Abstract
Climate change and the growing demand for energy have prompted research on alternative eco-friendly energy sources. This study focused on the potential for biogas production from water hyacinth and banana peel waste through physicochemical characterization and batch anaerobic digestion tests. The water hyacinth and banana peel samples were dried, ground, and subjected to elemental, proximate, and fiber content analyses. Subsequently, banana peel waste, water hyacinth stems, and leaves were used for batch anaerobic digestion tests in 500 mL glass flask bottles for 21 days under mesophilic conditions in n = 3 trials. Kruskal–Wallis and Dunnett’s tests were performed to identify the significance of the differences in biogas yield among the samples. The analyses of the elemental, proximate, and fiber contents of water hyacinth and banana peels revealed that they possess a suitable chemical composition and essential nutrients for the production of high-yield biogas. The biogas yields from water hyacinth leaves, stems, and banana peels were 280.15, 324.79, and 334.82 mL/g VS, respectively. These findings indicate that water hyacinth and banana peel waste have significant potential for biogas production.
Keywords:
water hyacinth; banana peels; characterization; biogas; anaerobic digestion; biofuel; potential; Togo 1. Introduction
The pressing need to seek sustainable energy sources is evident, as the world is reaching a critical point in depleting fossil fuel reserves and is facing a surge in energy demand. Global decarbonization of the energy sector is necessary [1]. Since the last decade, there has been growing global interest in renewable energy sources as potential substitutes for fossil fuels. The development and deployment of renewable energy technologies are considered a backbone solution to climate crises, as they significantly drive down carbon dioxide (CO2) emissions. Biogas is a renewable and environmentally friendly fuel produced by the anaerobic digestion of organic materials, such as biomass, waste resources, industrial effluents, and wastewater sludge [2]. Anaerobic digestion is a biological process by which particulate organic matter is broken down into simple soluble forms owing to a robust microbial community in the absence of oxygen, thereby releasing biogas [3]. Biogas presents a significant solution to the energy crisis, as it boasts a multitude of applications (including electricity generation, clean cooking fuel, and transportation fuel when upgraded) and relies on readily accessible organic materials for its production.
Water hyacinth (WH), also known as Eichhornia crassipes, is the world’s most harmful and dreadful aquatic invasive plant, covering a wide area of water bodies with thousands of tons of fresh biomass [4]. Water hyacinth invasiveness disturbs aquatic systems and jeopardizes the livelihoods of people who engage in economic activities in water bodies [5]. In Lomé (Togo), water hyacinth has caused the dramatic flooding of lakes and their vicinities in recent years during heavy rainfall periods, owing to disturbances in the water flow. The invasiveness of the water hyacinth represents a serious hindrance to the navigation, traffic, and recreation of people in water bodies. Figure 1 shows the water hyacinth invading a lake in the city of Lomé, Togo. The elimination of water hyacinth from water sources presents a substantial logistical and financial challenge. Nevertheless, the rapid growth of water hyacinth makes it an ideal candidate for conversion into energy products, particularly biogas. Furthermore, Togo is a significant producer of banana, particularly in the southern region of the country. The production of bananas in Togo has experienced a noteworthy increase from 12,000 tons in 1973 to 24,358 tons in 2022, with an annual average global increase rate of 1.48% [6]. The generation of considerable quantities of bananas results in substantial amounts of banana peel waste, which is frequently disposed of at landfill sites. However, in the pursuit of a circular economy and energy access in the country, this waste can be repurposed for biogas production.

Figure 1.
Water hyacinth plants covering a lake in Lomé.
Studies have been conducted to characterize water hyacinth and banana peel biomass for conversion into energy products [7,8]. Wauton et al. [9] reported that dried water hyacinth was composed of 27.90 wt% hemicellulose, 26.50 wt% cellulose, and 6.10 wt% lignin, whereas C, H, O, and N were the main chemical elements. Kabenge et al. [10] conducted a study to assess the potential of banana peel waste as a slow pyrolysis feedstock. Their research revealed the following composition of banana peels: 8 wt% volatile matter, 18.38 C/N, 58.08 wt% organic matter, 41.38 wt% hemicellulose, 9.9 wt% cellulose, and 8.9 wt% lignin.
In the last decade, research has focused on the anaerobic digestion of water hyacinth and banana peels as potential substrates for biogas production [11,12]. Hudakorn et al. [13] reported a methane yield of 237 L/kg of VS from the anaerobic digestion of water hyacinth leaves and the juice of water hyacinth stems at a food-to-microorganism ratio (F/M) of 1:1. Nugraha et al. [14] reported that up to 151.548 mL/g of VS biogas was obtained from water hyacinth at a food-to-microorganism ratio (F/M) of 10.01. Recently, Manigandan et al. [15] examined the effects of pretreatment on biogas production from various water hyacinth samples. They reported that pre-treated water hyacinth samples exhibited a higher biogas yield, with the maximum cumulative yield reaching 209 mL on the 19th day. Achinas et al. [16] reported a maximum biogas yield of 112.18 mL/g of VS from the batch anaerobic digestion of banana peels at a concentration of 10 g/L, with 10% cow manure as the inoculum. This study aimed to analyze the biomass of water hyacinth and banana peels and to determine the biogas output from these materials through batch anaerobic digestion tests. A specific methodology was employed in which all components of the water hyacinth were examined to gain a more comprehensive understanding of the plant’s composition and to assess its suitability for biogas production.
2. Materials and Methods
2.1. Substrates Collection and Preparation
Water hyacinth plants of the species Eichhornia crassipes were harvested from the shore of a lake in Lomé, Togo. The samples were thoroughly washed with tap water and rinsed with distilled water to remove any potential dirt on their surface. Banana peels of the species Musa acuminata Cola were collected from the students’ hostel on the campus of the Université de Lomé in Lomé. Water hyacinth and banana peel samples were naturally dried in sunlight for seven consecutive days. After sun-drying, water hyacinth was separated into leaves, stems, and roots. Subsequently, samples of water hyacinth leaves (WHL), water hyacinth stems (WHS), water hyacinth roots (WHR), and banana peels (BP) were subjected to thermal pretreatment consisting of oven-drying (BINDER) for 24 h at 105 °C. The samples were ground into small particles and stored in separate airtight plastic bottles for subsequent use in the experiments.
2.2. Characterization of the Substrates
2.2.1. Ultimate Analysis
Elemental analysis of the ground samples was performed using laser-induced breakdown spectroscopy (LIBS), as documented in [17], to analyze the elemental composition of each sample (WHL, WHS, WHR, and BP). Five replicates were analyzed for each sample.
2.2.2. Proximate Analysis
Proximate analysis was performed following the DIN EN 15935 (2012-11) [18] to determine the volatile solids (VS), ash content (AC), moisture content (MC), and total solids (TS) of the samples. Three stages of analysis were conducted to fully characterize the samples. The first stage consisted of analyzing the TS and MC of freshly harvested water hyacinth and banana peels to evaluate the amount of water in the substrates. In the second stage, the water hyacinth plant was separated into different parts, such as roots, stems, and leaves, to analyze the mass percentage of each portion. Finally, the ground and stored samples of WHL, WHS, WHR, and BP (as described in Section 2.1) were analyzed before their use in batch anaerobic digestion tests.
In the first stage, fresh WH and BP samples were collected in two bowls and weighed on a weight scale (KERN, ABT 320-4M). After weighing, the two samples were successively dried in sunlight for seven consecutive days and in an oven (BINDER) at 105 °C for 24 h to achieve constant mass. The final masses of the samples were recorded after oven-drying. The moisture content (MCf) and total solids (TSf) of the fresh samples were calculated using Equations (1) and (2).
where mf = mass of the fresh sample with the bowl, md = mass of the dried sample with the bowl, and mbowl = mass of bowl.
In the second stage, fresh water hyacinth was dried under sunlight for seven days. After sun-drying, the mass of the sample was recorded (msample). The samples were then separated into roots, stems, and leaves, as described previously. The mass of each part was then recorded, and the mass percentage of each part was calculated using Equations (3)–(5).
where mWHL = weight of water hyacinth leaves, mWHS = weight of water hyacinth stems, mWHR = weight of water hyacinth roots, %_WHL = share of water hyacinth leaves, %_WHS = share of water hyacinth stems, and %_WHR = share of water hyacinth roots.
Finally, to determine the VS, MC, AC, and TS contents of the dried, ground, and stored samples, three replicates of each sample (WHL, WHS, WHR, and BP) were taken in different crucibles, and their weights were recorded (mf) using a weight scale (KERN, ABT 320-4M). The crucibles containing the samples were dried in an oven (BINDER) at 105 °C to a constant weight. Constant weights of the crucibles with the dried samples were recorded (md). Subsequently, the samples were calcined in a muffle furnace (Venticell, Armin BAACK, Labortechnik) at 220 °C for 30 min and then for two hours at 550 °C. After incineration, the hot crucibles were cooled using a desiccator. After cooling the crucibles, their weights were recorded (mash). Equations (6)–(9) were used to determine the volatile solids (VS), ash content (AC), moisture content (MC), and total solids (TS) of the samples.
2.2.3. Fiber Content Analysis
The fractions of cellulose, hemicellulose, and lignin in WHL, WHS, WHR, and BP were investigated using an in-house method modeled after the prescribed method of VDLUFA, Book of Methods III, 2nd Supplement, Hamburg 1988 (VDLUFA 1988), as documented in [18]. The method consists of determining neutral detergent fiber (NDF), acid detergent fiber (ADF), and acid detergent lignin (ADL). FibreBags were used, and their empty weights were recorded (m1). Then, one gram of each sample was placed in a FibreBag, and the mass of the FibreBag with the sample was recorded as m2. A glass spacer was introduced into the FibreBag, and both were placed in a sample carousel. Then, all FibreBags were plunged for 5 min in a beaker containing approximately 300 mL of acetone solution to remove fats. Afterward, the samples were placed in a Fibretherm (FT 12) to be washed with a 1.3 L ADF solution. After the completion of the Fibretherm operation, the samples were dried in an oven (BINDER) overnight at 105 °C, and the mass of each sample in the crucible was recorded (m4). The mass of the empty crucible was recorded as m3. The FibreBags were then ashed at 500 °C for two hours in a muffle furnace (Venticell Armin BAACK, Labortechnik). After cooling in a desiccator, the weights of the samples in the crucible were recorded as m5.
For ADL determination, dried samples (m4) were hung on a carousel. The carousel with the Fibrebags was placed in a five-liter beaker containing 72% sulfuric acid at room temperature. The FibreBags were then rinsed with hot water to the neutral point and dried for 24 h at 105 °C, and their mass was recorded as m7.
Similarly, for NDF determination, after the removal and drying of the samples for approximately two minutes in the exhaust, the Fibretherm was switched on to wash the samples with NDF solution. After the Fibretherm operation was completed, the spacer was carefully removed from each FibreBag. The FibreBag with the sample was rolled up, placed in a crucible, and dried at 105 °C for 24 h (m8). Subsequently, the FibreBag containing the sample was ashed at 500 °C and cooled, and the weight was recorded (m9). The fiber content was calculated using Equations (10)–(15).
%_Hemicellulose = NDF − ADF
%_Lignin = ADL
%_Cellulose = NDF − (%_Hemicellulose + %_Lignin)
ADF = share of acid detergent fiber (%TS), ADL = share of acid detergent lignin (%TS), and NDF = share of neutral detergent fiber (%TS).
2.3. Biogas Production Test
2.3.1. Inoculum Collection
The inoculum used for the batch anaerobic digestion tests was obtained from a mesophilic biogas plant in Rostock, Germany, where the experiments were conducted. The inoculum was left untreated for the batch tests.
2.3.2. Batch Anaerobic Digestion Tests of Water Hyacinth Leaves, Water Hyacinth Stems, and Banana Peels
Gas-tight seals equipped with an automated gas measurement system were used for batch anaerobic digestion tests conducted in accordance with the Association of German Engineers (VDI) 4630: “Fermentation of organic materials” standard [19]. Specifically, 15 glass flasks with a volume of 500 mL were cleaned and prepared for three replicate batch experiments. A magnetic stirring rod was introduced into all bottles. The inoculum was stirred until homogeneity was achieved. To achieve an F/M ratio of 0.4 between the inoculum and the substrate, a constant mass of 200 g of inoculum was used for all the samples. Homogenous inoculum was fed into all fifteen (15) flask bottles. The pretreatment applied to the substrates is described in Section 2.1. A total of 5 g of cellulose, 6 g of ground WHL, 7 g of ground WHS, and 6 g of ground BP samples were added to the bottles in triplicate. The three (3) remaining bottles contained only the inoculum and served as blank samples. Finally, 100 g of tap water was added to each bottle. Cellulose and blank bottles were used as digestion controls. The bottles were sealed using gas-tight seals equipped with an automated gas measurement system. All bottles were placed in a temperature-regulated water bath (Julabo TW20) set at a mesophilic temperature of 37 °C as shown in Figure 2. The bottles were stirred every day for one week and then every two days for the remaining time (two weeks) using a magnetic stirrer.
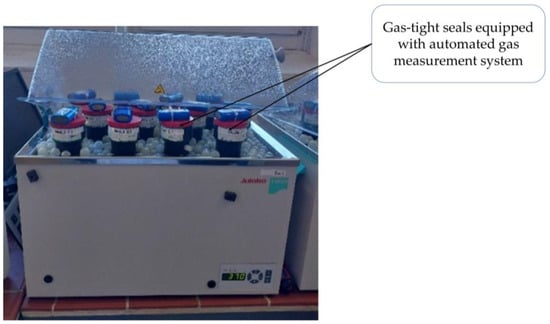
Figure 2.
Bottles containing the substrates placed in a temperature-regulated water bath (Julabo TW20).
The cumulative biogas yield was calculated using Equation (16). The average volume of biogas produced in the blank samples was subtracted from that obtained from the substrate samples. The obtained data were normalized to standard temperature and pressure (STP) conditions.
Ybiogas: biogas yield (mL/g VS), Vbiogas: cumulative biogas volume (mL), MVS substrate: mass of the VS of the substrate (g).
2.3.3. Statistical Significance Tests of the Differences in Cumulative Biogas Yield
Statistical analysis was conducted using R version 4.2.2 to determine the significance of the difference in biogas yield between the substrates and the controls, in addition to the simple observation of the cumulative biogas yield of both groups. The objective of this analysis was to investigate the influence of substrate composition, particularly regarding nutrients such as carbohydrates, on biogas yield during anaerobic digestion. To achieve this, the Kruskal–Wallis test was conducted using data on the cumulative biogas yield of the samples. Dunnett’s test was performed as a post-hoc analysis with Bonferroni correction. Dunnett’s test was used to identify specific sample pairs that were significantly different from each other.
3. Results and Discussion
3.1. Ultimate Analysis Results
The mean score measurements of the elemental analysis of water hyacinth leaves, stems, and banana peels using the LIBS method are shown in Table 1. Chemical elements include carbon (C), hydrogen (H), oxygen (O), potassium (K), sodium (Na), calcium (Ca) and silicon (Si).

Table 1.
Elemental composition of WHL, WHS, and BP.
As presented in Table 1, the standard error of the mean for most elements is too large. This was because of the five measurements performed, and not all measurements produced a score. Carbohydrate synthesis, which is essential for biogas production, particularly methane production through anaerobic digestion, is influenced by the presence of carbon and hydrogen. The minerals found in the different biomasses (K, Na, Ca, Si, P, and Mg) can contribute to improving cell growth and the formation of bioparticles (granular) inside the fermenter while increasing the hydrolytic capacity [20]. In general, the presence of trace minerals, which are micronutrients, can alleviate the nutritional requirements of the bacterial community in the fermentation medium. Biochar, which is known to contain a high amount of minerals, is introduced into anaerobic digestion as a biocatalyst to enhance methane formation by mitigating ammonia stress and augmenting the archaeal community [21]. Minerals act as biocatalysts to maintain equilibrium through bioprocesses during anaerobic digestion. Minerals such as K and Ca behave as enzymes and cofactors in the fermentation medium [22]. However, excessive amounts of minerals may inhibit the fermentation process through side reactions.
The fundamental composition of water hyacinth (Table 1) is similar to the findings reported by Sukarni et al. [23] from a similar analysis of WH. In the mentioned study, the mean and standard error were calculated based on four measurements, and the results obtained in wt% were C (14.4 ± 6.81), O (49.5 ± 6.71), Na (0.58 ± 0.40), Mg (1.96 ± 1.04), Al (2.32 ± 1.71), Zr (2.24 ± 1.33), Cl (5.58 ± 1.94), K (8.26 ± 2.62), Ca (4.73 ± 0.63), Si (5.33 ± 4.52), Ti (0.27 ± 0.27), and Fe (4.71 ± 4.32). Hence, it was concluded that the geographical location and origin of WH could affect its chemical composition.
3.2. Proximate Analysis Results
An analysis of the freshwater hyacinth sample revealed that it had over 94.16% moisture and 5.84% dry matter. Similarly, an analysis of the fresh BP sample revealed a moisture content of 88.11% and a dry matter content of 11.89%. Moreover, the analysis of the mass distribution of water hyacinth plants showed that stems had the highest mass share, followed by leaves, as shown in Figure 3.
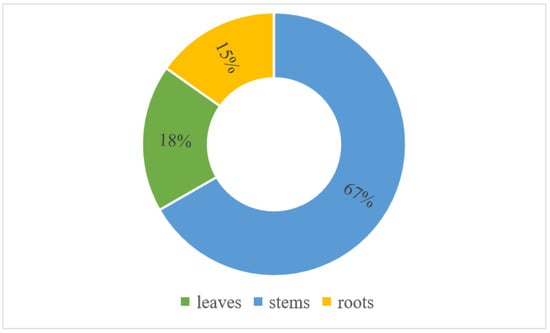
Figure 3.
Mass distribution of water hyacinth biomass.
The water content of the freshwater hyacinth investigated in this study was consistent with the findings reported by Omondi et al. [24] for the same substrate sampled from Lake Victoria in Kenya. In the previous study, the reported moisture content of freshwater hyacinth plants was 91%. This disparity may be attributed to the age of the harvested water hyacinth and its origin. The presence of moisture in water hyacinth and banana peels renders them suitable substrates for anaerobic digestion. The anaerobic digestion process necessitates a substantial quantity of water in the medium; consequently, highly wet substrates are preferred to minimize the consumption of potable water. In terms of the distribution of mass in the water hyacinth, stems accounted for the greatest proportion of mass (Figure 3). This suggests that the potential for biogas production from water hyacinth resides in the stems.
The mean score measurements of the proximate analysis of the dried and ground samples of the water hyacinth parts and banana peels are shown in Table 2.

Table 2.
Proximate analysis of water hyacinth parts and banana peels.
The results of the proximate analysis of the dried water hyacinth parts were consistent with those reported by Elsamadony et al. [25]. In the mentioned study, the VS and TS of water hyacinth as a whole plant were 73.08 wt% and 97.54 wt%, respectively, on a dry basis. The high VS content (70–80 wt%) shown by the different samples (BP, WHS, WHL, and WHR) (Table 2) constitutes valuable potential for biogas production via anaerobic digestion. The majority of VS (fermentable VS) degrades to produce biogas. In contrast, the relatively elevated AC in water hyacinth stems and roots may inhibit the digestion process. Yi et al. [26] reported that increasing the TS content of feedstock during anaerobic digestion increases the bacterial community and methane yield. Hence, a high TS percentage in different substrates is beneficial for bacterial growth and biogas production.
3.3. Fiber Content Analysis Results
The cellulose, hemicellulose, and lignin content of the different substrates are listed in Table 3.

Table 3.
Fiber analysis of WH and BP samples.
The results shown in Table 3 were compared with those reported by Cheng et al. [27], who performed a similar analysis on water hyacinth leaves, stems, and roots sampled from the Fuchun River in Hangzhou, China. They found cellulose content was in the range of 17–28.91 wt%, hemicellulose content in the range of 15–30.80 wt%, and lignin content in the range of 4–17.44 wt% for water hyacinth roots, water hyacinth stems, and water hyacinth leaves. The gap observed between the results of this study and the aforementioned studies may be explained by the degree of maturity of the plants, as well as their geographical location and origin. The degree of maturity influences the rigidity of plants and thus can influence their fiber composition. Lara et al. [28] found similar results, which were 8.64 wt% cellulose, 14.16 wt% hemicellulose, and 9.26 wt% lignin portions in banana peel biomass.
The cellulose and hemicellulose contents (Table 3) in WHL, WHS, and WHR were consistent with the VS contents found in these substrates. The cellulose and hemicellulose contents contribute considerably to biogas production as they hydrolyze into sugars (glucose and xylose, respectively), which are important nutrients for microbial activity. However, a high lignin content can delay the hydrolysis of cellulose and hemicellulose, lowering the biogas yield while extending the fermentation time.
3.4. Biogas Production from Water Hyacinth Leaves, Water Hyacinth Stems, and Banana Peels
The cumulative biogas production curves are shown in Figure 4. Biogas was generated in all the bottles to varying extents shortly after the experiment commenced. In the control bottle containing cellulose, biogas production exhibited exponential growth during the initial week, whereas stable progression was observed in the control bottle (blank). Over the same period, the production of biogas in the banana peel bottle exhibited a substantial increase, while the rise in the WHS and WHL bottles was moderate. Following the first week, biogas production in all bottles stabilized and proceeded at a steady pace.
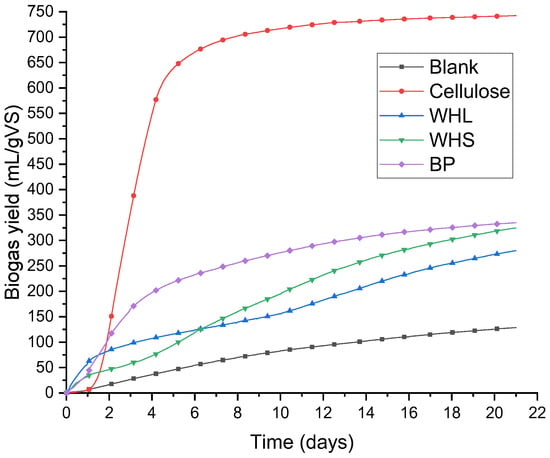
Figure 4.
Cumulative biogas yield over time.
As observed in Figure 5, the net biogas yields from water hyacinth leaves, water hyacinth stems, and banana peels in the first week were 131.23, 140.18, and 244.23 mL/g of VS, respectively. In the second week, water hyacinth stems produced the highest biogas yield of 121.57 mL/g of VS, while water hyacinth leaves and banana peels produced, respectively, 78.84 mL/g of VS and 62.88 mL/g of VS. Three weeks after the start of the experiments, on the 21st day, the cumulative biogas yields for WHL, WHS, BP, cellulose, and blank samples were recorded. The results, as shown in Figure 4 and Figure 5, indicate that BP had the highest biogas yield of 334.82 mL/g of VS, followed by WHS with a yield of 324.79 mL/g of VS. WHL had a yield of 280.15 mL/g of VS, whereas the cellulose sample yielded 742.27 mL/g of VS. The blank sample showed the lowest yield (128.58 mL/g VS).
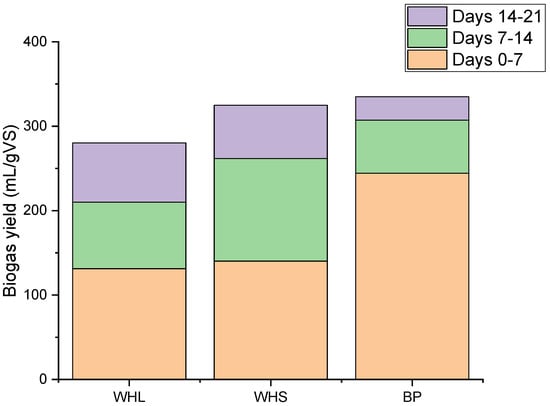
Figure 5.
Cumulative weekly biogas yield from water hyacinth leaves, stems, and banana peels.
As shown in Figure 4, the cellulose sample experienced a substantial and exponential increase in biogas yield, suggesting that there was an adequate supply of nutrients for the bacteria in the anaerobic medium. These nutrients were conveniently accessible to the bacterial consortium, which swiftly consumed them to produce biogas. On the other hand, the blank sample exhibited lower biogas production, likely due to the scarcity of nutrients necessary for microbial activity.
Biogas production from banana peels was higher than that from water hyacinth leaves and stems. One possible reason for this is the high VS content of banana peels (80.63%). The VS content serves as the primary source of biogas in anaerobic digestion, providing a variety of essential nutrients. As depicted in Figure 5, after the first week, biogas production for each sample demonstrated a substantial decrease in cumulative weekly production, reflecting the depletion of available nutrients.
The cumulative biogas yields of WHL and WHS were compared with the results reported by Nugraha et al. [14] in their study on biogas production from water hyacinth. They recorded a maximum of 151.91 mL/g of VS at an optimum F/M ratio of 10.01 over a hydraulic retention time of 160 days. The biogas yield from the banana peel sample was compared with the results obtained by Achinas et al. [16]. In this study, a maximum biogas yield of 112.18 mL/g of VS was reported from the batch anaerobic digestion of banana peels at a concentration of 10 g/L, with 10% cow manure as inoculum.
The disparities in biogas production between the present investigation and previous reports can be attributed to various factors, including substrate conditions (wet or dry), type of pretreatment applied to the substrate, hydraulic retention time, and frequency of stirring. Pretreatment is particularly crucial for lignocellulosic materials, such as water hyacinth and banana peels, to ensure optimal performance throughout the anaerobic digestion process (hydrolysis, acidogenesis, acetogenesis, and methanogenesis), resulting in the highest possible biogas yield. Although the samples were dried and ground, additional chemical pretreatment may further improve the biogas production.
3.5. Statistical Significance of the Differences in Biogas Yield and Impact of the Substrate Composition
The chi-squared value (the test statistic value) found for the Kruskal–Wallis test was 2561.4. This value was typically high, indicating a major difference in biogas yield among the different samples. The p-value was less than 2.2 × 10−16 (p-value < 2.2 × 10−16). The p-value represents the probability that the observed differences in biogas yield among the samples occurred simply by coincidence. The extremely low p-value (much lower than the common alpha level p-value < 0.05) rejects the null hypothesis of the Kruskal–Wallis test, which assumes that the mean values of the cumulative biogas yields of the different samples are equal. In conclusion, there was a significant statistical difference in the cumulative biogas yield among the WHL, WHS, BP, cellulose, and blank samples, as shown in Figure 6.
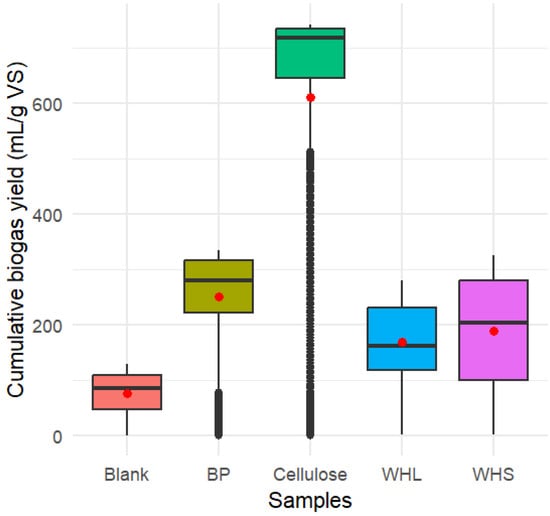
Figure 6.
Cumulative biogas yield by sample with mean points.
Since the Kruskal–Wallis test was significant, Dunnett’s test was performed for post-hoc analysis with Bonferroni correction to identify specific pairs of samples with significant statistical differences in biogas yield, as presented in Table 4. The differences in cumulative biogas yield were statistically significant among all pairs of samples. These substantial differences highlight the impact of the substrates’ compositions on biogas productivity during anaerobic digestion. The higher values of the statistical mean difference in biogas yield between cellulose and other substrates emphasize the role of nutrient content in biogas production. Cellulose, as the primary nutrient in the substrate, is the crucial component for biogas production [29].

Table 4.
Comparison of samples’ biogas productivity by pairs (Bonferroni).
3.6. Utilization Pathways and Valorization of Products and By-Products from Anaerobic Digestion
The utilization of water hyacinth and banana peel wastes for the production of biogas offers a sustainable solution in the local context by providing multiple options for the utilization of the produced biogas and the further processing of the effluents. As illustrated in Figure 7, the biogas generated can be utilized for sustainable electricity generation to support the national grid, as well as for clean cooking fuel to reduce reliance on imported petroleum products. The effluents from the digester can be composted [30] to produce organic fertilizer to support soil amendment, either directly used for nutrients and the recovery of fine chemical [31], or processed through hydrothermal carbonization (HTC)/hydrothermal gasification (HTG) to produce biochar and syngas [32] (which can be used for electricity generation or upgraded to transportation fuels through the Fischer–Tropsch process).
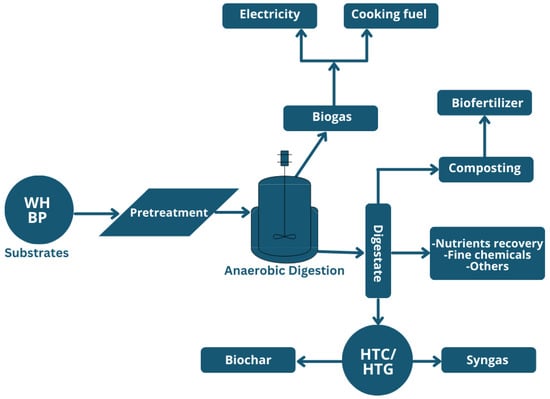
Figure 7.
Utilization pathways of the product and by-products from anaerobic digestion.
4. Conclusions
The physicochemical properties of water hyacinth and banana peel as potential substrates for biogas production were investigated. The analysis of the chemical elements of these biomasses revealed that, apart from C, H, and O, water hyacinth and banana peels are also composed of minerals such as K, Na, Ca, and Si in small proportions. The proximate and fiber content analysis of the dried samples showed a high VS content, with important proportions of cellulose and hemicellulose necessary for biogas production. Moreover, batch anaerobic digestion tests of the different substrates showed a high biogas yield, with the highest yield recorded from banana peels. Statistical analysis of biogas yields showed that there were significant differences in biogas production among the substrates as a factor of cellulose content. Water hyacinth and banana peels are valuable resources for biogas production in the context of circular economy and energy transition. However, feedstock availability can be a drawback for large-scale or industrial applications. The seasonality of water hyacinth plants and banana fruits can hinder resource availability for continuous large-scale biogas production from these substrates. Further research should focus on the water hyacinth and banana peel supply chains for biogas production and the life cycle analysis of water hyacinth and banana peel conversion into biogas.
Author Contributions
All the authors contributed to the preparation of this study. Conceptualization, D.G., J.S., D.M.K., S.N., P.K., K.A. and E.M.; methodology, D.G., J.S., S.N. and D.M.K.; validation, D.G., J.S. and S.N.; formal analysis, D.G., J.S., D.M.K. and S.N.; investigation, D.G., J.S., D.M.K. and S.N.; Resources, D.G., D.M.K., K.A., J.S. and S.N.; data curation D.G., J.S. and D.M.K.; writing original draft preparation, D.G.; writing review and editing, D.G., J.S., D.M.K., S.N., P.K., E.M. and K.A.; visualization, D.G., D.M.K., J.S. and S.N.; Supervision, S.N., D.M.K., J.S. and K.A.; funding acquisition, D.M.K., S.N. and K.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the German Federal Ministry of Education and Research (BMBF) through the West African Science Service Centre on Climate Change and Adapted Land Use (WASCAL), a Master’s scholarship grant. Open-access funding was provided by the University of Rostock.
Data Availability Statement
Raw data supporting the conclusions of this article will be made available by the authors upon request.
Acknowledgments
The authors are grateful to the German Federal Ministry of Education and Research (BMBF) and the West African Science Service Centre on Climate Change and Adapted Land Use (WASCAL) for funding this study. The authors would like to thank the University of Lomé and the University of Rostock for providing the necessary research facilities for this study.
Conflicts of Interest
The authors declare no conflicts of interest.
Nomenclature
| AC | Ash content |
| VS | Volatile solid |
| TS | Total solid |
| MC | Moisture content |
| ADL | Acid detergent lignin |
| NDF | Neutral detergent fiber |
| ADF | Acid detergent fiber |
| WH | Water hyacinth |
| WHS | Water hyacinth stems |
| WHL | Water hyacinth leaves |
| WHR | Water hyacinth roots |
| BP | Banana peels |
| BMBF | German Federal Ministry of Education and Research |
| HTC | Hydrothermal carbonisation |
| HTG | Hydrothermal gasification |
| WASCAL | West African Science Service Centre on Climate Change and Adapted Land Use |
References
- Osman, A.I.; Deka, T.J.; Baruah, D.C.; Rooney, D.W. Critical challenges in biohydrogen production processes from the organic feedstocks. Biomass Convers. Biorefinery 2023, 13, 8383–8401. [Google Scholar] [CrossRef]
- Ellacuriaga, M.; García-Cascallana, J.; Gómez, X. Biogas Production from Organic Wastes: Integrating Concepts of Circular Economy. Fuels 2021, 2, 144–167. [Google Scholar] [CrossRef]
- Barua, V.B.; Rathore, V.; Kalamdhad, A.S. Anaerobic co-digestion of water hyacinth and banana peels with and without thermal pretreatment. Renew. Energy 2019, 134, 103–112. [Google Scholar] [CrossRef]
- Ruan, T.; Zeng, R.; Yin, X.-Y.; Zhang, S.-X.; Yang, Z.-H. Water Hyacinth (Eichhornia crassipes) Biomass as a Biofuel Feedstock by Enzymatic Hydrolysis. BioResources 2016, 11, 2372–2380. [Google Scholar] [CrossRef]
- Asante, E.; Asiedu, N.Y.; Sarpong, S.; Agyemang, E.O.; Ajani, I.; Ntiamoah, A.; Adjaottor, A.A.; Addo, A. Modeling and assessment of the techno-economic analysis of biogas and its potential for the generation of electricity from water hyacinth biomass. J. Eng. Appl. Sci. 2024, 71, 1–19. [Google Scholar] [CrossRef]
- Togo Bananas Production. 1961–2023. Available online: https://knoema.com/atlas/Togo/topics/Agriculture/Crops-Production-Quantity-tonnes/Bananas-production (accessed on 19 February 2024).
- George, S.; Thomas, S.; Nedumpillil, N.N.; Jose, S. Extraction and Characterization of Fibers from Water Hyacinth Stem Using a Custom-Made Decorticator. J. Nat. Fibers 2023, 20, 2212927. [Google Scholar] [CrossRef]
- Arivendan, A.; Thangiah, W.J.J.; Das, R.; Ahamad, D.; Chithra, G.K. Effect of water hyacinth (Eichhornia crassipes) plant into water bodies and its composite materials for commercial applications. Proc. Inst. Mech. Eng. Part C J. Mech. Eng. Sci. 2023, 237, 5381–5390. [Google Scholar] [CrossRef]
- Wauton and William-Ebi. Available online: www.jmess.org (accessed on 21 February 2024).
- Kabenge, I.; Omulo, G.; Banadda, N.; Seay, J.; Zziwa, A.; Kiggundu, N. Characterization of Banana Peels Wastes as Potential Slow Pyrolysis Feedstock. J. Sustain. Dev. 2018, 11, 14. [Google Scholar] [CrossRef]
- Daniel, F.; Sekar, M.; Gavurová, B.; Govindasamy, C.; R, K.M.; P, B.; R, P.T. Recovering biogas and nutrients via novel anaerobic co-digestion of pre-treated water hyacinth for the enhanced biogas production. Environ. Res. 2023, 231, 116216. [Google Scholar] [CrossRef]
- Wirngo, H.Y.; Bamgboye, A.I.; Ngwabie, N.M. Biogas production from water hyacinth (Eichhornia crassipes) harvested from River Wouri, Douala, Cameroon. J. Appl. Nat. Sci. 2024, 16, 883–889. [Google Scholar] [CrossRef]
- Hudakorn, T.; Sritrakul, N. Biogas and biomass pellet production from water hyacinth. Energy Rep. 2020, 6, 532–538. [Google Scholar] [CrossRef]
- Nugraha, W.D.; Syafrudin; Pradita, L.L.; A Matin, H.H. Budiyono Biogas Production from Water Hyacinth (Eichhornia crassipes): The Effect of F/M Ratio. IOP Conf. Ser. Earth Environ. Sci. 2018, 150, 012019. [Google Scholar] [CrossRef]
- Manigandan, S.; R, P.T.; Anderson, A.; Maryam, A.; Mahmoud, E. Benefits of pretreated water hyacinth for enhanced anaerobic digestion and biogas production. Int. J. Thermofluids 2023, 19, 100369. [Google Scholar] [CrossRef]
- Achinas, S.; Krooneman, J.; Euverink, G.J.W. Enhanced Biogas Production from the Anaerobic Batch Treatment of Banana Peels. Engineering 2019, 5, 970–978. [Google Scholar] [CrossRef]
- Tosin, R.; Monteiro-Silva, F.; Martins, R.; Cunha, M. A New Approach for Element Characterization of Grapevine Tissue with Laser-Induced Breakdown Spectroscopy. Horticulturae 2024, 10, 82. [Google Scholar] [CrossRef]
- Jan Liebetrau, E.; Pfeiffer, D. Biomass Energy Use Collection of Methods for Biogas; Report; DBFZ: Leipzig, Germany, 2020; Volume 457, pp. 50–106. [Google Scholar]
- Verein Deutscher Ingenieure. VDI 4630: Fermentation of Organic Materials: Characterisation of the Substrate, Sampling, Collection of Material Data, Fermentation Tests; Beuth Verlag: Berlin, Germany, 2016. [Google Scholar]
- Rajagopalan, G.; He, J.; Yang, K.L. Direct fermentation of xylan by Clostridium strain BOH3 for the production of butanol and hydrogen using optimized culture medium. Bioresour. Technol. 2014, 154, 38–43. [Google Scholar] [CrossRef]
- Hassan, S.; Ngo, T.; Khudur, L.S.; Krohn, C.; Dike, C.C.; Hakeem, I.G.; Shah, K.; Surapaneni, A.; Ball, A.S. Biosolids-Derived Biochar Improves Biomethane Production in the Anaerobic Digestion of Chicken Manure. Resources 2023, 12, 123. [Google Scholar] [CrossRef]
- Pérez-Rangel, M.; Barboza-Corona, J.E.; Buitrón, G.; Valdez-Vazquez, I. Essential Nutrients for Improving the Direct Processing of Raw Lignocellulosic Substrates Through the Dark Fermentation Process. Bioenergy Res. 2020, 13, 349–357. [Google Scholar] [CrossRef]
- Sukarni, S.; Zakaria, Y.; Sumarli, S.; Wulandari, R.; Permanasari, A.A.; Suhermanto, M. Physical and Chemical Properties of Water Hyacinth (Eichhornia crassipes) as a Sustainable Biofuel Feedstock. IOP Conf. Series: Mater. Sci. Eng. 2019, 515, 012070. [Google Scholar] [CrossRef]
- Omondi, E.A.; Ndiba, P.K.; Njuru, P.G. Characterization of water hyacinth (E. crassipes) from Lake Victoria and ruminal slaughterhouse waste as co-substrates in biogas production. SN Appl. Sci. 2019, 1, 1–10. [Google Scholar] [CrossRef]
- Elsamadony, M.; Tawfik, A. Maximization of hydrogen fermentative process from delignified water hyacinth using sodium chlorite. Energy Convers. Manag. 2018, 157, 257–265. [Google Scholar] [CrossRef]
- Yi, J.; Dong, B.; Jin, J.; Dai, X. Effect of Increasing Total Solids Contents on Anaerobic Digestion of Food Waste under Mesophilic Conditions: Performance and Microbial Characteristics Analysis. PLoS ONE 2014, 9, e102548. [Google Scholar] [CrossRef] [PubMed]
- Su, H.; Cheng, J.; Zhou, J.; Song, W.; Cen, K. Hydrogen production from water hyacinth through dark- and photo- fermentation. Int. J. Hydrogen Energy 2010, 35, 8929–8937. [Google Scholar] [CrossRef]
- Lara, M.A.; Méndez, E.F.; Malagón, D.H.; Bernal, J.M.; Montoya, D. Evaluation of production of hydrogen in a batch bioreactor using Clostridium butyricum DSM 2478 from banana peel. Chem. Eng. Trans. 2020, 79, 265–270. [Google Scholar] [CrossRef]
- Mirmohamadsadeghi, S.; Karimi, K.; Azarbaijani, R.; Yeganeh, L.P.; Angelidaki, I.; Nizami, A.-S.; Bhat, R.; Dashora, K.; Vijay, V.K.; Aghbashlo, M.; et al. Pretreatment of lignocelluloses for enhanced biogas production: A review on influencing mechanisms and the importance of microbial diversity. Renew. Sustain. Energy Rev. 2021, 135, 110173. [Google Scholar] [CrossRef]
- Wang, Y.; Tang, Y.; Yuan, Z. Improving food waste composting efficiency with mature compost addition. Bioresour. Technol. 2022, 349, 126830. [Google Scholar] [CrossRef]
- Proskynitopoulou, V.; Garagounis, I.; Vourros, A.; Toursidis, P.D.; Lorentzou, S.; Zouboulis, A.; Panopoulos, K. Nutrient recovery from digestate: Pilot test experiments. J. Environ. Manag. 2024, 353, 120166. [Google Scholar] [CrossRef]
- Mikusińska, J.; Kuźnia, M.; Czerwińska, K.; Wilk, M. Hydrothermal Carbonization of Digestate Produced in the Biogas Production Process. Energies 2023, 16, 5458. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).