Geochemical and Thermodynamic Study of Formation Water for Reservoir Management in Bibi Hakimeh Oil and Gas Field, Iran
Abstract
1. Introduction
2. Previous Studies
3. Materials and Methods
4. Results and Analysis
4.1. Thermodynamic Analysis
4.2. Geochemical Analysis
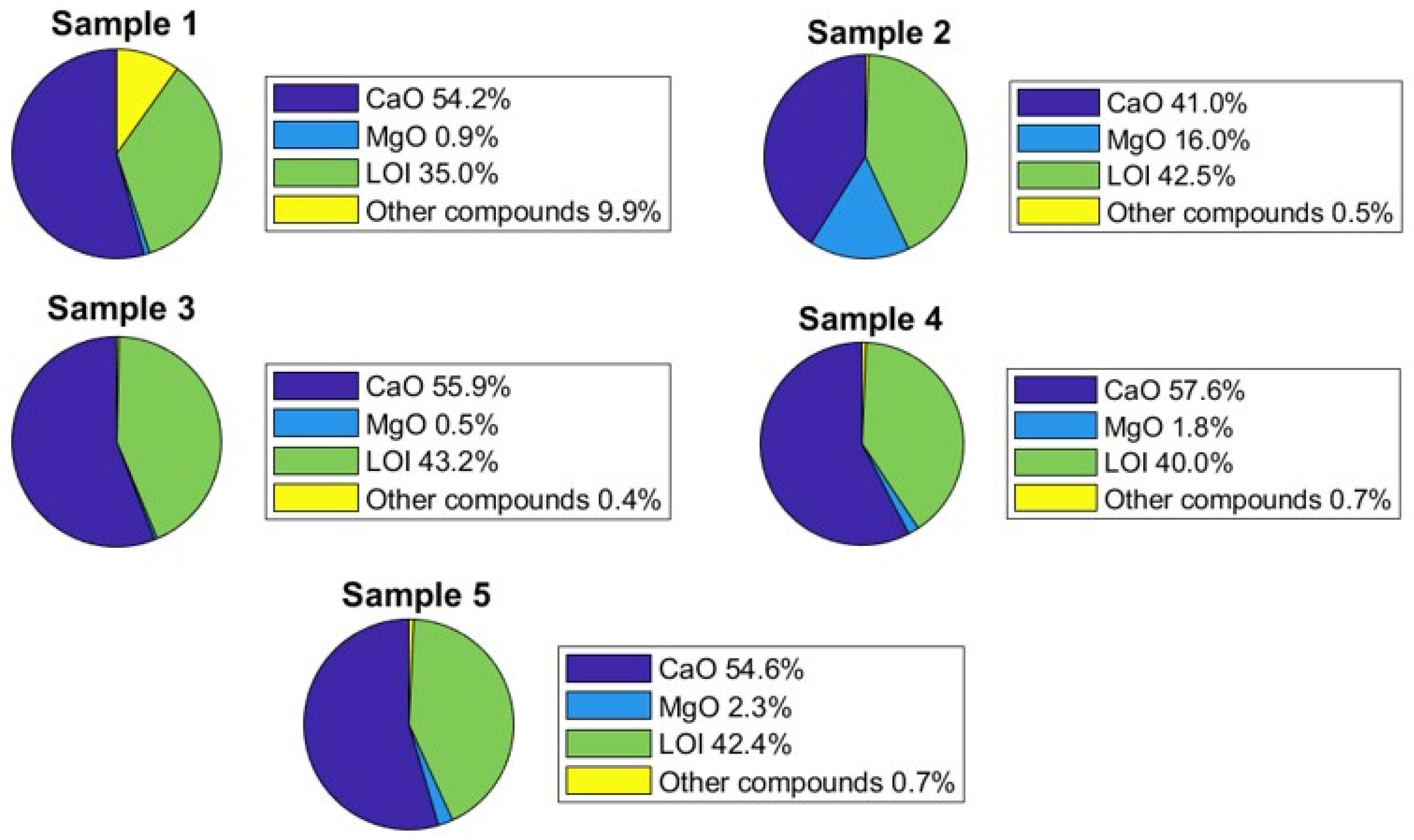
4.3. Water and Formation Rock
5. Discussion and Conclusions
- -
- The hydrochemical analysis and water geochemistry of five wells at the Bibi Hakimeh oil and gas field pointed to strong geochemical interactions and potential production consequences, with the following key conclusions:
- -
- A comparison between the water formation and Bibi Hakimeh formation rock indicates that, except for calcium (Ca2+) and magnesium (Mg2+) ions, the source of ions in the water reservoir is not the rock reservoir itself but rather original marine water.
- -
- Under the temperature and pressure conditions of the Bibi Hakimeh oil field, the precipitation of calcium carbonate and calcium sulfate is to be expected.
- -
- The concentration of mineral ions such as calcium, sodium, magnesium, sulfate, chloride, and bicarbonate in the water of the Bibi Hakimeh oil field formation can significantly impact the oil exploitation process.
- -
- The geochemical properties of water from the Bibi Hakimeh oil and gas field formation suggest suitable conditions for gas storage and production.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hashemi, S.H.; Niknam, A.; Karimian Torghabeh, A.; Pimentel, N. Thermodynamic and geochemical studies of formation water in Rag-e Sefid oil and gas field, Iran. AIMS Geosci. 2023, 9, 578–594. [Google Scholar] [CrossRef]
- Hirasaki, G.J. Wettability: Fundamentals and Surface Forces. SPE Form Eval. 1991, 6, 217–226. [Google Scholar] [CrossRef]
- Endean, H.; Shelton, R. Water Initiated Problems in Production Operations; Champion Technologies, Inc.: Houston, TX, USA, 1991. [Google Scholar]
- Hashemi, S.H.; Din Mohammad, M.; Mousavi Dehghani, S.A. Thermodynamic Prediction of Ba and Sr Sulfates Scale Formation in Waterflooding Projects in Oil Reservoirs. J. Miner. Resour. Eng. 2019, 4, 23–37. [Google Scholar]
- Hashemi, S.H.; Hashemi, S.A. Prediction of Scale formation according to water injection operations in Nosrat Oil Field. Model. Earth Syst. Environ. 2020, 6, 585–589. [Google Scholar] [CrossRef]
- Hashemi, S.H.; Mousavi Dehghani, S.A.; Khodadadi, H.; Dinmohammad, M.; Hosseini, S.M.; Hashemi, S.H. Optimization of Extended UNIQUAC Parameter for Activity Coefficients of Ions of an Electrolyte System Using Genetic Algorithms. Korean Chem. Eng. Res. 2017, 55, 652–659. [Google Scholar]
- Hashemi, S.H.; Mousavi Dehghani, S.A.; Samimi, S.E.; Dinmohammad, M.; Hashemi, S.A. Performance comparison of GRG algorithm with evolutionary algorithms in an aqueous electrolyte system. Model. Earth Syst. Environ. 2020, 6, 2103–2110. [Google Scholar] [CrossRef]
- Hashemi, S.H. Thermodynamic Study of water activity of Single Strong Electrolytes. J. Appl. Comput. Mech. 2017, 3, 150–157. [Google Scholar]
- Hashemi, S.H.; Bagheri, M.; Hashemi, S.A. Thermodynamic study of the effect of concentration and ionic strength on osmotic coefficient of aqueous sulfate and chloride solutions at 298.15 K. Model. Earth Syst. Environ. 2020, 6, 2189–2196. [Google Scholar] [CrossRef]
- Hashemi, S.H.; Dinmohammad, M.; Bagheri, M. Optimization of Extended UNIQUAC model parameter for mean activity coefficient of aqueous chloride solutions using Genetic+PSO. J. Chem. Pet. Eng. 2020, 54, 1–12. [Google Scholar]
- Mitchell, W.; Grist, M.; Boyle, J. Chemical Treatments Associated With North Sea Projects. J. Pet. Technol. 1980, 32, 904–912. [Google Scholar] [CrossRef]
- Jordan, M.; Graff, J.; Cooper, N. Development and Deployment of a Scale Squeeze Enhancer and Oil-Soluble Scale Inhibitor to Avoid Deferred Oil Production Losses. In Proceedings of the International Symposium on Formation Damage Control, Lafayette, LA, USA, 23–24 February 2000. SPE 58725. [Google Scholar]
- Moghadasi, J.; Jamialahmadi, M.; Muller-Steinhagen, H.; Sharif, A. Scale Formation in Oil Reservoir and Production Equipment. In Proceedings of the SPE European Formation Damage Conference, The Hague, The Netherlands, 13–14 May 2003; SPE 82233. pp. 1–12. [Google Scholar]
- Nasr-El-Din, H.; Al-Saiari, H.; Al-Hajji, H. A Single-Stage Acid Treatment to Remove and Mitigate Calcium Carbonate Scale; SPE International: Aberdeen, UK, 2004; SPE 87454. [Google Scholar]
- Raju, K. Successful Scale Mitigation Strategies. In Proceedings of the SPE International Symposium on Oilfield, The Woodlands, TX, USA, 20–22 April 2009. SPE 121679. [Google Scholar]
- Al-Roomi, Y.M.; Hussain, K.F. Potential kinetic model for scaling and scale inhibition mechanism. Desalination 2016, 393, 186–195. [Google Scholar] [CrossRef]
- Ghalib, H.B.; Almallah, I.A.R. Scaling simulation resulting from mixing predicted model between Mishrif formation water and different waters injection in Basrah oil field, southern Iraq. Model. Earth Syst. Environ. 2017, 3, 1557–1569. [Google Scholar] [CrossRef]
- Wang, Q.; Liang, F.; Al-Nasser, W.; Al-Dawood, F.; Al-Shafai, T.; Al-Badairy, H.; Shen, S.; Al-Ajwad, H. Laboratory study on efficiency of three calcium carbonate scale inhibitors in the presence of EOR chemicals. Petroleum 2018, 4, 375–384. [Google Scholar] [CrossRef]
- Ghalib, H.B.; Al-Hawash, A.B.; Muttashar, W.R.; Bozdag, A.; Al-Saady, A.A. Determining the effect of mineral scaling formation under different injection water sources on the performance of Mishrif carbonate reservoir in Halfaya oilfield, Southern Iraq. J. Petrol. Explor. Prod. Technol. 2023, 13, 1265–1282. [Google Scholar] [CrossRef]
- Abtahi, S.T. Bibi Hakima oil field development plan. Explor. Prod. 2009, 55, 13–16. [Google Scholar]
- Ebadi, N.; Yaram Taghlousohrabi, M.; Rahimi, T.; Varnasari, N. Performance and development of fractures in the Asmari reservoir, one of the southwestern fields of Bibi Hakimeh field. In Proceedings of the International Conference on Research in Science and Technology, Pathum Thani, Thailand, 4–6 November 2015. [Google Scholar]
- Beydoun, Z.R.; Hughes Clark, M.W.; Stonely, R. Petroleum in Zagros Basin: A late Tertiary foreland basin overprinted on to the outer edge of vast hydrocarbon rich Paleozoic Mesozoic passive margin shelf. Am. Assoc. Pet. Geol. J. 1992, 23, 309–339. [Google Scholar]
- ASTM D511-14(2021)e1; Standard Test Methods for Calcium and Magnesium in Water. ASTM International: West Conshohocken, PA, USA, 2021. Available online: https://www.astm.org/Standards/D511.htm (accessed on 15 September 2024).
- Available online: https://www.astm.org/catalogsearch/result/?q=ASTM-D512+ (accessed on 15 September 2024).
- Available online: https://www.hach.com/p-ferrover-iron-reagent-powder-pillows-10-ml-pk1000/2105728?srsltid=AfmBOoocrgNdmrXICnE7rtZtqWfyhZpv-P3fGzmOdvYixEZDU4H2B_2d (accessed on 15 September 2024).
- Available online: https://www.hach.com/asset-get.download-en.jsa?id=7639983901&srsltid=AfmBOorhfPkmEckMOw6O9-zpUntM_2lF_kwzRTUIueKPbqv-fVk51KCJ (accessed on 15 September 2024).
- Betz, L. Betz Handbook of Industrial Water Conditioning, 6th ed.; Betz: Trevose, PA, USA, 1962. [Google Scholar]
- Collins, A.G. Geochemistry of Oilfield Waters. In Developments in Petroleum Science; Elsevier Scientific Publishing Company: Oxford, NY, USA, 1975. [Google Scholar]
- Awadh, S.M. Chemical, physical characterization and salinity distribution of the oilfield water in the Upper Sandstone Member of the Zubair reservoir at Rumaila North Oilfield, Southern Iraq. Iran. J. Oil Gas Sci. Technol. 2018, 7, 20–39. [Google Scholar]
- Hem, J.D. Study and Interpretation of the Chemical Characteristics of Natural Water. USGS Water Supply Pap. 1985, 253, 2254. [Google Scholar]
- Taylor, E.W. The Examination of Water and Water Supplies; Church Hill Ltd., Press: Subiaco, WA, USA, 1958. [Google Scholar]
- De Choudens-Sanchez, V.; Gonzalez, L.A. Calcite and aragonite precipitation under controlled instantaneous supersaturation: Elucidating the role of CaCO3 saturation state and Mg/Ca ratio on calcium carbonate polymorphism. J. Sediment. Res. 2009, 79, 363–376. [Google Scholar] [CrossRef]
- Osborn, S.G.; Vengosh, A.; Warner, N.R.; Jackson, R.B. Methane contamination of drinking water accompanying gas-well drilling and hydraulic fracturing. Proc. Natl. Acad. Sci. USA 2011, 108, 8172–8176. [Google Scholar] [CrossRef]
- Rice, C.; Flores, R.; Stricker, G.; Ellis, M. Chemical and stable isotopic evidence for water/rock interaction and biogenic origin of coalbed methane, Fort Union Formation, Powder River Basin, Wyoming and Montana USA. Int. J. Coal Geol. 2008, 76, 76–85. [Google Scholar] [CrossRef]
- Mirzaee, S.Y.; Zarasvandi, A.; Orang, M. Geochemical effect of Asmari Oil Reservoirs on Masjed-e-Solaiman Karstic Water Resources. Adv. Appl. Geol. 2016, 5, 1–14. [Google Scholar]
- Ranasinghe, P.; Dissanayake, C.; Rupasinghe, M. Application of geochemical ratios for delineating gem-bearing areas in high grade metamorphic terrains. Appl. Geochem. 2005, 20, 1489–1495. [Google Scholar] [CrossRef]
- Zheng, Y.-F. Metamorphic chemical geodynamics in continental subduction zones. Chem. Geol. 2012, 328, 5–48. [Google Scholar] [CrossRef]
- Yu, H.; Wang, Z.; Rezaee, R.; Zhang, Y.; Nwidee, L.N.; Liu, X.; Verrall, M.; Iglauer, S. Formation water geochemistry for carbonate reservoirs in Ordos basin, China: Implicationsfor hydrocarbon preservation by machine learning. J. Pet. Sci. Eng. 2020, 185, 106673. [Google Scholar] [CrossRef]
- Folk, R.L.; Land, L.S. Mg/Ca ratio and salinity: Two controls over crystallization of dolomite. AAPG Bull. 1975, 59, 60–68. [Google Scholar]
- Koleini, M.M.; Mehraban, M.F.; Ayatollahi, S. Effects of low salinity water on calcite/brine interface: A molecular dynamics simulation study. Colloids Surf. A Physicochem. Eng. Asp. 2018, 537, 61–68. [Google Scholar] [CrossRef]
- Lear, C.H.; Rosenthal, Y.; Slowey, N. Benthic foraminiferal Mg/Ca-paleothermometry: A revised core-top calibration. Geochim. Et Cosmochim. Acta 2002, 66, 3375–3387. [Google Scholar] [CrossRef]
- Nielsen, M.R.; Sand, K.K.; Rodriguez-Blanco, J.D.; Bovet, N.; Generosi, J.; Dalby, K.N.; Stipp, S.L.S. Inhibition of calcite growth: Combined effects of Mg2+ and SO42–. Cryst. Growth Des. 2016, 16, 6199–6207. [Google Scholar] [CrossRef]
- Shariatpanahi, S.F.; Strand, S.; Austad, T. Initial wetting properties of carbonate oil reservoirs: Effect of the temperature and presence of sulfate in formation water. Energy Fuels 2011, 25, 3021–3028. [Google Scholar] [CrossRef]
- Wilkinson, B.H.; Algeo, T.J. Sedimentary carbonate record of calcium-magnesium cycling. Am. J. Sci. 1989, 289, 1158–1194. [Google Scholar] [CrossRef]
- Van Voast, W.A. Geochemical signature of formation waters associated with coalbed methane. AAPG Bull. 2003, 87, 667–676. [Google Scholar] [CrossRef]
- Chen, T.; Neville, A.; Yuan, M. Calcium carbonate scale formation—Assessing the initial stages of precipitation and deposition. J. Pet. Sci. Eng. 2005, 46, 185–194. [Google Scholar] [CrossRef]
- Kurita, Y.; Nakada, T.; Kato, A.; Doi, H.; Mistry, A.C.; Chang, M.H.; Romero, M.F.; Hirose, S. Identification of intestinal bicarbonate transporters involved in formation of carbonate precipitates to stimulate water absorption in marine teleost fish. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2008, 294, R1402–R1412. [Google Scholar] [CrossRef] [PubMed]
- Palandri, J.L.; Reed, M.H. Reconstruction of in situ composition of sedimentary formation waters. Geochim. Et Cosmochim. Acta 2001, 65, 1741–1767. [Google Scholar] [CrossRef]
- Xu, T.; Zhu, C.; Jiang, X.; Tang, B.; Wang, X.; Zhang, H.; Zhang, B. Chemical Characteristics of Ordovician Formation Water and Its Relationship with Hydrocarbons in Halahatang Depression, Tarim Basin, NW China. Water 2022, 14, 756. [Google Scholar] [CrossRef]
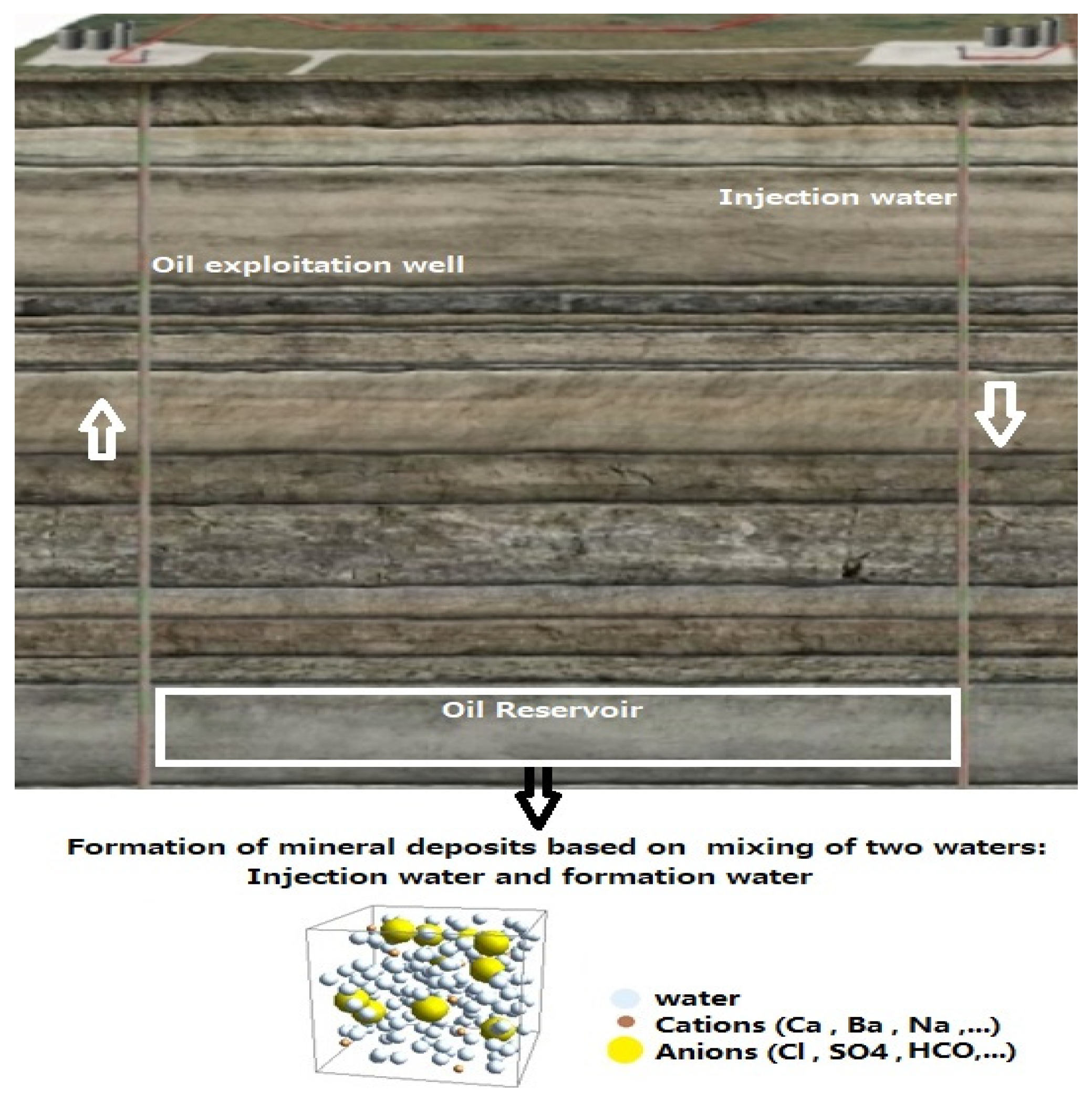
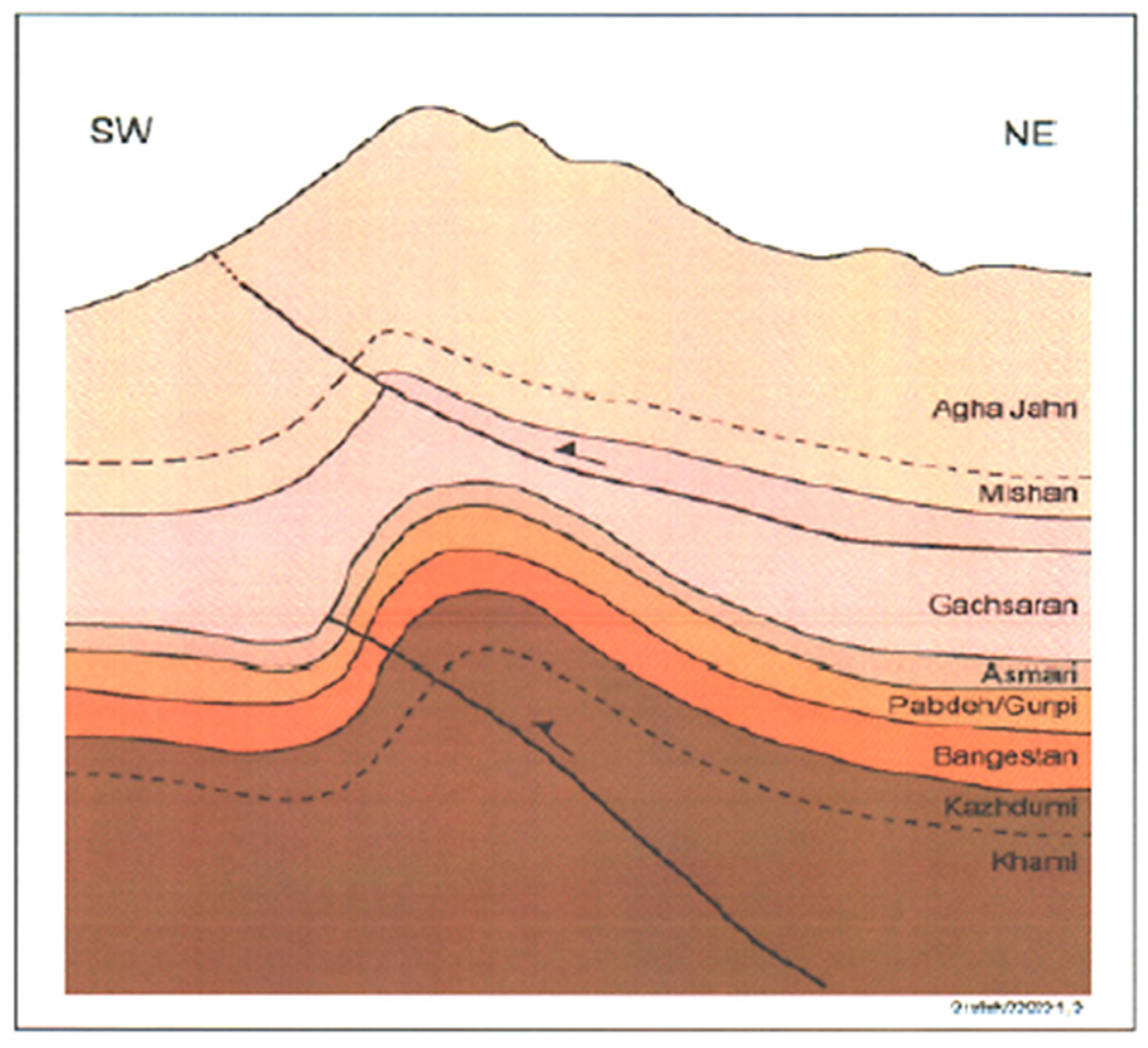
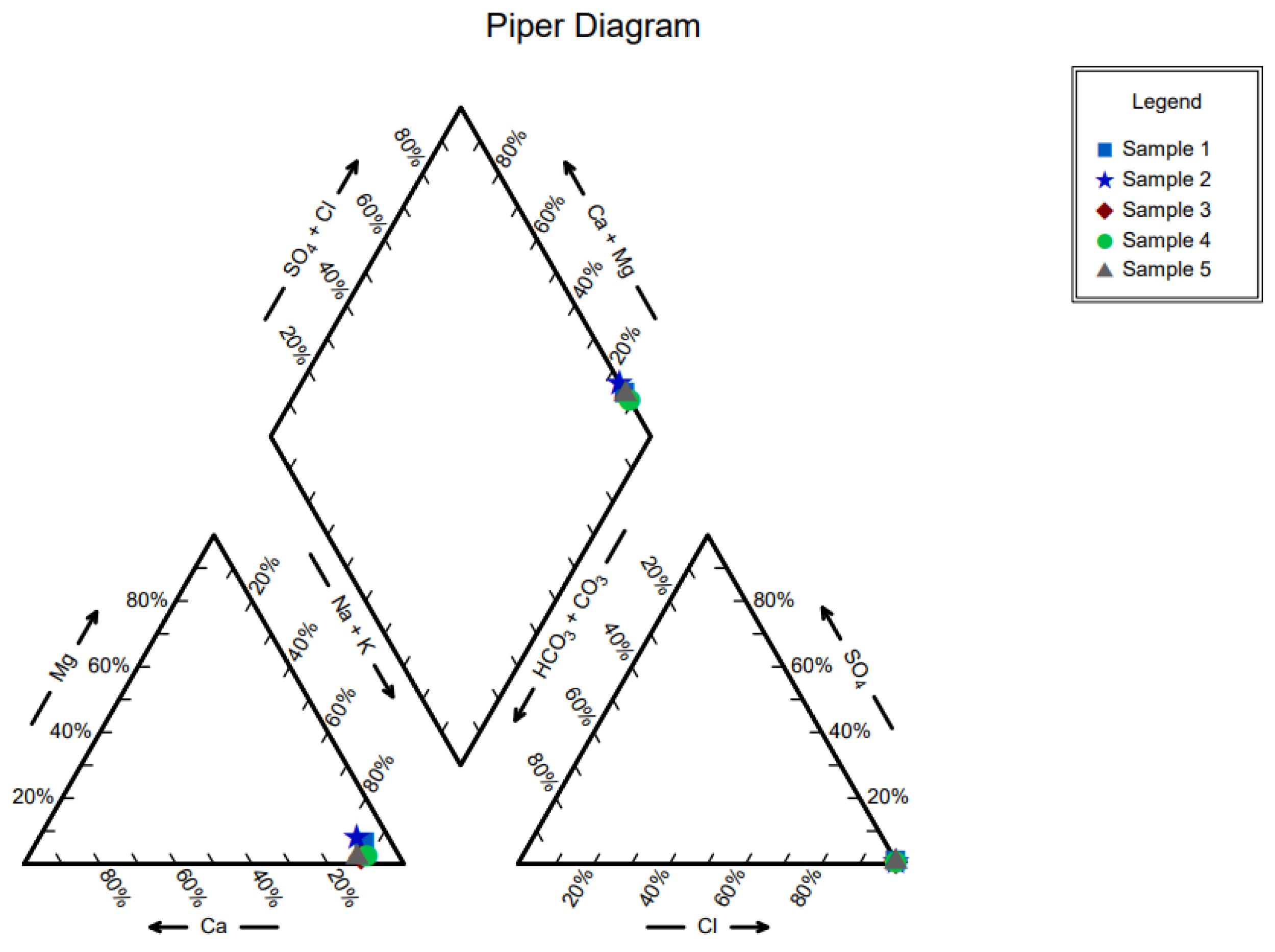
| Ions (mg/Lit) | Sample1 | Sample 2 | Sample 3 | Sample 4 | Sample 5 |
|---|---|---|---|---|---|
| Na+ | 22,979 | 31,671 | 76,379 | 78,311 | 75,413 |
| Ca2+ | 1640 | 2720 | 7600 | 6400 | 8400 |
| Mg2+ | 923 | 1628 | 972 | 1215 | 972 |
| Fe2+ | 0 | 0 | 0 | 0 | 0 |
| Cl− | 90,525 | 143,775 | 133,125 | 134,900 | 133,125 |
| SO42− | 1300 | 1200 | 1000 | 1000 | 1000 |
| HCO3− | 122 | 122 | 610 | 244 | 488 |
| Total dissolved solids | 117,489 | 181,116 | 219,686 | 222,070 | 219,398 |
| pH (at 25 °C) | 6.37 | 6.99 | 6.16 | 6.48 | 6.19 |
| Well | Deposition (% Saturation *) | Survey Data | |||
|---|---|---|---|---|---|
| 3000 Psia and 176 F | 3130 Psia and 180 F | 3200 Psia and 182 F | 3300 Psia and 186 F | ||
| Sample 1 | CaSO4 | 38.73 | 40.18 | 40.92 | 42.44 |
| CaSO4.2H2O | 11.27 | 10.94 | 10.78 | 10.46 | |
| CaCO3 | 100 | 100 | 100 | 100 | |
| MgCO3 | 30.72 | 33.14 | 34.41 | 37.07 | |
| Sample 2 | CaSO4 | 43.22 | 44.93 | 45.81 | 47.6 |
| CaSO4.2H2O | 10.81 | 10.52 | 10.37 | 10.08 | |
| CaCO3 | 100 | 100 | 100 | 100 | |
| MgCO3 | 30.83 | 33.26 | 34.53 | 37.19 | |
| Sample 3 | CaSO4 | 89.46 | 92.88 | 94.63 | 98.2 |
| CaSO4.2H2O | 20.17 | 19.59 | 19.31 | 18.74 | |
| CaCO3 | 100 | 100 | 100 | 100 | |
| MgCO3 | 7.18 | 7.74 | 8.04 | 8.66 | |
| Sample 4 | CaSO4 | 75.28 | 78.16 | 79.64 | 82.65 |
| CaSO4.2H2O | 16.83 | 16.35 | 16.11 | 15.64 | |
| CaCO3 | 100 | 100 | 100 | 100 | |
| MgCO3 | 10.51 | 11.34 | 11.77 | 12.68 | |
| Sample 5 | CaSO4 | 100 | 100 | 100 | 100 |
| CaSO4.2H2O | 22.58 | 21.13 | 20.44 | 19.12 | |
| CaCO3 | 100 | 100 | 100 | 100 | |
| MgCO3 | 6.46 | 6.98 | 7.25 | 7.82 | |
| Well | Deposition (mg/L) | Survey Data | |||
|---|---|---|---|---|---|
| 3000 Psia and 176 F | 3130 Psia and 180 F | 3200 Psia and 182 F | 3300 Psia and 186 F | ||
| Sample 1 | CaCO3 | 34.6 | 35.6 | 36 | 36.98 |
| Sample 2 | CaCO3 | 40.49 | 41.49 | 41.98 | 42.97 |
| Sample 3 | CaCO3 | 370.6 | 373.73 | 375.28 | 378.31 |
| Sample 4 | CaCO3 | 119.48 | 121.20 | 122.06 | 123.74 |
| Sample 5 | CaSO4 | 0.167 | 49.92 | 74.02 | 120.73 |
| CaCO3 | 291.79 | 294.3 | 295.5 | 297.99 | |
| Ratios | Formation Water | ||||
|---|---|---|---|---|---|
| Sample 1 | Sample 2 | Sample 3 | Sample 4 | Sample 5 | |
| Na+/Cl− | 0.254 | 0.22 | 0.574 | 0.58 | 0.566 |
| (Ca + Mg)/SO4 | 1.97 | 3.62 | 8.57 | 7.61 | 9.37 |
| (Cl− − Na+)/Mg2+ | 73.18 | 68.86 | 58.38 | 46.57 | 59.37 |
| HCO−/Cl− | 0.0013 | 0.00085 | 0.0046 | 0.0018 | 0.0036 |
| (HCO− − CO3)/Ca2+ | 0.07 | 0.04 | 0.08 | 0.04 | 0.06 |
| Mg2+/Ca2+ | 0.56 | 0.6 | 0.13 | 0.19 | 0.12 |
| Ca/Cl | 0.018 | 0.189 | 0.0571 | 0.047 | 0.064 |
| Mg/Cl | 0.0102 | 0.0113 | 0.0073 | 0.009 | 0.0073 |
| SO4/Cl | 0.0143 | 0.0083 | 0.0075 | 0.0074 | 0.00751 |
| SO4/HCO3 | 10.655 | 9.836 | 1.639 | 4.098 | 2.049 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hashemi, S.H.; Torghabeh, A.K.; Niknam, A.; Hashemi, S.A.; Gharaie, M.H.M.; Pimentel, N. Geochemical and Thermodynamic Study of Formation Water for Reservoir Management in Bibi Hakimeh Oil and Gas Field, Iran. Fuels 2025, 6, 11. https://doi.org/10.3390/fuels6010011
Hashemi SH, Torghabeh AK, Niknam A, Hashemi SA, Gharaie MHM, Pimentel N. Geochemical and Thermodynamic Study of Formation Water for Reservoir Management in Bibi Hakimeh Oil and Gas Field, Iran. Fuels. 2025; 6(1):11. https://doi.org/10.3390/fuels6010011
Chicago/Turabian StyleHashemi, Seyed Hossein, Amir Karimian Torghabeh, Abbas Niknam, Seyed Abdolrasoul Hashemi, Mohamad Hosein Mahmudy Gharaie, and Nuno Pimentel. 2025. "Geochemical and Thermodynamic Study of Formation Water for Reservoir Management in Bibi Hakimeh Oil and Gas Field, Iran" Fuels 6, no. 1: 11. https://doi.org/10.3390/fuels6010011
APA StyleHashemi, S. H., Torghabeh, A. K., Niknam, A., Hashemi, S. A., Gharaie, M. H. M., & Pimentel, N. (2025). Geochemical and Thermodynamic Study of Formation Water for Reservoir Management in Bibi Hakimeh Oil and Gas Field, Iran. Fuels, 6(1), 11. https://doi.org/10.3390/fuels6010011







