Experimental Investigation on the Effect of Heating Oil and Tyre Pyrolysis Oil Combustion in an Evaporative Combustion Chamber
Abstract
1. Introduction
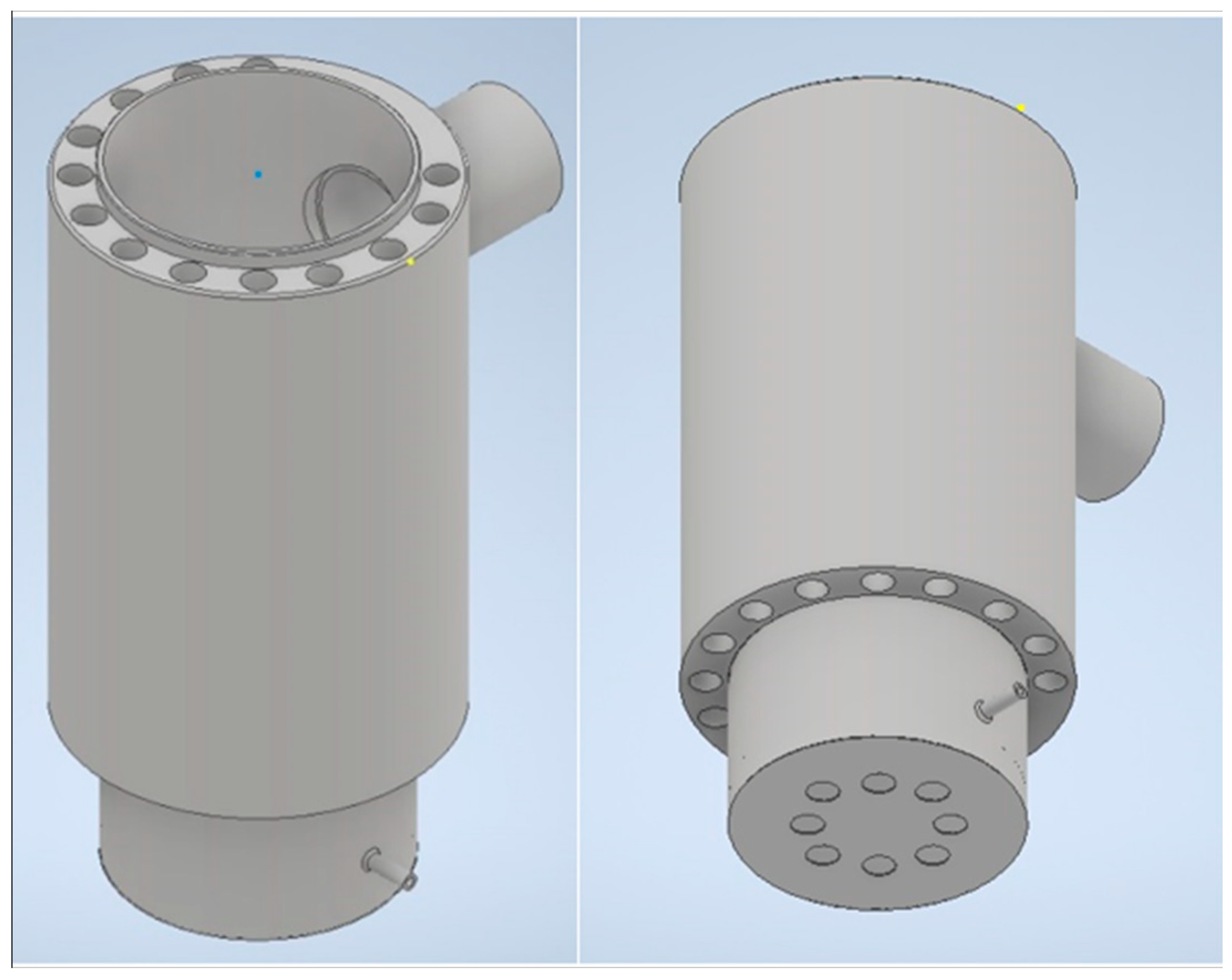
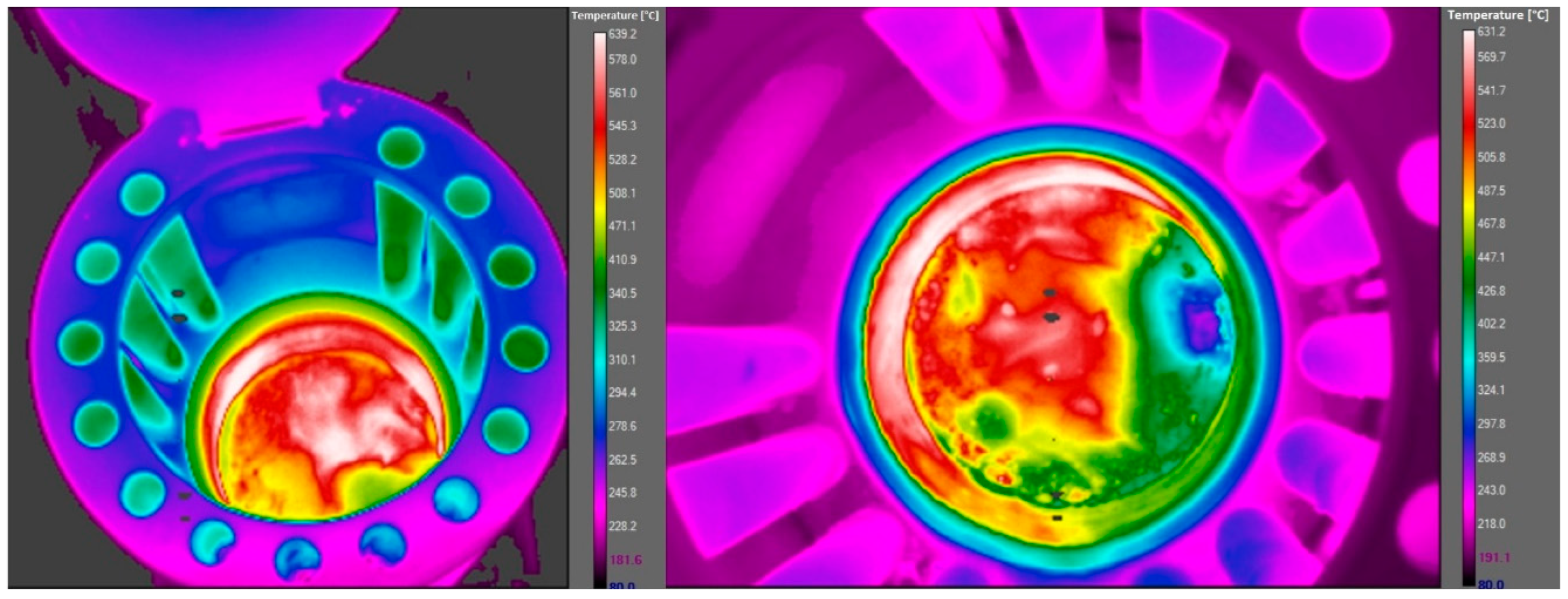
1.1. Technical Parameters of the Combustion Chamber
1.2. Experimental Setup
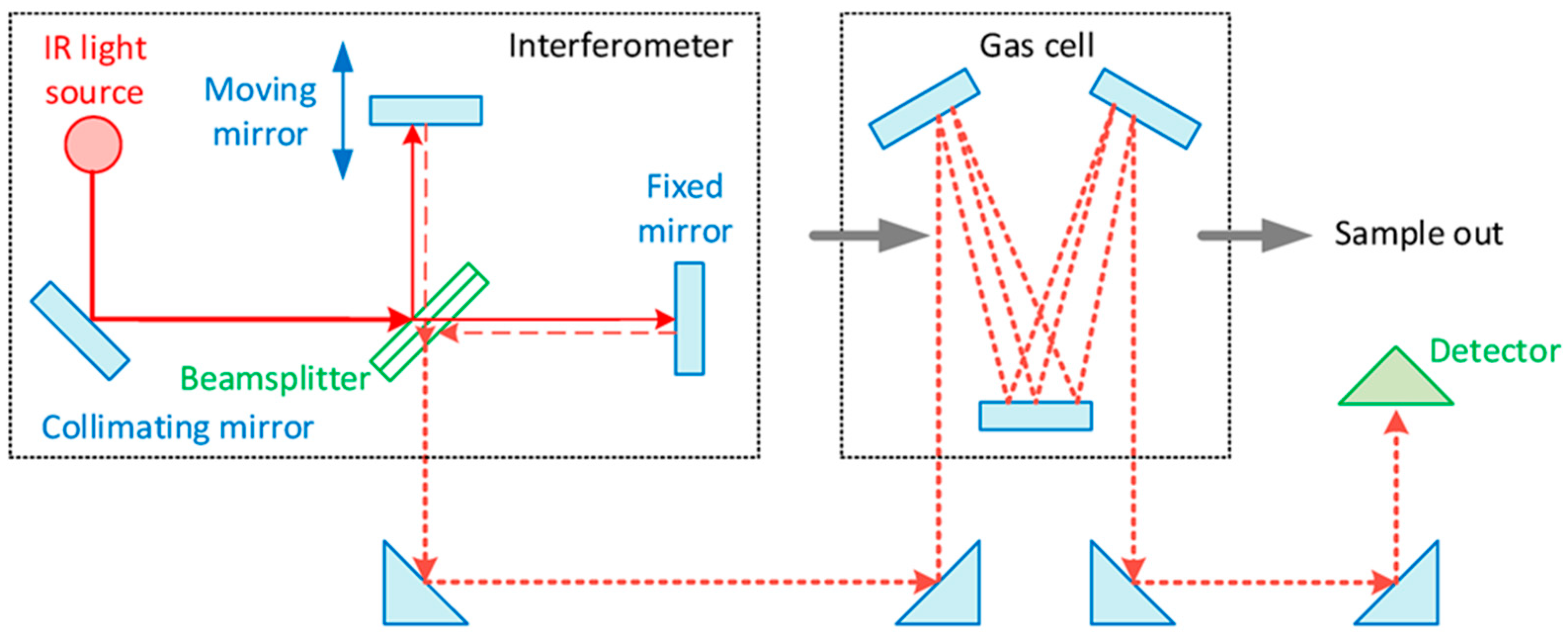
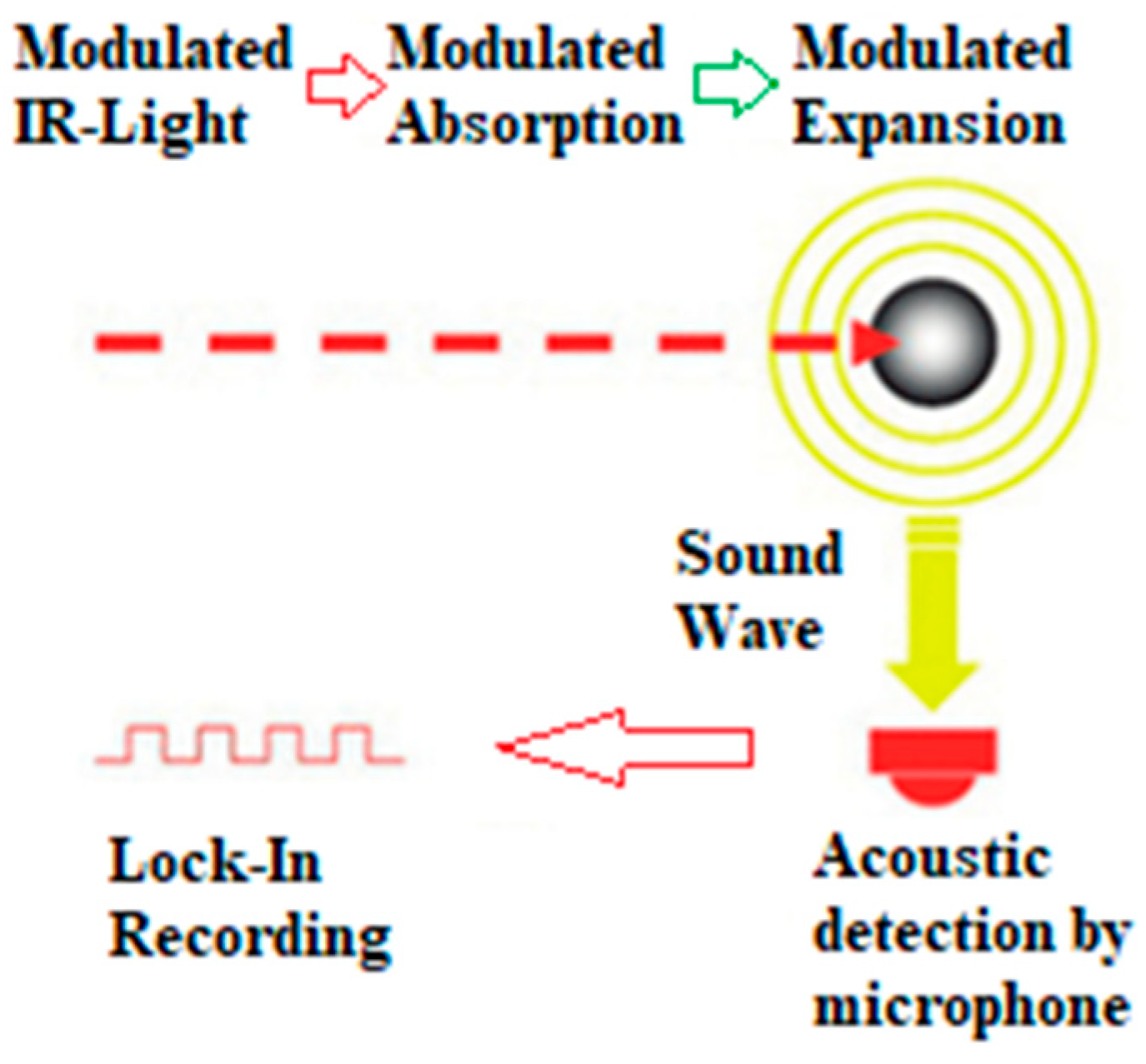
2. Exhaust Gas and Particulate Matter Measurement Method
3. Measurement Points
3.1. Soot Emission
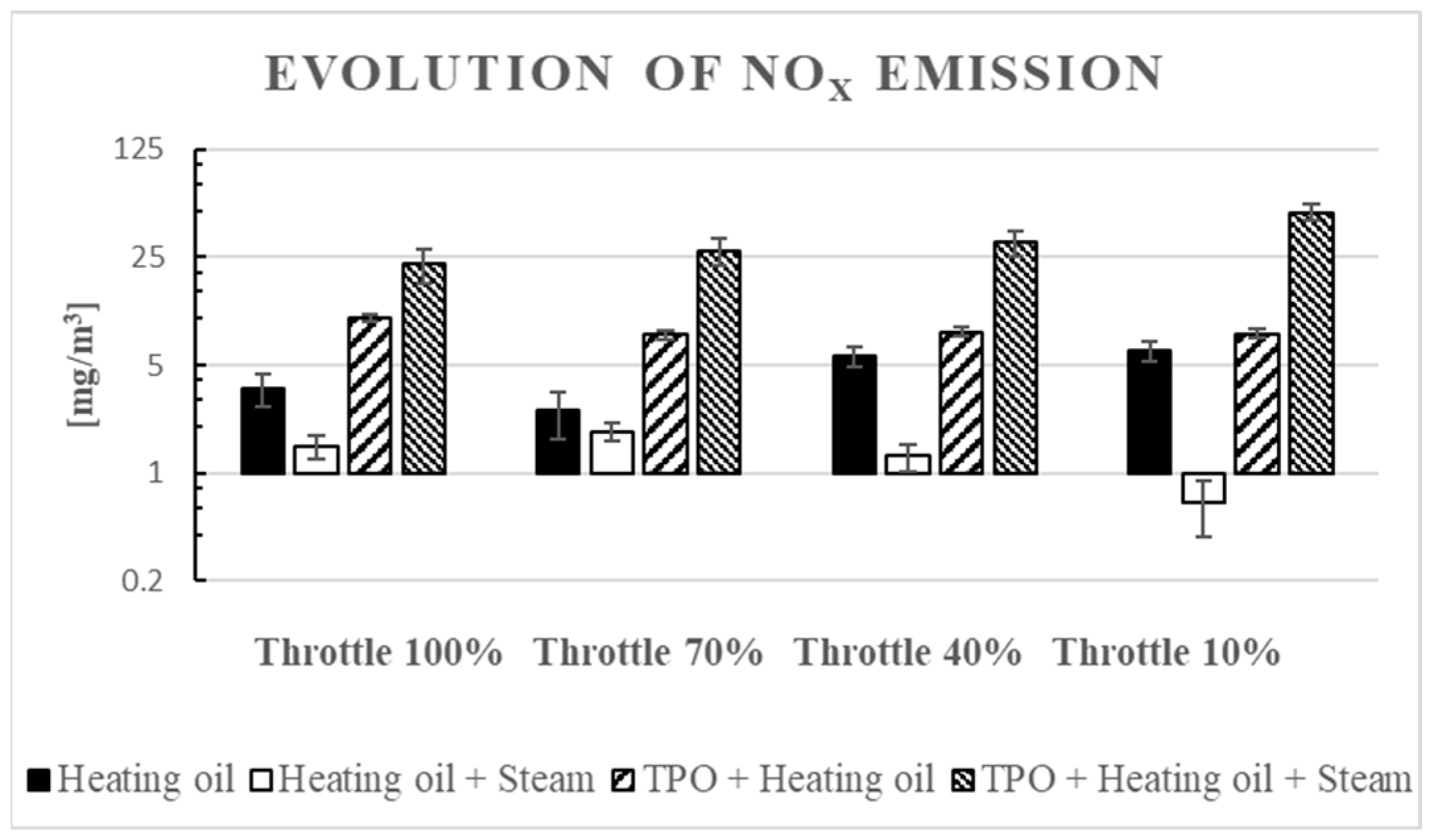
3.2. NOx Emission
3.3. CO2 Emission
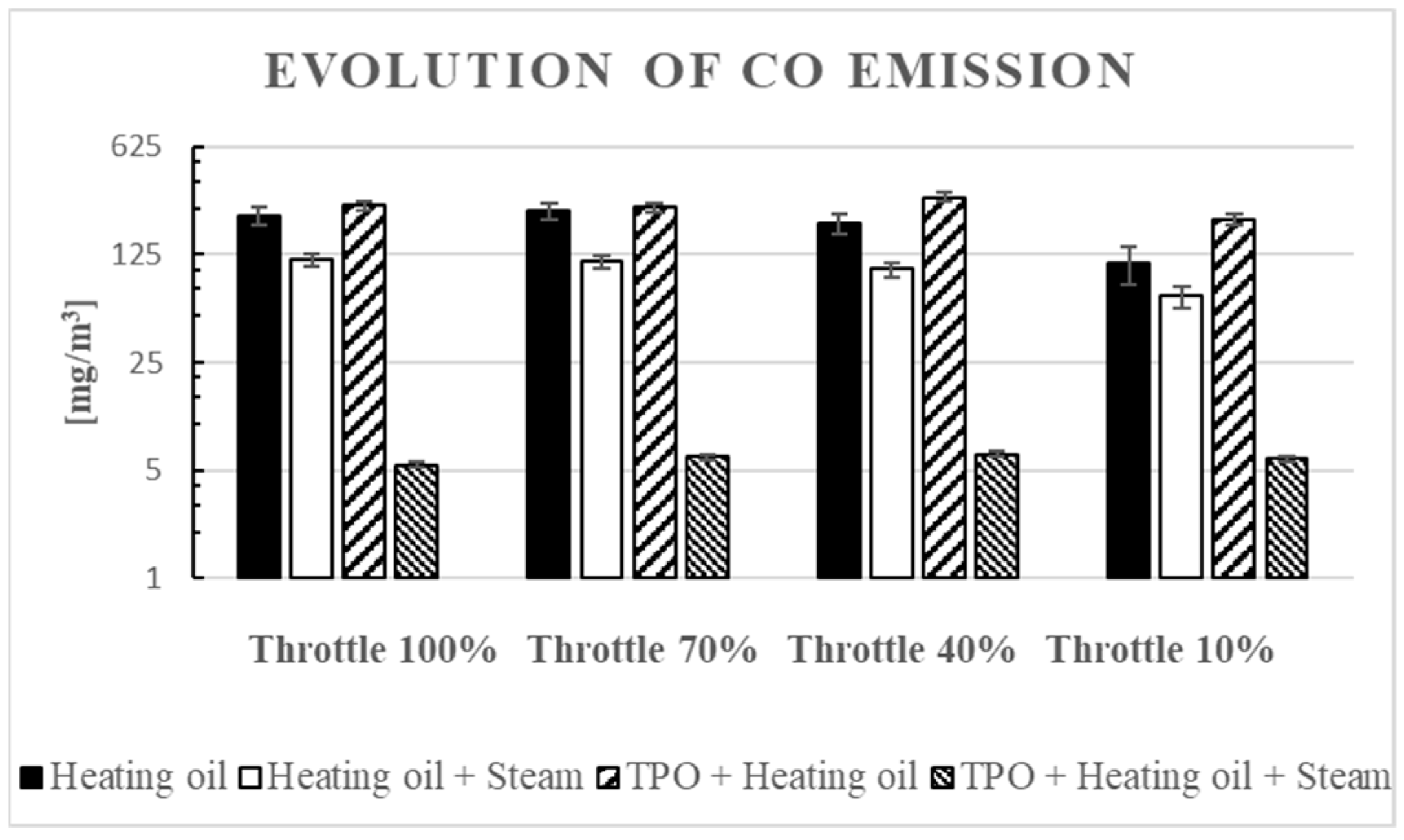
3.4. CO Emission
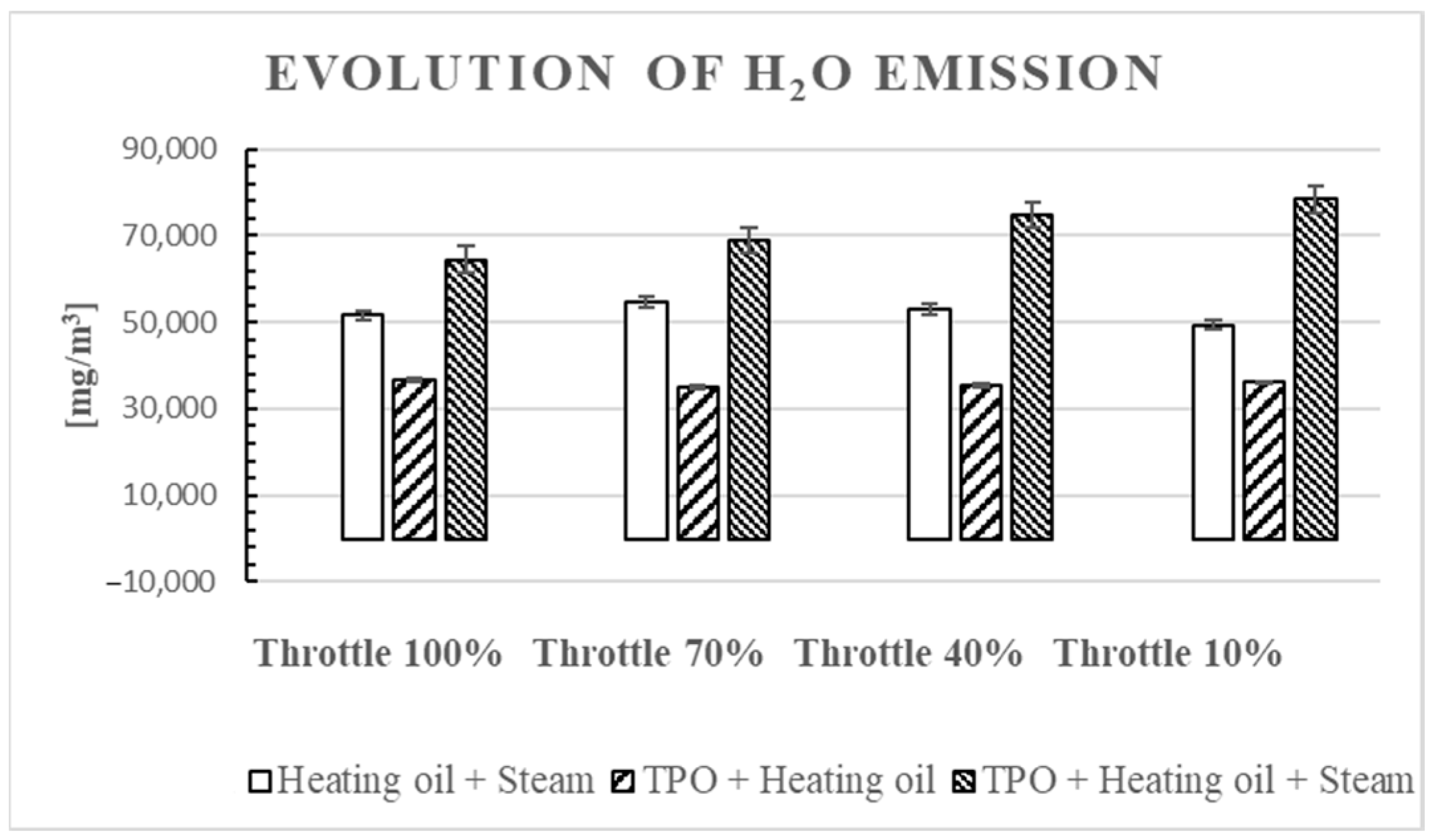
3.5. Water Concentration
4. Conclusions
Funding
Data Availability Statement
Conflicts of Interest
Nomenclature
| AVL | Anstalt für Verbrennungskraftmaschinen List |
| EU | European Union |
| TPO | Tire Pyrolysis Oil |
| H2O | Just Add Water |
| PM | Particulate Matter |
| NO | Nitrogen Oxides |
| CO | Carbone Monoxide |
| CO2 | Carbon Dioxide |
| HC | Hydrogen Carbon |
| FTIR | Fourier Transform Infrared Spectroscopy |
| TLC | Thermophoretic loss compensation |
| MSZ | Hungarian Standard |
| MOL | Hungarian Oil and Gas Company |
| NOx | Nitrous oxide |
References
- Rodríguez-Fernández, L.; Fernández Carvajal, A.B.; Ruiz-Gómez, L.M. Evolution of European Union’s energy security in gas supply during Russia–Ukraine gas crises (2006–2009). Energy Strategy Rev. 2020, 30, 100518. [Google Scholar] [CrossRef]
- Von Homeyer, I.; Oberthür, S.; Jordan, A.J. EU climate and energy governance in times of crisis: Towards a new agenda. J. Eur. Public Policy 2021, 28, 959–979. [Google Scholar] [CrossRef]
- Jirušek, M. The attitude of the Visegrad Group Countries towards Russian Infrastructural Projects in the gas sector. Energy Policy 2020, 139, 111340. [Google Scholar] [CrossRef]
- Ederington, L.H.; Fernando, C.S.; Hoelscher, S.; Lee, T.K.; Linn, S.C. A Review of the Evidence on the Relation Between Crude Oil Prices and Petroleum Product Prices. SSRN Electron. J. 2018, 1–47. [Google Scholar] [CrossRef]
- Armas, O.; Ballesteros, R.; Martos, F.; Agudelo, J. Characterization of light duty Diesel engine pollutant emissions using water-emulsified fuel. Fuel 2005, 84, 1011–1018. [Google Scholar] [CrossRef]
- Kökkülünk, G.; Gonca, G.; Ayhan, V.; Cesur, İ.; Parlak, A. Theoretical and experimental investigation of diesel engine with steam injection system on performance and emission parameters. Appl. Therm. Eng. 2013, 54, 161–170. [Google Scholar] [CrossRef]
- Lin, C.Y.; Wang, K.H. Diesel engine performance and emission characteristics using the three-phase emulsions as fuel. Fuel 2004, 83, 537–545. [Google Scholar] [CrossRef]
- Bak, J.; Clausen, S. FTIR emission spectroscopy methods and procedures for real time quantitative gas analysis in industrial environments. Meas. Sci. Technol. 2001, 13, 150–156. [Google Scholar] [CrossRef]
- Kondor, I.P.; Zöldy, M.; Mihály, D. Experimental Investigation on the Performance and Emission Characteristic of Cmpression Ignition Engine Using Waste Based Tire Pyrolysis Fuel and Diesel Fuel Blends. Energies 2021, 14, 7903. [Google Scholar] [CrossRef]
- Gonca, G.; Sahin, B.; Parlak, A.; Ust, Y.; Ayhan, V.; Cesur, İ.; Boru, B. The effects of steam injection on the performance and emission parameters of a Miller cycle diesel engine. Energy 2014, 78, 266–275. [Google Scholar] [CrossRef]
- Grob, B.; Schmid, J.; Ivleva, N.P.; Niessner, R. Conductivity for Soot Sensing: Possibilities and Limitations. Anal. Chem. 2012, 84, 3586–3592. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Fuehrhapter, R.; Waschl, H.; Del Re, L. Calibration and Performance of a Novel In-situ Soot Sensor for Production Engines. Procedia Eng. 2016, 168, 43–46. [Google Scholar] [CrossRef]
- Pyshyev, S.; Lypko, Y.; Chervinskyy, T.; Fedevych, O.; Kuatzynski, M.; Pstrowska, K. Application of tyre derived pyrolysis oil as a fuel component. South Afr. J. Chem. Eng. 2023, 43, 342–347. [Google Scholar] [CrossRef]
- Hadia, F.; Wadhah, S.; Ammar, H.; Ahmed, O. Investigation of combined effects of compression ratio and steam injection on performance, combustion and emissions characteristics of HCCI engine. Case Stud. Therm. Eng. 2017, 10, 262–271. [Google Scholar] [CrossRef]
- Xue, J.; Grift, T.E.; Hansen, A.C. Effect of biodiesel on engine performances and emissions. Renew. Sustain. Energy Rev. 2011, 15, 1098–1116. [Google Scholar] [CrossRef]
- Bayramoglu, K.; Nuran, M. Energy, exergy, sustainability evaluation of the usage of pyrolytic oil and conventional fuels in diesel engines. Process Saf. Environ. Prot. 2024, 181, 324–333. [Google Scholar] [CrossRef]
- Froissart, M.; Ochrymiuk, T. Novel wet combustion chamber concept CFD studies with triple water inlet. Energy 2023, 278 Pt A, 127854. [Google Scholar] [CrossRef]
- Zembi, J.; Battistani, M.; Mariani, F.; Irimescu, A.; Vaglieco, B.M.; Merola, S.S. Investigations on the impact of port water injection on soot formation in a DISI engine through CFD simulations and optical methods. Fuel 2023, 337, 127170. [Google Scholar] [CrossRef]
- Wu, J.; Lv, Y.; Kang, Z.; Lv, Y.; Deng, J.; Li, L.; Wu, Z. A novel approach of water injection optimization applied in gasoline engines: An investigation on heated water injection. Fuel 2023, 340, 127607. [Google Scholar] [CrossRef]




| Parameter | TPO | Heating Oil | Diesel | Standard |
|---|---|---|---|---|
| Color | Black | Red | Yellow | |
| Cetane | <30 | ≥51.0 | ≥51.0 | MSZ EN ISO 5165 |
| Sulfur [mg/kg] | 8348 | ≤10 | ≤10 | MSZ EN ISO 20846 |
| Water [mg/kg] | 1804 | ≤200 | ≤200 | MSZ EN ISO 12937 |
| Pensky-Martens Flashpoint | <24 | >55 | >55 | MSZ EN 2719 |
| Caloric Value [Mj/kg] | 40.8 | 42.7 | 42.7 | |
| Ash [%/m/m] | 0.005 | ≤0.01 | ≤0.01 | MSZ EN ISO 6245 |
| Viscosity [mm2/s] | 4.443 | 2.00–4.50 | 2.00–4.50 | MSZ EN ISO 2104 |
| Mechanical impurities [mg/kg] | 0.027–0.16 | ≤24 | ≤24 | MSZ EN 12662 |
| Density [kg/m3] | 926 | 830 | 830 | |
| Coke residue [%/m/m] | 0.6 | ≤0.30 | ≤0.30 | EN ISO 10370 |
| Cold Filter Point (CFPP) | ≤5 | ≤5 | MSZ EN 116 | |
| Polycycle carbohydrates | 22–35 | <8.0 | <8.0 | MSZ EN 12916 |
| Maximum steam capacity | 6 kg/h |
| Working pressure | 3 bar |
| Water inlet pressure | 4 bar |
| Boiler capacity | 15 L |
| Maximum steam pressure | 6 bar |
| Steam temperature | 354 °C |
| Feed water temperature | 20 °C |
| Warm-up time | 15 min |
| Heating power | 5 kW |
| Supply voltage | 380 V |
| Measured Components | NO and NOx | THC and CH4 | CO2 | CO |
|---|---|---|---|---|
| Reproducibility | ≤0.5% of range full scale | ≤0.5% of range full scale | ≤0.5% of range full scale | ≤0.5% of range full scale |
| Linearity | ≤2% of measured value (10–100% of range full scale) ≤1% of range full scale whichever is smaller | ≤2% of measured value (10–100% of range full scale) ≤1% of range full scale whichever is smaller | ≤2% of measured value (10–100% of range full scale) ≤1% of range full scale whichever is smaller | ≤2% of measured value (10–100% of range full scale) ≤1% of range full scale whichever is smaller |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kondor, I.P. Experimental Investigation on the Effect of Heating Oil and Tyre Pyrolysis Oil Combustion in an Evaporative Combustion Chamber. Fuels 2024, 5, 210-221. https://doi.org/10.3390/fuels5020012
Kondor IP. Experimental Investigation on the Effect of Heating Oil and Tyre Pyrolysis Oil Combustion in an Evaporative Combustion Chamber. Fuels. 2024; 5(2):210-221. https://doi.org/10.3390/fuels5020012
Chicago/Turabian StyleKondor, István Péter. 2024. "Experimental Investigation on the Effect of Heating Oil and Tyre Pyrolysis Oil Combustion in an Evaporative Combustion Chamber" Fuels 5, no. 2: 210-221. https://doi.org/10.3390/fuels5020012
APA StyleKondor, I. P. (2024). Experimental Investigation on the Effect of Heating Oil and Tyre Pyrolysis Oil Combustion in an Evaporative Combustion Chamber. Fuels, 5(2), 210-221. https://doi.org/10.3390/fuels5020012







