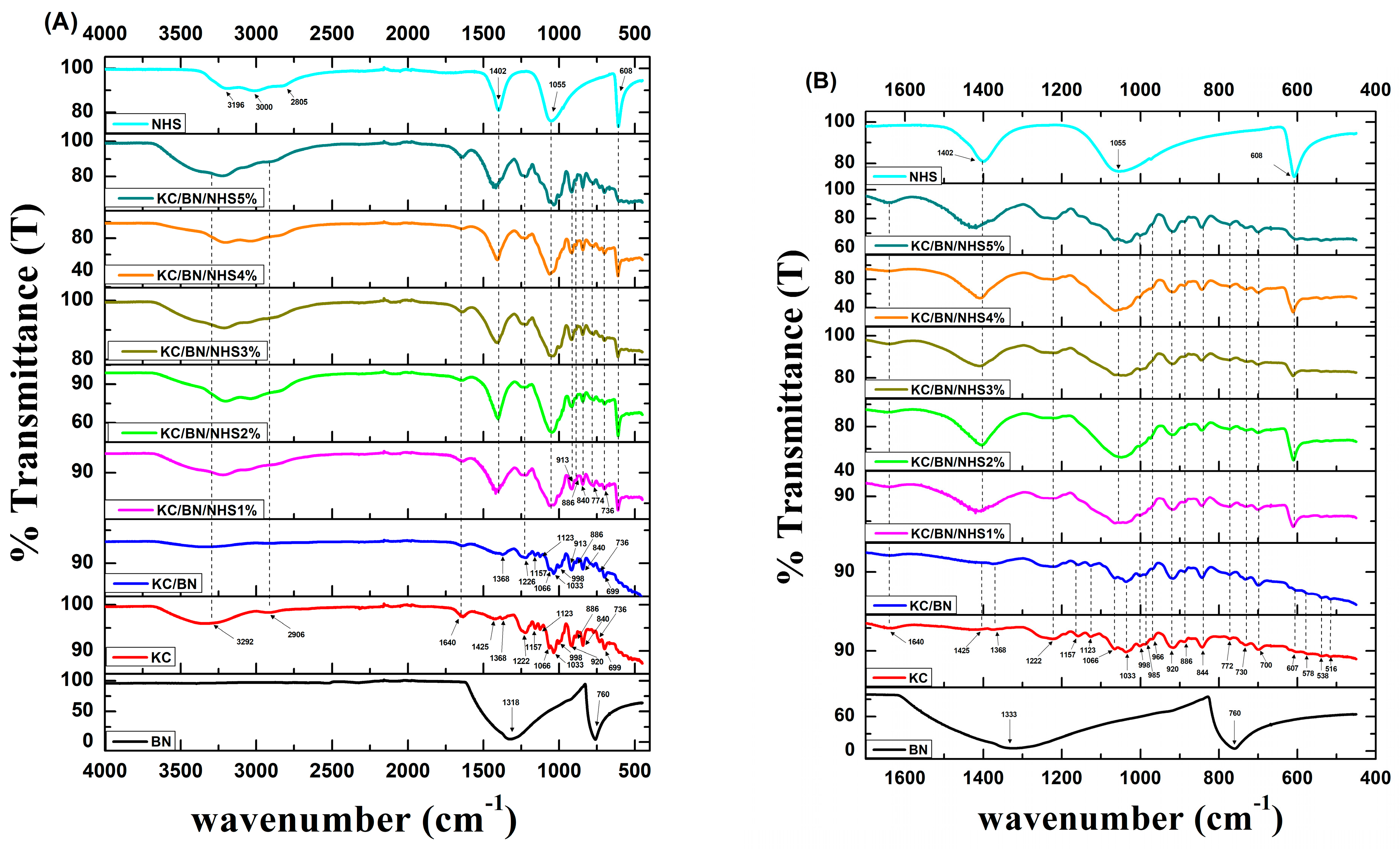
3.2. Chemical Composition
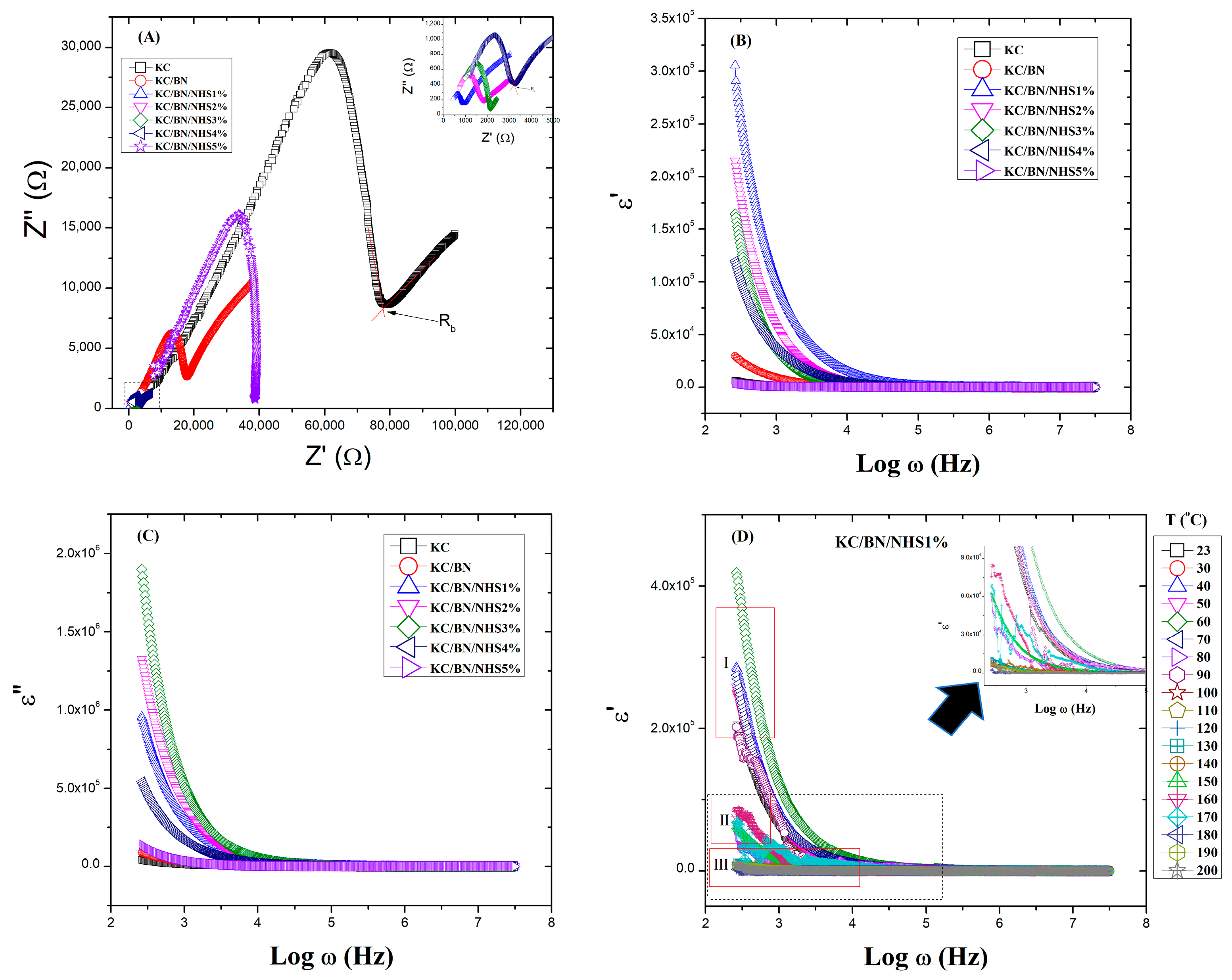
3.3. Electrical Properties
Dielectric Study
3.4. Morphology and Composition Analysis Samples (SEM-EDS)
3.5. Identification of Structural Phases in Membranes Through X-Rays
3.6. Surface Energy
3.7. Mechanical Properties
3.8. Implications
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Acronyms
| κ-Carrageenan | (KC) |
| Boron nitride | (BN) |
| Ammonium sulfate | (NHS) |
| Thermogravimetric analysis | (TGA) |
| Differential scanning calorimetry | (DSC) |
| X-ray diffraction | (XRD) |
| Scanning electron microscopy | (SEM) |
| Energy-dispersive X-ray spectroscopy | (EDS) |
| Fourier transform infrared spectroscopy | (FTIR) |
| Impedance spectroscopy | (IS) |
| Ammonium chloride | (NH4Cl) |
| Ammonium bromide | (NH4Br) |
| O-methylene phosphonic κ-carrageenan | (OMPC) |
| Carboxycellulose nanofibers | (CNFs) |
| Poly(perfluorosulfonic acids) | (PFAs) |
| Perfluorosulfonic acid ionomers | (PFSIs) |
| KC/BN/NHS1, 2, 3, 4, and 5% | κ-carrageenan/boron nitride/variation in the concentration of ammonium sulfate |
References
- Samsudin, A.M.; Roschger, M.; Wolf, S.; Hacker, V. Preparation and Characterization of QPVA/PDDA Electrospun Nanofiber Anion Exchange Membranes for Alkaline Fuel Cells. Nanomaterials 2022, 12, 3965. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.I.; Shanableh, A.; Shahida, S.; Lashari, M.H.; Manzoor, S.; Fernandez, J. SPEEK and SPPO Blended Membranes for Proton Exchange Membrane Fuel Cells. Membranes 2022, 12, 263. [Google Scholar] [CrossRef]
- Peighambardoust, S.J.; Rowshanzamir, S.; Amjadi, M. Review of the proton exchange membranes for fuel cell applications. Int. J. Hydrogen Energy 2010, 35, 9349–9384. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, H.; Wu, J.; Zhang, J.; Boston, A.L.; London, H.L. PEM Fuel Cell Testing and Diagnosis; Newnes: London, UK, 2013. [Google Scholar]
- Fan, L.; Tu, Z.; Chan, S.H. Recent development of hydrogen and fuel cell technologies: A review. Energy Rep. 2021, 7, 8421–8446. [Google Scholar] [CrossRef]
- Bonato, M.; Corrà, F.; Bellio, M.; Guidolin, L.; Tallandini, L.; Irato, P.; Santovito, G. Pfas environmental pollution and antioxidant responses: An overview of the impact on human field. Int. J. Environ. Res. Public. Health 2020, 17, 8020. [Google Scholar] [CrossRef] [PubMed]
- Lukić Bilela, L.; Matijošytė, I.; Krutkevičius, J.; Alexandrino, D.A.M.; Safarik, I.; Burlakovs, J.; Gaudêncio, S.P.; Carvalho, M.F. Impact of per- and polyfluorinated alkyl substances (PFAS) on the marine environment: Raising awareness, challenges, legislation, and mitigation approaches under the One Health concept. Mar. Pollut. Bull. 2023, 194, 115309. [Google Scholar] [CrossRef]
- Kurwadkar, S.; Dane, J.; Kanel, S.R.; Nadagouda, M.N.; Cawdrey, R.W.; Ambade, B.; Struckhoff, G.C.; Wilkin, R. Per- and polyfluoroalkyl substances in water and wastewater: A critical review of their global occurrence and distribution. Sci. Total Environ. 2022, 809, 151003. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Luan, P.; Li, Q.; Cheng, Z.; Xiang, P.; Liu, D.; Hou, Y.; Yang, Y.; Zhu, H. Biopolymers Derived from Trees as Sustainable Multifunctional Materials: A Review. Adv. Mater. 2021, 33, e2001654. [Google Scholar] [CrossRef]
- George, A.; Sanjay, M.R.; Srisuk, R.; Parameswaranpillai, J.; Siengchin, S. A comprehensive review on chemical properties and applications of biopolymers and their composites. Int. J. Biol. Macromol. 2020, 154, 329–338. [Google Scholar] [CrossRef]
- Abouricha, S.; Aziam, H.; Noukrati, H.; Sel, O.; Saadoune, I.; Lahcini, M.; Ben Youcef, H. Biopolymers-Based Proton Exchange Membranes For Fuel Cell Applications: A Comprehensive Review. ChemElectroChem 2024, 11, e202300648. [Google Scholar] [CrossRef]
- Doobi, F.A.; Mir, F.Q. Exploring the development of natural biopolymer (chitosan)-based proton exchange membranes for fuel cells: A review. Results Surf. Interfaces 2024, 15, 100218. [Google Scholar] [CrossRef]
- Ma, J.; Sahai, Y. Chitosan biopolymer for fuel cell applications. Carbohydr. Polym. 2013, 92, 955–975. [Google Scholar] [CrossRef] [PubMed]
- Dalwadi, S.; Goel, A.; Kapetanakis, C.; Salas-De La Cruz, D.; Hu, X. The Integration of Biopolymer-Based Materials for Energy Storage Applications: A Review. Int. J. Mol. Sci. 2023, 24, 3975. [Google Scholar] [CrossRef]
- Nur, N.F.; Shyuan, L.K.; Mohamad, A.B.; Kadhum, A.A.H. Review on biopolymer membranes for fuel cell applications. Appl. Mech. Mater. 2013, 291–294, 614–617. [Google Scholar] [CrossRef]
- Myrzakhmetov, B.; Akhmetova, A.; Bissenbay, A.; Karibayev, M.; Pan, X.; Wang, Y.; Bakenov, Z.; Mentbayeva, A. Review: Chitosan-based biopolymers for anion-exchange membrane fuel cell application. R. Soc. Open Sci. 2023, 10, 230843. [Google Scholar] [CrossRef] [PubMed]
- Samoila, P.; Grecu, I.; Asandulesa, M.; Cojocaru, C.; Harabagiu, V. Bio-based ionically cross-linked alginate composites for PEMFC potential applications. React. Funct. Polym. 2021, 165, 104967. [Google Scholar] [CrossRef]
- Shaari, N.; Kamarudin, S.K. Chitosan and alginate types of bio-membrane in fuel cell application: An overview. J. Power Sources 2015, 289, 71–80. [Google Scholar] [CrossRef]
- Liew, J.W.Y.; Loh, K.S.; Ahmad, A.; Lim, K.L.; Wan Daud, W.R. Synthesis and characterization of modified κ-carrageenan for enhanced proton conductivity as polymer electrolyte membrane. PLoS ONE 2017, 12, e0185313. [Google Scholar] [CrossRef]
- Christwardana, M.; Kuntolaksono, S.; Septevani, A.A.; Hadiyanto, H. Starch—Carrageenan based low-cost membrane permeability characteristic and its application for yeast microbial fuel cells. Int. J. Renew. Energy Dev. 2024, 13, 303–314. [Google Scholar] [CrossRef]
- Esmaeili, C.; Ghasemi, M.; Heng, L.Y.; Hassan, S.H.A.; Abdi, M.M.; Daud, W.R.W. Synthesis and application of polypyrrole/carrageenan nano-bio composite as a cathode catalyst in microbial fuel cells. Carbohydr. Polym. 2014, 114, 253–259. [Google Scholar] [CrossRef]
- Prado-Fernández, J.; Rodríguez-Vázquez, J.A.; Tojo, E.; Andrade, J.M. Quantitation of κ-, ι- and λ-carrageenans by mid-infrared spectroscopy and PLS regression. Anal. Chim. Acta 2003, 480, 23–37. [Google Scholar] [CrossRef]
- Belton, P.S.; Wilson, R.H. Interaction of group I cations with iota and kappa carrageenans studied by Fourier transform infrared spectroscopy. Int. J. Biol. Macromol. 1986, 8, 247–251. [Google Scholar] [CrossRef]
- Necas, J.; Bartosikova, L. Carrageenan: A review. Vet. Med. 2013, 58, 187–205. [Google Scholar] [CrossRef]
- Tasende, M.G.; Manriquez-Hernandez, J. Carrageenan Properties and Applications: A Review. In Carrageenans: Sources and Extraction Methods, Molecular Structure, Bioactive Properties and Health Effects; Nova Science Publishers: Hauppauge, NY, USA, 2016. [Google Scholar]
- An, D.; Cheng, S.; Jiang, C.; Duan, X.; Yang, B.; Zhang, Z.; Li, J.; Liu, Y.; Wong, C.-P. A novel environmentally friendly boron nitride/lignosulfonate/natural rubber composite with improved thermal conductivity. J. Mater. Chem. C Mater. 2020, 8, 4801–4809. [Google Scholar] [CrossRef]
- Cheng, Z.L.; Ma, Z.S.; Ding, H.L.; Liu, Z. Environmentally friendly, scalable exfoliation for few-layered hexagonal boron nitride nanosheets (BNNSs) by multi-time thermal expansion based on released gases. J. Mater. Chem. C Mater. 2019, 7, 14701–14708. [Google Scholar] [CrossRef]
- Mehmet Ay, G.; Göncü, Y.; Ay, N. Environmentally friendly material: Hexagonal boron nitride. J. Boron 2016, 1, 66–73. [Google Scholar]
- Arenal, R.; Lopez-Bezanilla, A. Boron nitride materials: An overview from 0D to 3D (nano)structures. Wiley Interdiscip. Rev. Comput. Mol. Sci. 2015, 5, 299–309. [Google Scholar] [CrossRef]
- Han, W.; Ma, Z.; Liu, S.; Ge, C.; Wang, L.; Zhang, X. Highly-dispersible boron nitride nanoparticles by spray drying and pyrolysis. Ceram. Int. 2017, 43, 10192–10200. [Google Scholar] [CrossRef]
- Konzock, O.; Zaghen, S.; Fu, J.; Kerkhoven, E.J. Urea is a drop-in nitrogen source alternative to ammonium sulphate in Yarrowia lipolytica. IScience 2022, 25, 105703. [Google Scholar] [CrossRef] [PubMed]
- Chien, S.H.; Gearhart, M.M.; Villagarcía, S. Comparison of ammonium sulfate with other nitrogen and sulfur fertilizers in increasing crop production and minimizing environmental impact: A review. Soil. Sci. 2011, 176, 327–335. [Google Scholar] [CrossRef]
- Hassan, M.A.; Gouda, M.E.; Sheha, E. Investigations on the electrical and structural properties of PVA doped with (NH4)2SO4. J. Appl. Polym. Sci. 2010, 116, 1213–1217. [Google Scholar] [CrossRef]
- Vilela, C.; Morais, J.D.; Silva, A.C.Q.; Muñoz-Gil, D.; Figueiredo, F.M.L.; Silvestre, A.J.D.; Freire, C.S.R. Flexible nanocellulose/lignosulfonates ion-conducting separators for polymer electrolyte fuel cells. Nanomaterials 2020, 10, 1713. [Google Scholar] [CrossRef] [PubMed]
- Kawabata, T.; Matsuo, Y. Chitin Based Fuel Cell and Its Proton Conductivity. Mater. Sci. Appl. 2018, 9, 779–789. [Google Scholar] [CrossRef]
- Pasini Cabello, S.D.; Mollá, S.; Ochoa, N.A.; Marchese, J.; Giménez, E.; Compañ, V. New bio-polymeric membranes composed of alginate-carrageenan to be applied as polymer electrolyte membranes for DMFC. J. Power Sources 2014, 265, 345–355. [Google Scholar] [CrossRef]
- Mohy Eldin, M.S.; Farag, H.A.; Tamer, T.M.; Konsowa, A.H.; Gouda, M.H. Development of novel iota carrageenan-g-polyvinyl alcohol polyelectrolyte membranes for direct methanol fuel cell application. Polym. Bull. 2020, 77, 4895–4916. [Google Scholar] [CrossRef]
- Vijaya, N.; Selvasekarapandian, S.; Sornalatha, M.; Sujithra, K.S.; Monisha, S. Proton-conducting biopolymer electrolytes based on pectin doped with NH4X (X = Cl, Br). Ionics 2017, 23, 2799–2808. [Google Scholar] [CrossRef]
- Shukur, M.F.; Kadir, M.F.Z.; Ahmad, Z.; Ithnin, R. Dielectric studies of proton conducting polymer electrolyte based on chitosan/PEO blend doped with NH 4NO 3. Adv. Mater. Res. 2012, 488–489, 583–587. [Google Scholar] [CrossRef]
- Moniha, V.; Alagar, M.; Selvasekarapandian, S.; Sundaresan, B.; Boopathi, G. Conductive bio-polymer electrolyte iota-carrageenan with ammonium nitrate for application in electrochemical devices. J. Non Cryst. Solids 2018, 481, 424–434. [Google Scholar] [CrossRef]
- Li, S.; Cai, G.; Wu, S.; Raut, A.; Borges, W.; Sharma, P.R.; Sharma, S.K.; Hsiao, B.S.; Rafailovich, M. Sustainable Plant-Based Biopolymer Membranes for PEM Fuel Cells. Int. J. Mol. Sci. 2022, 23, 15245. [Google Scholar] [CrossRef]
- Smitha, B.; Sridhar, S.; Khan, A.A. Chitosan-sodium alginate polyion complexes as fuel cell membranes. Eur. Polym. J. 2005, 41, 1859–1866. [Google Scholar] [CrossRef]
- Sigwadi, R.; Dhlamini, M.S.; Mokrani, T.; Ṋemavhola, F.; Nonjola, P.F.; Msomi, P.F. The proton conductivity and mechanical properties of Nafion®/ ZrP nanocomposite membrane. Heliyon 2019, 5, e02240. [Google Scholar] [CrossRef] [PubMed]
- Zakuwan, S.Z.; Ahmad, I. Effects of hybridized organically modified montmorillonite and cellulose nanocrystals on rheological properties and thermal stability of K-carrageenan bio-nanocomposite. Nanomaterials 2019, 9, 1547. [Google Scholar] [CrossRef]
- Surowiec, J.; Bogoczek, R. Studies on the thermal stability of the perfluorinated cation-exchange membrane nafion-417. J. Therm. Anal. 1988, 33, 1097–1102. [Google Scholar] [CrossRef]
- Lee, E.J.; Shin, D.S.; Kim, H.E.; Kim, H.W.; Koh, Y.H.; Jang, J.H. Membrane of hybrid chitosan-silica xerogel for guided bone regeneration. Biomaterials 2009, 30, 743–750. [Google Scholar] [CrossRef] [PubMed]
- Kundu, S.; Simon, L.C.; Fowler, M.; Grot, S. Mechanical properties of NafionTM electrolyte membranes under hydrated conditions. Polymer 2005, 46, 11707–11715. [Google Scholar] [CrossRef]
- Sonef, Y.; Ekdunge, P.; Simonsson, D. Proton Conductivily of Nafion 117 as Measured by a Four-Electrode AC Impedance Method. J. Electrochem. Soc. 1996, 143, 1254. [Google Scholar] [CrossRef]
- Che Balian, S.R.; Ahmad, A.; Mohamed, N.S. The effect of lithium iodide to the properties of carboxymethyl κ-carrageenan/carboxymethyl cellulose polymer electrolyte and dye-sensitized solar cell performance. Polymers 2016, 8, 163. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.L.; Yu, T.L.; Han, F.H. A method for improving ionic conductivity of nafion membranes and its application to PEMFC. J. Polym. Res. 2006, 13, 379–385. [Google Scholar] [CrossRef]
- Walkowiak-Kulikowska, J.; Wolska, J.; Koroniak, H. Polymers application in proton exchange membranes for fuel cells (PEMFCs). Phys. Sci. Rev. 2017, 293-347, 293–347. [Google Scholar] [CrossRef]
- Karaca, A.; Galkina, I.; Sohn, Y.J.; Wippermann, K.; Scheepers, F.; Glüsen, A.; Shviro, M.; Müller, M.; Carmo, M.; Stolten, D. Self-Standing, Ultrasonic Spray-Deposited Membranes for Fuel Cells. Membranes 2023, 13, 522. [Google Scholar] [CrossRef]
- Di Virgilio, M.; Basso Peressut, A.; Arosio, V.; Arrigoni, A.; Latorrata, S.; Dotelli, G. Functional and Environmental Performances of Novel Electrolytic Membranes for PEM Fuel Cells: A Lab-Scale Case Study. Clean. Technol. 2023, 5, 74–93. [Google Scholar] [CrossRef]
- Krafft, M.P.; Riess, J.G. Per- and polyfluorinated substances (PFASs): Environmental challenges. Curr. Opin. Colloid. Interface Sci. 2015, 20, 192–212. [Google Scholar] [CrossRef]
- Lindstrom, A.B.; Strynar, M.J.; Libelo, E.L. Polyfluorinated compounds: Past, present, and future. Environ. Sci. Technol. 2011, 45, 7954–7961. [Google Scholar] [CrossRef] [PubMed]
- Mudumbi, J.B.N.; Ntwampe, S.K.O.; Matsha, T.; Mekuto, L.; Itoba-Tombo, E.F. Recent developments in polyfluoroalkyl compounds research: A focus on human/environmental health impact, suggested substitutes and removal strategies. Environ. Monit. Assess. 2017, 189, 402. [Google Scholar] [CrossRef] [PubMed]
- Chavez-Baldovino, E.; Malca-Reyes, C.A.; Masso, R.; Feng, P.; Camacho, A.; Sarmiento, J.; Negrón, J.I.B.; Pagán-Torres, Y.J.; Díaz-Vázquez, L.M. Optimizing Sustainable Energy Generation in Ethanol Fuel Cells: An Exploration of Carrageenan with TiO 2 Nanoparticles and Ni/CeO 2 Composites. ACS Omega 2023, 8, 20642–20653. [Google Scholar] [CrossRef]
- Lizundia, E.; Urruchi, A.; Vilas, J.L.; León, L.M. Increased functional properties and thermal stability of flexible cellulose nanocrystal/ZnO films. Carbohydr. Polym. 2016, 136, 250–258. [Google Scholar] [CrossRef] [PubMed]
- Petkova, V.; Pelovski, Y.; Hristova, V. Introduction thermal analysis for identification of e-beam nanosize ammonium sulfate. J. Therm. Anal. Calorim. 2005, 82, 813–817. [Google Scholar] [CrossRef]
- Sadeghi, M. Synthesis of a biocopolymer carrageenan-g-poly(aam-co-ia)/ montmorilonite superabsorbent hydrogel composite. Braz. J. Chem. Eng. 2012, 29, 295–305. [Google Scholar] [CrossRef]
- Zhou, F.; Wang, D.; Zhang, J.; Li, J.; Lai, D.; Lin, S.; Hu, J. Preparation and Characterization of Biodegradable κ-Carrageenan Based Anti-Bacterial Film Functionalized with Wells-Dawson Polyoxometalate. Foods 2022, 11, 586. [Google Scholar] [CrossRef]
- Wang, N.; Teng, H.; Li, L.; Zhang, J.; Kang, P. Synthesis of phosphated K-carrageenan and its application for flame-retardant waterborne epoxy. Polymers 2018, 10, 1268. [Google Scholar] [CrossRef]
- Mishra, D.K.; Tripathy, J.; Behari, K. Synthesis of graft copolymer (k-carrageenan-g-N,N-dimethylacrylamide) and studies of metal ion uptake, swelling capacity and flocculation properties. Carbohydr. Polym. 2008, 71, 524–534. [Google Scholar] [CrossRef]
- Pereira, L.; Amado, A.M.; Critchley, A.T.; van de Velde, F.; Ribeiro-Claro, P.J.A. Identification of selected seaweed polysaccharides (phycocolloids) by vibrational spectroscopy (FTIR-ATR and FT-Raman). Food Hydrocolloids 2009, 23, 1903–1909. [Google Scholar] [CrossRef]
- Muthu, R.N.; Rajashabala, S.; Kannan, R. Synthesis, characterization of hexagonal boron nitride nanoparticles decorated halloysite nanoclay composite and its application as hydrogen storage medium. Renew. Energy 2016, 90, 554–564. [Google Scholar] [CrossRef]
- Madakbaş, S.; Çakmakçi, E.; Kahraman, M.V. Preparation and thermal properties of polyacrylonitrile/hexagonal boron nitride composites. Thermochim. Acta 2013, 552, 1–4. [Google Scholar] [CrossRef]
- Iger, S.J.; Bewilogua, K.; Klages, C.-P. Infrared spectroscopic investigations on h-BN and mixed h/c-BN thin films. Thin Solid Films 1994, 245, 50–55. [Google Scholar] [CrossRef]
- Şen, M.; Erboz, E.N. Determination of critical gelation conditions of κ-carrageenan by viscosimetric and FT-IR analyses. Food Res. Int. 2010, 43, 1361–1364. [Google Scholar] [CrossRef]
- Mobarak, N.N.; Ramli, N.; Ahmad, A.; Rahman, M.Y.A. Chemical interaction and conductivity of carboxymethyl κ-carrageenan based green polymer electrolyte. Solid. State Ion. 2012, 224, 51–57. [Google Scholar] [CrossRef]
- Fan, L.; Peng, K.; Li, M.; Wang, L.; Wang, T. Preparation and properties of carboxymethyl κ -carrageenan / alginate blend fibers. J. Biomater. Sci. Polym. Ed. 2013, 24, 1099–1111. [Google Scholar] [CrossRef] [PubMed]
- Daud, J.M.; Warzukni, N.S.; Salim, R.M.; Zuberdi, A.M. Semi-refined κ-carrageenan: Part 1. Chemical modification of semi-refined κ-carrageenan via graft copolymerization method, optimization process and characterization of its super absorbent hydrogel. Orient. J. Chem. 2015, 31, 973–980. [Google Scholar] [CrossRef]
- Pereira, L.; Sousa, A.; Coelho, H.; Amado, A.M.; Ribeiro-Claro, P.J.A. Use of FTIR, FT-Raman and13C-NMR spectroscopy for identification of some seaweed phycocolloids. Biomol. Eng. 2003, 20, 223–228. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, W.; Mahmood, K.; Mizanur, M.; Khan, R.; Yee, T.C. Effects of Reaction Temperature on the Synthesis and Thermal Properties of Carrageenan Ester. J. Phys. Sci. 2014, 25, 123–138. [Google Scholar] [CrossRef]
- Selvin, P.C.; Perumal, P.; Selvasekarapandian, S.; Monisha, S.; Boopathi, G.; Chandra, M.V.L. Study of proton-conducting polymer electrolyte based on K-carrageenan and NH 4 SCN for electrochemical devices. Ionics 2018, 24, 3535–3542. [Google Scholar] [CrossRef]
- Huang, X.; Wang, S.; Zhu, M.; Yang, K.; Jiang, P.; Bando, Y.; Golberg, D.; Zhi, C. Thermally conductive, electrically insulating and melt-processable polystyrene/boron nitride nanocomposites prepared by in situ reversible addition fragmentation chain transfer polymerization. Nanotechnology 2015, 26, 015705. [Google Scholar] [CrossRef]
- Hema, M.; Selvasekarapandian, S.; Arunkumar, D.; Sakunthala, A.; Nithya, H. Author ’ s personal copy FTIR, XRD and ac impedance spectroscopic study on PVA based polymer electrolyte doped with NH4X ( X = Cl, Br, I). J. Non Cryst. Solids 2009, 355, 84–90. [Google Scholar] [CrossRef]
- Moniha, V.; Alagar, M.; Selvasekarapandian, S.; Sundaresan, B.; Hemalatha, R.; Boopathi, G. Synthesis and characterization of bio-polymer electrolyte based on iota-carrageenan with ammonium thiocyanate and its applications. J. Solid. State Electrochem. 2018, 22, 3209–3223. [Google Scholar] [CrossRef]
- Bantang, J.P.O.; Bigol, U.G.; Camacho, D.H. Gel and Film Composites of Silver Nanoparticles in κ-, ι-, and λ-Carrageenans: One-Pot Synthesis, Characterization, and Bioactivities. Bionanoscience 2021, 11, 53–66. [Google Scholar] [CrossRef]
- Zhi, C.; Bando, Y.; Tang, C.; Honda, S.; Sato, K.; Kuwahara, H.; Golberg, D. Characteristics of boron nitride nanotube-polyaniline composites. Angew. Chem.—Int. Ed. 2005, 44, 7929–7932. [Google Scholar] [CrossRef]
- Harrison, H.; Lamb, J.T.; Nowlin, K.S.; Guenthner, A.J.; Ghiassi, K.B.; Kelkar, A.D.; Alston, J.R. Quantification of hexagonal boron nitride impurities in boron nitride nanotubes: Via FTIR spectroscopy. Nanoscale Adv. 2019, 1, 1693–1701. [Google Scholar] [CrossRef] [PubMed]
- Dash, S.; Swain, S.K. Effect of nanoboron nitride on the physical and chemical properties of soy protein. Compos. Sci. Technol. 2013, 84, 39–43. [Google Scholar] [CrossRef]
- Kubota, Y.; Watanabe, K.; Tsuda, O.; Taniguchi, T. Deep Ultraviolet Light–EmittingHexagonal Boron Nitride Synthesizedat Atmospheric Pressure. Science (1979) 2007, 317, 932–934. [Google Scholar] [CrossRef]
- Paleckiene, R.; Sviklas, A.; Šlinkšiene, R. Physicochemical properties of a microelement fertilizer with amino acids. Russ. J. Appl. Chem. 2007, 80, 352–357. [Google Scholar] [CrossRef]
- Mohod, A.V.; Gogate, P.R. Improved crystallization of ammonium sulphate using ultrasound assisted approach with comparison with the conventional approach. Ultrason. Sonochem. 2018, 41, 310–318. [Google Scholar] [CrossRef] [PubMed]
- Nelmes, R.J. An X-ray Diffraction Determination of the Crystal Structure of Ammonium Hydrogen Sulphate above the Ferroeleetrie Transition. Acta Cryst. 1971, 27, 272–281. [Google Scholar] [CrossRef]
- Schlemper, E.O.; Hamilton, W.C. Neutron-diffraction study of the structures of ferroelectric and paraelectric ammonium sulfate. J. Chem. Phys. 1966, 44, 4498–4509. [Google Scholar] [CrossRef]
- Sen Gupta, S.; Karan, S.; Gupta, S.P.S. Growth and Defect Characterisation in Single Crystals of Ferroelectric Ammonium Sulphate. Jpn. J. Appl. Phys. 2000, 39, 2736. [Google Scholar] [CrossRef]
- Pakdel, A.; Bando, Y.; Golberg, D. Plasma-assisted interface engineering of boron nitride nanostructure films. ACS Nano 2014, 8, 10631–10639. [Google Scholar] [CrossRef]
- Achari, P.F.; Bejagam, K.K.; Singh, S.; Deshmukh, S.A. Development of non-bonded interaction parameters between hexagonal boron-nitride and water. Comput. Mater. Sci. 2019, 161, 339–345. [Google Scholar] [CrossRef]
- Moustafa, M.; Abu-Saied, M.A.; Taha, T.H.; Elnouby, M.; El Desouky, E.A.; Alamri, S.; Shati, A.; Alrumman, S.; Alghamdii, H.; Al-Khatani, M.; et al. Preparation and characterization of super-absorbing gel formulated from κ-carrageenan–potato peel starch blended polymers. Polymers 2021, 13, 4308. [Google Scholar] [CrossRef]
- Nogueira, L.F.B.; Maniglia, B.C.; Pereira, L.S.; Tapia-Blácido, D.R.; Ramos, A.P. Formation of carrageenan-CaCO3 bioactive membranes. Mater. Sci. Eng. C 2016, 58, 1–6. [Google Scholar] [CrossRef]
- Blumenthal, R.N.; Whitmore, D.H.; Narayanan, U.H.; Sundararajan, K.; Kasabekar, M.S.; Nagasubramanian, G. Thermal Stability of Nafion® in Simulated Fuel Cell Environments Thermodynamic Study of Phase Equilibria in the TitaniumOxygen System Within the TiO 1.95 TiO 2 Region Measurement of Thickness of Electroplates Taking Advantage of the Impedance Change in the Stripping Cell Thermal Stability of Nafion® in Simulated Fuel Cell Environments; Academic Press: Cambridge, MA, USA, 1996; Volume 143. [Google Scholar]
- Iwai, Y.; Yamanishi, T. Thermal stability of ion-exchange Nafion N117CS membranes. Polym. Degrad. Stab. 2009, 94, 679–687. [Google Scholar] [CrossRef]
- Lage, L.G.; Delgado, P.G.; Kawano, Y. Thermal Stability and Decomposition of Nafion ® Membranes with Different Cations Using High-Resolution Thermogravimetry. J. Therm. Anal. Calorim. 2004, 75, 521–530. [Google Scholar] [CrossRef]
- Garaev, V.; Pavlovica, S.; Reinholds, I.; Vaivars, G. Mechanical properties and XRD of Nafion modified by 2-hydroxyethylammonium ionic liquids. IOP Conf. Ser. Mater. Sci. Eng. 2013, 49, 012058. [Google Scholar] [CrossRef]
- Satterfield, M.B.; Majsztrik, P.W.; Ota, H.; Benziger, J.B.; Bocarsly, A.B. Mechanical properties of Nafion and titania/Nafion composite membranes for polymer electrolyte membrane fuel cells. J. Polym. Sci. B Polym. Phys. 2006, 44, 2327–2345. [Google Scholar] [CrossRef]
- Di Noto, V.; Gliubizzi, R.; Negro, E.; Pace, G. Effect of SiO2 on relaxation phenomena and mechanism of ion conductivity of [Nafion/(SiO2)x] composite membranes. J. Phys. Chem. B 2006, 110, 24972–24986. [Google Scholar] [CrossRef] [PubMed]
- Kuwertz, R.; Kirstein, C.; Turek, T.; Kunz, U. Influence of acid pretreatment on ionic conductivity of Nafion® membranes. J. Memb. Sci. 2016, 500, 225–235. [Google Scholar] [CrossRef]


| Property | Biomembranes | Nafion Commercial |
|---|---|---|
| Thermal Stability | Up to 200 °C [43] | Up to 380 °C [44] |
| Up to 150 °C [45] | Up to 280 [46] | |
| Mechanical Strength | 72.29 MPa (Tensile Strength) [43] | ~1 MPa (Stress) [44] |
| ~1 MPa (Stress) [47] | 3.23 MPa/% (Young’s modulus) [48] | |
| Ionic Conductivity | 4.2 × 10−2 S/cm [43] | 1.5 × 10−2 S/cm at 30 °C and 70% RH [44] |
| 3.16 × 10−2 S/cm [49] | 7.8 × 10−2 S/cm [50] | |
| 3.89 × 10−2 S/cm [51] | 3.7 × 10−2 S/cm 75 °C, 95% RH [52] |
| Wavenumber (cm−1) | Functional Group | References |
|---|---|---|
| 3392 | O–H (stretching) | [22,70,71] |
| 1640 | Polymer-bound water | [22,70] |
| 1222 | O=S=O (asymmetric stretching) | [22,23,70,71,72,73,74] |
| 1064 | C–O + C–OH | [22,70] |
| 1037 | C–OH + S=O | [70] |
| 1002 | Glicosidic bonds | [22] |
| 920 | 3,6-anhydro-D-galactose | [71,73,74] |
| 844 | C4–O–S group in galactose (stretching) | [22,70,71,72,73] |
| 730 | C–O–C α(1,3) (stretching) | [22,70] |
| 700 | Sulfates in C4, galactose | [75] |
| Membranes | Conductivity (Scm−1) |
|---|---|
| KC | 1.22 × 10−6 |
| KC/BN | 4.87 × 10−6 |
| KC/BN/NHS1% | 7.82 × 10−5 |
| KC/BN/NHS2% | 5.13 × 10−5 |
| KC/BN/NHS3% | 5.05 × 10−5 |
| KC/BN/NHS4% | 2.65 × 10−5 |
| KC/BN/NHS5% | 3.27 × 10−6 |
| Samples | Elements | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| B | C | N | O | Na | S | Cl | K | |||||||||||
| Mass% | Atom% | Mass% | Atom% | Mass% | Atom% | Mass% | Atom% | Mass% | Atom% | Mass% | Atom% | Mass% | Atom% | Mass% | Atom% | Total Mass% | Total Atom% | |
| KC | 0 | 0 | 46.8 | 60.8 | 0 | 0 | 29 | 28.4 | 1 | 0.7 | 7.8 | 3.9 | 2.5 | 1 | 12.9 | 5.2 | 100 | 100 |
| BN | 54.6 | 61 | 0 | 0 | 45.4 | 39 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 100 | 100 |
| KC/BN | 4.2 | 6.4 | 45.9 | 58 | 0.7 | 0.6 | 27 | 25.5 | 1 | 0.8 | 7.2 | 3.3 | 2.5 | 1 | 11.5 | 4.4 | 100 | 100 |
| NHS | 0 | 0 | 0 | 0 | 10.9 | 17.5 | 29.5 | 41.1 | 0 | 0 | 59.6 | 41.4 | 0 | 0 | 0 | 0 | 100 | 100 |
| KC/BN/NHS1% | 8.7 | 12.7 | 33.9 | 44.6 | 7.8 | 8.6 | 24.1 | 23.6 | 0.8 | 0.6 | 11 | 4.4 | 2.7 | 1.1 | 11 | 4.4 | 100 | 100 |
| KC/BN/NHS2% | 19.6 | 25.9 | 31 | 37 | 16.4 | 16.8 | 13.5 | 11.9 | 0.6 | 0.4 | 11.7 | 5.2 | 2.4 | 1 | 4.8 | 1.8 | 100 | 100 |
| KC/BN/NHS3% | 8.7 | 11.8 | 39.2 | 48 | 11.4 | 12 | 21.3 | 19.5 | 0.9 | 0.6 | 13.2 | 6 | 2 | 0.9 | 3.3 | 1.2 | 100 | 100 |
| KC/BN/NHS4% | 9.6 | 13.3 | 36.7 | 45.7 | 10.5 | 11.2 | 21.3 | 20 | 0.9 | 0.5 | 14.2 | 6.6 | 2.4 | 1 | 4.4 | 1.7 | 100 | 100 |
| KC/BN/NHS5% | 0 | 0 | 38 | 54.3 | 11 | 10.6 | 23.2 | 21.5 | 0 | 0 | 18 | 8.6 | 0 | 0 | 9.8 | 5 | 100 | 100 |
| Membranes | Surface Tension (mN/m) | Contact Angle (°) |
|---|---|---|
| KC | 68.3 | 19.9 |
| KC/BN | 50.3 | 55.2 |
| KC/BN/NHS1% | 55.9 | 45 |
| KC/BN/NHS2% | 57.4 | 42.7 |
| KC/BN/NHS3% | 54.9 | 64.1 |
| KC/BN/NHS4% | 54.4 | 48.1 |
| KC/BN/NHS5% | 54.6 | 47.2 |
| Membranes | Ultimate Tensile Stress (MPa) | Elongation at Break (%) | Young’s Modulus (MPa) |
|---|---|---|---|
| KC | 7.36 | 6.5 | 2.20 |
| KC/BN | 10.96 | 7.2 | 3.73 |
| KC/BN/NHS1% | 6.66 | 4.8 | 3.02 |
| KC/BN/NHS2% | 2.29 | 3.6 | 1.56 |
| KC/BN/NHS3% | 1.64 | 3.0 | 1.32 |
| KC/BN/NHS4% | 1.59 | 4.1 | 0.72 |
| KC/BN/NHS5% | 1.04 | 2.4 | 0.57 |
| Property | κ-Carrageenan and BN | Traditional Nafion (commercial) | Comments/Notes |
|---|---|---|---|
| Thermal stability | Improved by BN | Around 280 °C [46,95,96,97] | BN improves thermal stability up to 160 °C, although Nafion has a higher operating temperature range of 280 °C. |
| Mechanical strength (Young’s modulus) | Enhanced by BN nanoparticles | Around 250 MPa [48,98] | BN improves the strength of the biopolymer with a value of 10.96 Mpa, but Nafion is inherently more robust, with values up to 250 MPa. |
| Ionic conductivity | Enhanced by NHS | ~0.1 S/cm at room temperature | NHS improves conductivity with the value of 7.82 × 10−5 S/cm; this is still lower than Nafion (~0.1 S/cm) but competitive for many applications. |
| Environmental impact | Biodegradable, lower toxicological profile | Persistent fluorinated compounds [56,57] | Significant advantage over Nafion due to sustainable and eco-friendly profile. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chavez-Baldovino, E.; Malca-Reyes, C.A.; Masso, R.; Feng, P.; Díaz-Vázquez, L.M. Development and Characterization of κ-Carrageenan and Boron Nitride Nanoparticle Membranes for Improved Ionic Conductivity in Fuel Cells. Fuels 2025, 6, 15. https://doi.org/10.3390/fuels6010015
Chavez-Baldovino E, Malca-Reyes CA, Masso R, Feng P, Díaz-Vázquez LM. Development and Characterization of κ-Carrageenan and Boron Nitride Nanoparticle Membranes for Improved Ionic Conductivity in Fuel Cells. Fuels. 2025; 6(1):15. https://doi.org/10.3390/fuels6010015
Chicago/Turabian StyleChavez-Baldovino, Ermides, Carlos A. Malca-Reyes, Roberto Masso, Peter Feng, and Liz M. Díaz-Vázquez. 2025. "Development and Characterization of κ-Carrageenan and Boron Nitride Nanoparticle Membranes for Improved Ionic Conductivity in Fuel Cells" Fuels 6, no. 1: 15. https://doi.org/10.3390/fuels6010015
APA StyleChavez-Baldovino, E., Malca-Reyes, C. A., Masso, R., Feng, P., & Díaz-Vázquez, L. M. (2025). Development and Characterization of κ-Carrageenan and Boron Nitride Nanoparticle Membranes for Improved Ionic Conductivity in Fuel Cells. Fuels, 6(1), 15. https://doi.org/10.3390/fuels6010015








