Effect of Blending Dimethyl Carbonate and Ethanol with Gasoline on Combustion Characteristics
Abstract
1. Introduction
2. Experiment
3. Results and Discussions
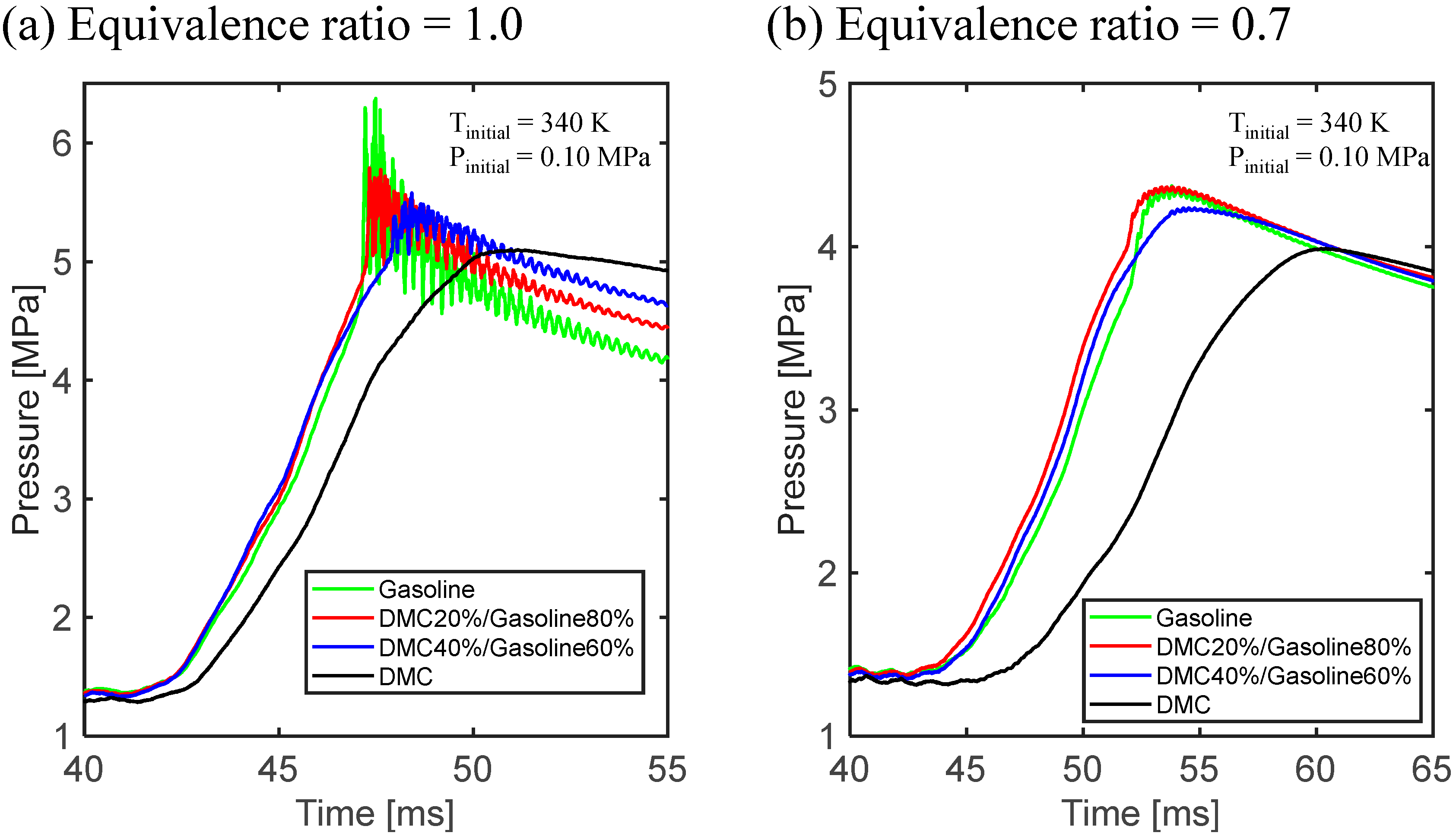
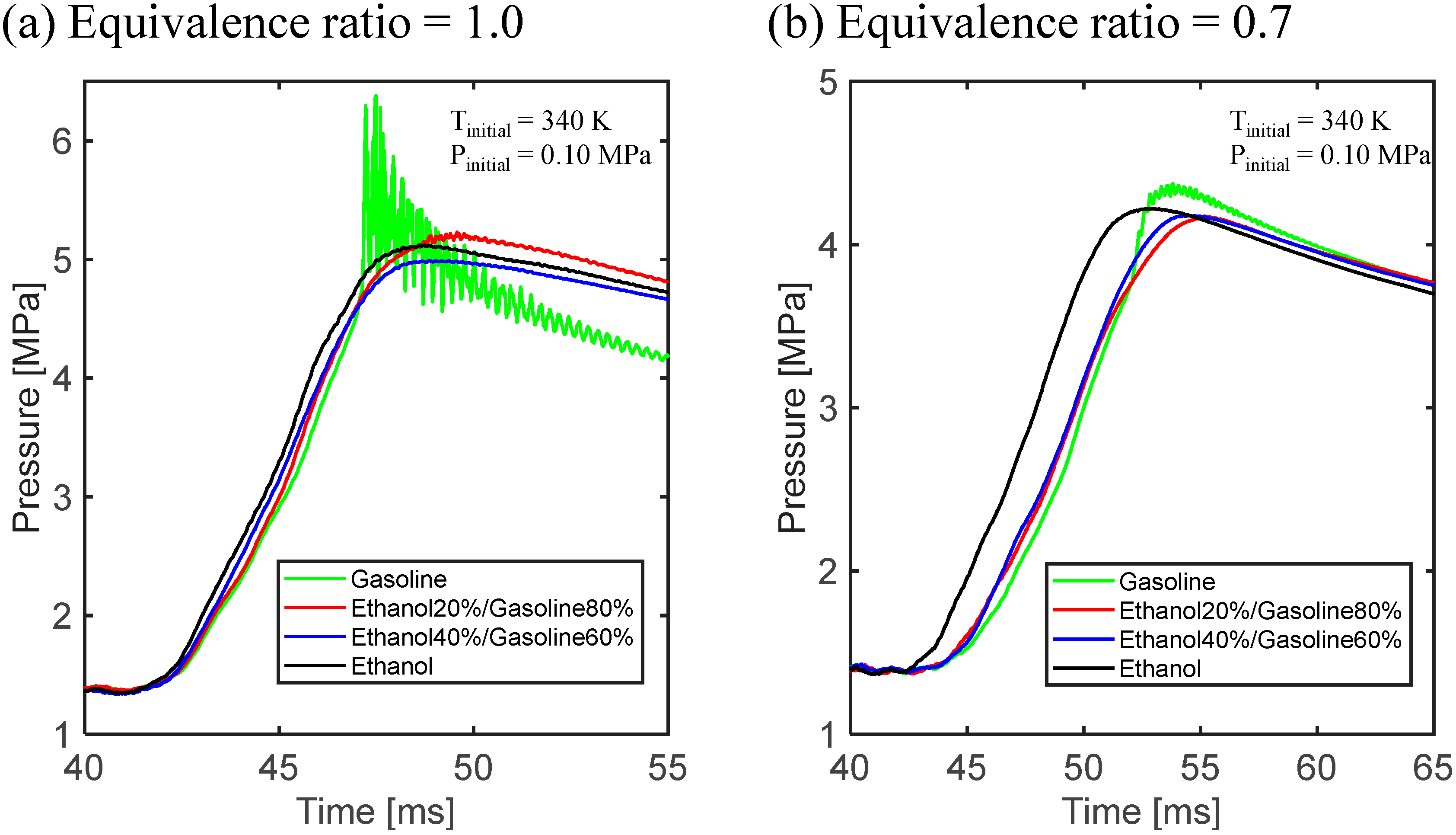
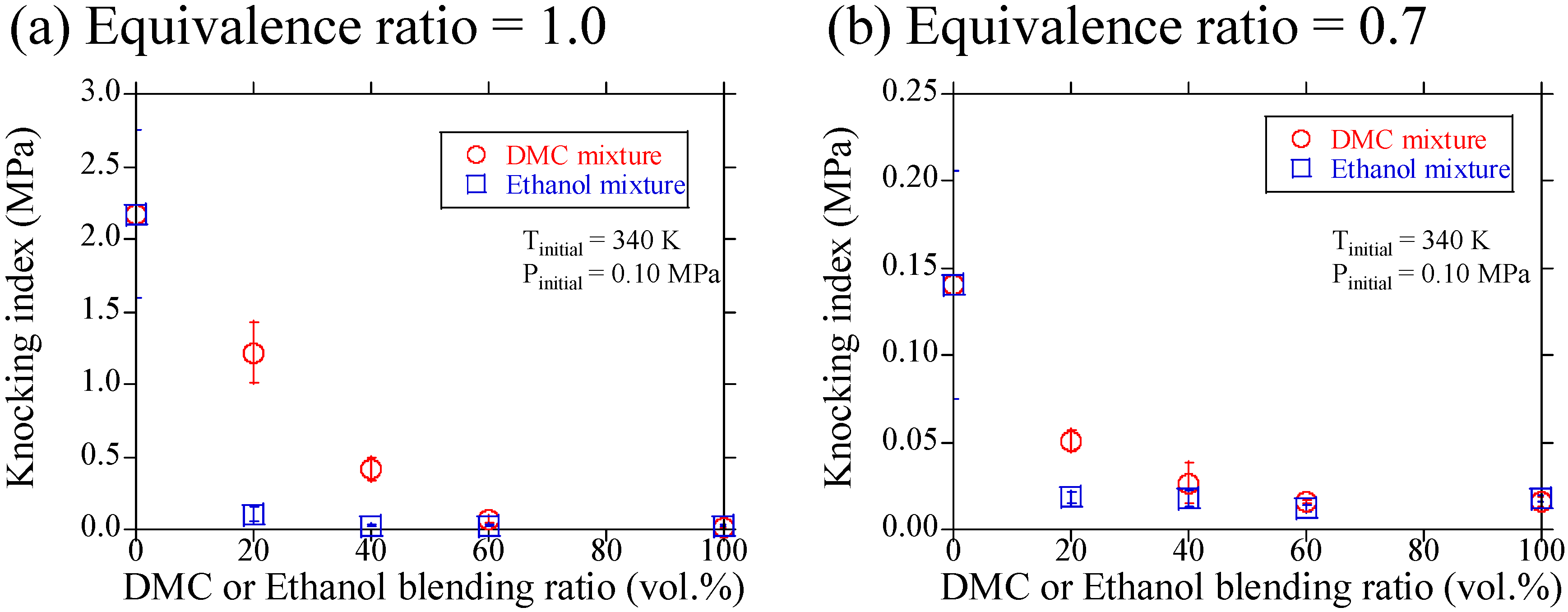
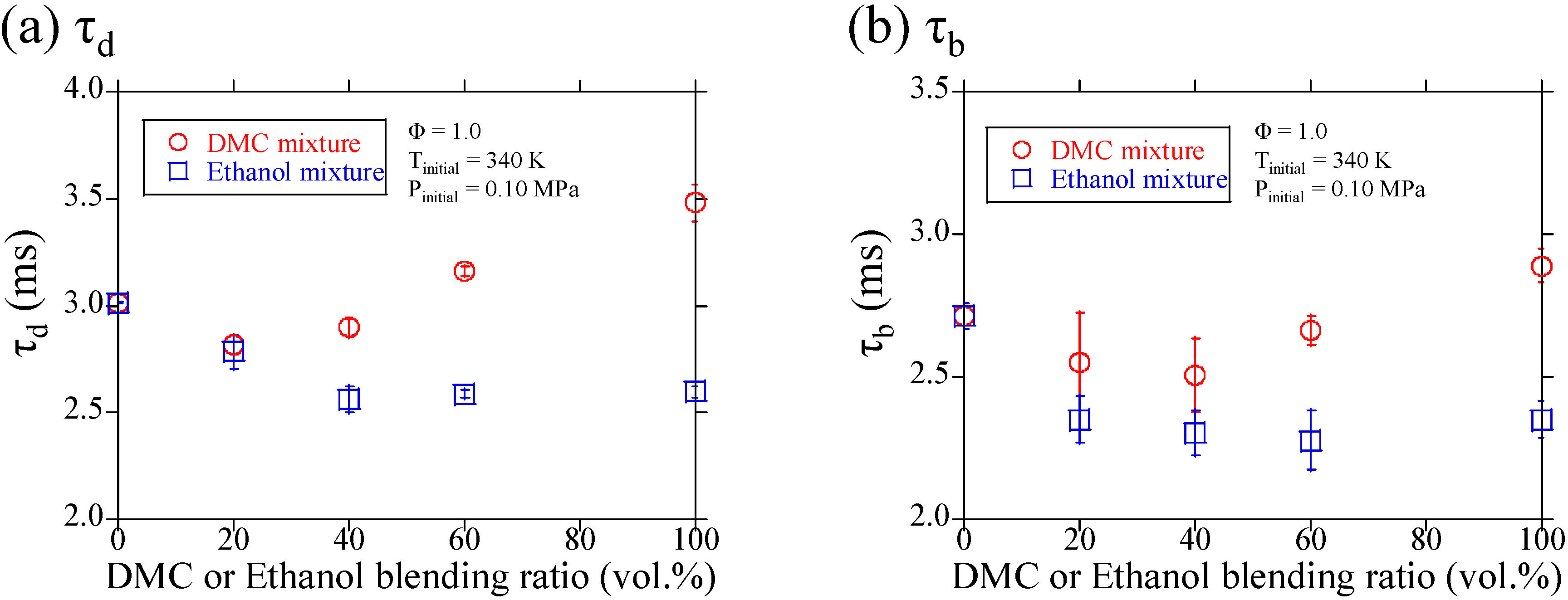
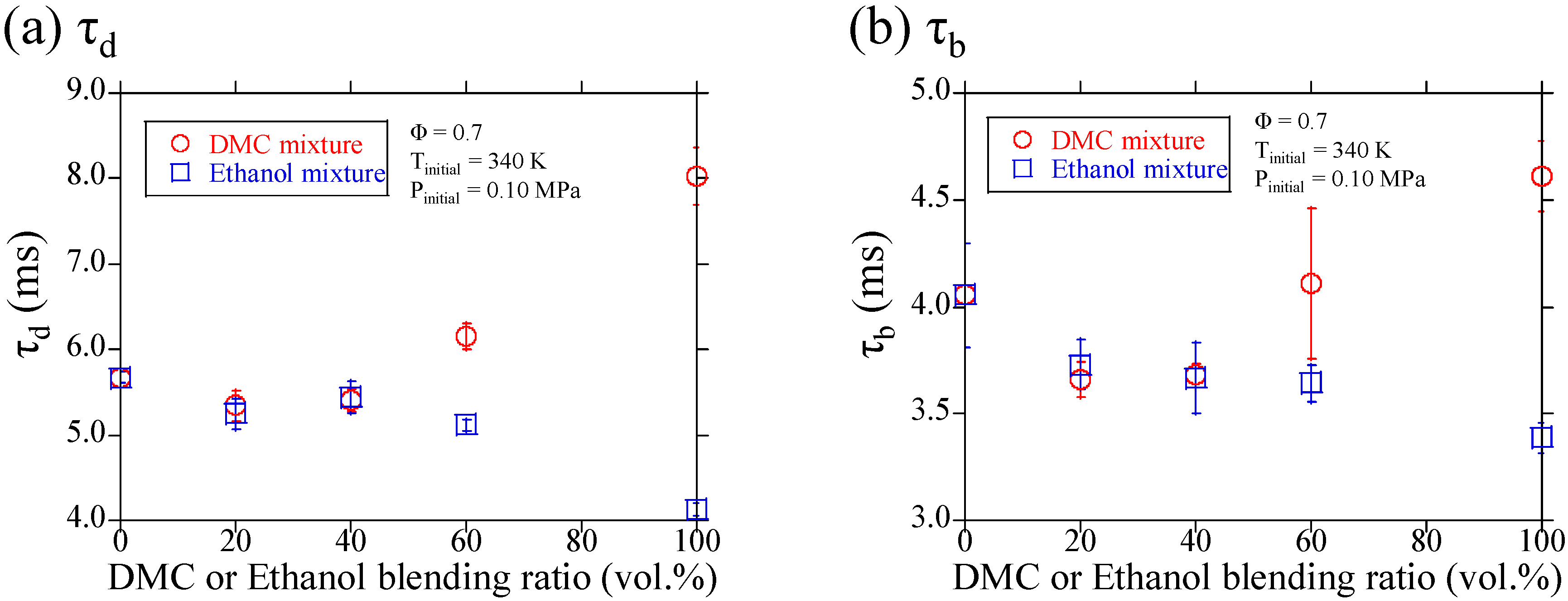
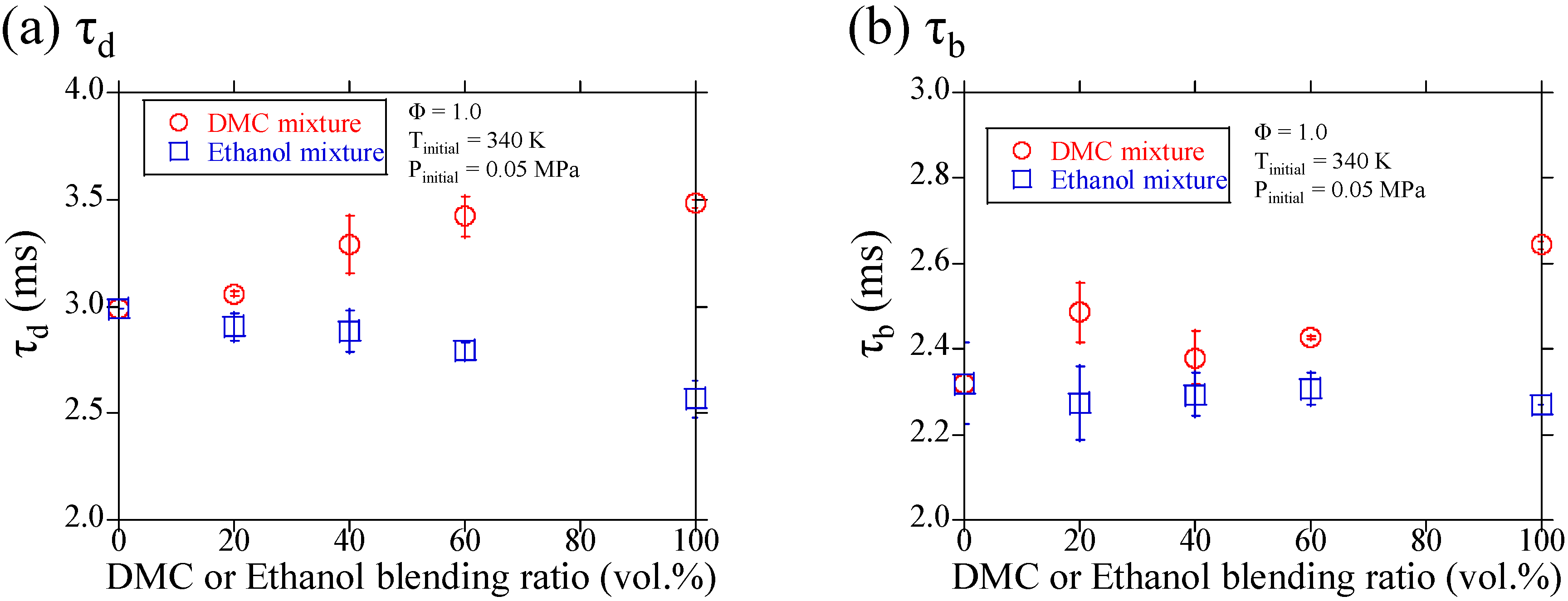
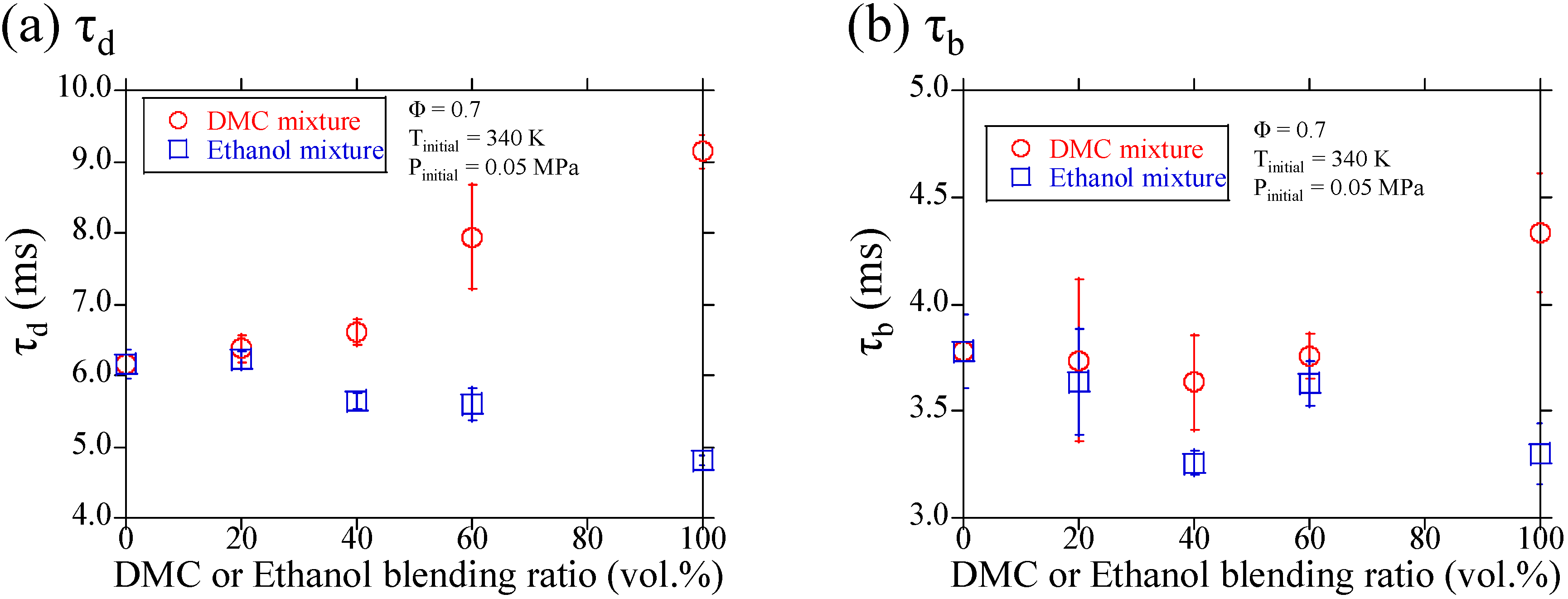
3.1. Experimental Results
3.2. Discussions
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- BP Energy Outlook, 2020 ed.; BP: London, UK, 2020.
- Ramirez, A.; Sarathy, S.M.; Gascon, J. CO2 derived E-fuels: Research trends, misconceptions, and future directions. Trends Chem. 2020, 2, 785–795. [Google Scholar] [CrossRef]
- Jeswani, H.K.; Chilvers, A.; Azapagic, A. Environmental sustainability of biofuels: A review. Proc. R. Soc. A 2020, 476, 20200351. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Fortes, M.; Schöneberger, J.C.; Boulamanti, A.; Tzimas, E. Methanol synthesis using captured CO2 as raw material: Techno-economic and environmental assessment. Appl. Energy 2016, 161, 718–732. [Google Scholar] [CrossRef]
- Artz, J.; Müller, T.E.; Thenert, K.; Kleinekorte, J.; Meys, R.; Sternberg, A.; Bardow, A.; Leitner, W. Sustainable Conversion of Carbon Dioxide: An Integrated Review of Catalysis and Life Cycle Assessment. Chem. Rev. 2018, 118, 434–504. [Google Scholar] [CrossRef]
- Zabed, H.; Sahu, J.N.; Boyce, A.N.; Faruq, G. Fuel ethanol production from lignocellulosic biomass: An overview on feedstocks and technological approaches. Renew. Sustain. Energy Rev. 2016, 66, 751–774. [Google Scholar] [CrossRef]
- Saeidi, S.; Najari, S.; Hessel, V.; Wilson, K.; Keil, F.J.; Concepción, P.; Suib, S.L.; Rodrigues, A.E. Recent advances in CO2 hydrogenation to value-added products—Current challenges and future directions. Prog. Energy Combust. Sci. 2021, 85, 100905. [Google Scholar] [CrossRef]
- Abdalla, A.O.G.; Liu, D. Dimethyl Carbonate as a Promising Oxygenated Fuel for Combustion: A Review. Energies 2018, 11, 1552. [Google Scholar] [CrossRef]
- Manzetti, S.; Andersen, O. A review of emission products from bioethanol and its blends with gasoline. Background for new guidelines for emission control. Fuel 2015, 140, 293–301. [Google Scholar] [CrossRef]
- Badia, J.H.; Badia, J.H.; Ramírez, E.; Bringué, R.; Cunill, F.; Delgado, J. New Octane Booster Molecules for Modern Gasoline Composition. Energy Fuels 2021, 35, 10949–10997. [Google Scholar] [CrossRef]
- van Lipzig, J.P.J.; Nilsson, E.J.K.; de Goey, L.P.H.; Konnov, A.A. Laminar burning velocities of n-heptane, iso-octane, ethanol and their binary and tertiary mixtures. Fuel 2011, 90, 2773–2781. [Google Scholar] [CrossRef]
- Awad, O.I.; Mamat, R.; Ibrahim, T.K.; Hammid, A.T.; Yusri, I.M.; Hamidi, M.A.; Humada, A.M.; Yusop, A.F. Overview of the oxygenated fuels in spark ignition engine: Environmental and performance. Renew. Sustain. Energy Rev. 2018, 91, 394–408. [Google Scholar] [CrossRef]
- Wen, L.-B.; Xin, C.-Y.; Yang, S.-C. The effect of adding dimethyl carbonate (DMC) and ethanol to unleaded gasoline on exhaust emission. Appl. Energy 2010, 87, 115–121. [Google Scholar] [CrossRef]
- Schifter, I.; González, U.; Díaz, L.; Sánchez-Reyna, G.; Mejía-Centeno, I.; González-Macías, C. Comparison of performance and emissions for gasoline-oxygenated blends up to 20 percent oxygen and implications for combustion on a spark-ignited engine. Fuel 2017, 208, 673–681. [Google Scholar] [CrossRef]
- Christensen, E.; Yanowitz, J.; Ratcliff, M.; McCormick, R.L. Renewable Oxygenate Blending Effects on Gasoline Properties. Energy Fuels 2011, 25, 4723–4733. [Google Scholar] [CrossRef]
- Broustail, G.; Seers, P.; Halter, F.; Moréac, G.; Mounaim-Rousselle, C. Experimental determination of laminar burning velocity for butanol and ethanol iso-octane blends. Fuel 2011, 90, 1–6. [Google Scholar] [CrossRef]
- Bogin, G.E., Jr.; Luecke, J.; Ratcliff, M.A.; Osecky, E.; Zigler, B.T. Effects of iso-octane/ethanol blend ratios on the observance of negative temperature coefficient behavior within the Ignition Quality Tester. Fuel 2016, 186, 82–90. [Google Scholar] [CrossRef]
- Barraza-Botet, C.L.; Wooldridge, M.S. Combustion chemistry of iso-octane/ethanol blends: Effects on ignition and reaction pathways, Combust. Flame 2018, 188, 324–336. [Google Scholar] [CrossRef]
- Foong, T.M.; Morganti, K.J.; Brear, M.J.; da Silva, G.; Yang, Y.; Dryer, F.L. The octane numbers of ethanol blended with gasoline and its surrogates. Fuel 2014, 115, 727–739. [Google Scholar] [CrossRef]
- Schifter, I.; González, U.; González-Macías, C. Effects of ethanol, ethyl-tert-butyl ether and dimethyl-carbonate blends with gasoline on SI engine. Fuel 2016, 183, 253–261. [Google Scholar] [CrossRef]
- Chan, J.H.; Tsolakis, A.; Herreros, J.M.; Kallis, K.X.; Hergueta, C.; Sittichompoo, S.; Bogarra, M. Combustion, gaseous emissions and PM characteristics of Di-Methyl Carbonate (DMC)-gasoline blend on gasoline Direct Injection (GDI) engine. Fuel 2020, 263, 116742. [Google Scholar] [CrossRef]
- Wagner, C.; Keskin, M.-T.; Pitsch, H.; Grill, M.; Bargende, M.; Cai, L. Potential Analysis and Virtual Development of SI Engines Operated with Synthetic Fuel DMC+. SAE Technical Paper 2020-01-0342. 2020. Available online: https://www.sae.org/publications/technical-papers/content/2020-01-0342/ (accessed on 23 October 2023).
- Chen, G.; Yu, W.; Fu, J.; Huang, J.M.Z.; Yang, J.; Wang, Z.; Jin, H.; Qi, F. Experimental and modeling study of the effects of adding oxygenated fuels to premixed n-heptane flames. Combust. Flame 2012, 159, 2324–2335. [Google Scholar] [CrossRef]
- Gao, Z.; Hu, E.; Xu, Z.; Huang, S.; Ku, J.; Huang, Z. Measurements and kinetic study on the ignition delay time of dimethyl carbonate/n-heptane/oxygen/argon mixtures. Combust. Sci. Technol. 2018, 190, 933–948. [Google Scholar] [CrossRef]
- Tan, Y.R.; Salamanca, M.; Bai, J.; Akroyd, J.; Kraft, M. Structural effects of C3 oxygenated fuels on soot formation in ethylene coflow diffusion flames. Combust. Flame 2021, 232, 111512. [Google Scholar] [CrossRef]
- Pacheco, M.A.; Marshall, C.L. Review of Dimethyl Carbonate (DMC) Manufacture and Its Characteristics as a Fuel Additive. Energy Fuels 1997, 11, 2–29. [Google Scholar] [CrossRef]
- Takahashi, E.; Nagano, Y.; Kitagawa, T.; Nishioka, M.; Nakamura, T.; Nakano, M. Demonstration of knock intensity mitigation through dielectric barrier discharge reformation in an RCEM. Combust. Flame 2020, 216, 185–193. [Google Scholar] [CrossRef]
- Takahashi, E.; Kuramochi, A.; Nishioka, M. Turbulent flame propagation enhancement by application of dielectric barrier discharge to fuel-air mixtures. Combust. Flame 2018, 192, 401–409. [Google Scholar] [CrossRef]
- Takahashi, E.; Sakamoto, S.; Imamura, O.; Ohkuma, Y.; Yamasaki, H.; Furutani, H.; Akihama, K. Fundamental characteristics of laser breakdown assisted long distance discharge ignition. J. Phys. D Appl. Phys. 2019, 52, 485501. [Google Scholar] [CrossRef]
- Takahashi, E.; Kato, S. Laser ablation ignition of flammable gas. Jpn. J. Appl. Phys. 2021, 60, 047001. [Google Scholar] [CrossRef]
- Vipavanich, C.; Chuepeng, S.; Skullong, S. Heat release analysis and thermal efficiency of a single cylinder diesel dual fuel engine with gasoline port injection. Case Stud. Therm. Eng. 2018, 12, 143–148. [Google Scholar] [CrossRef]
- Heywood, J.B. Internal Combustion Engine Fundamentals; McGraw-Hill Education: New York, NY, USA, 1988; pp. 372–373,382. [Google Scholar]
- Han, W.-Q.; Yao, C.-D. Research on high cetane and high octane number fuels and the mechanism for their common oxidation and auto-ignition. Fuel 2015, 150, 29–40. [Google Scholar] [CrossRef]
- Boot, M.D.; Tian, M.; Hensen, E.J.M.; Sarathy, S.M. Impact of fuel molecular structure on auto-ignition behavior—Design rules for future high performance gasolines. Prog. Energy Combust. Sci. 2017, 60, 1–25. [Google Scholar] [CrossRef]
- Yang, B.; Sun, W.; Moshammer, K.; Hansen, N. Review of the Influence of Oxygenated Additives on the Combustion Chemistry of Hydrocarbons. Energy Fuels 2021, 35, 13550–13568. [Google Scholar] [CrossRef]
- Kanayama, K.; Takahashi, S.; Morikura, S.; Nakamura, H.; Tezuka, T.; Maruta, K. Study on oxidation and pyrolysis of carbonate esters using a micro flow reactor with a controlled temperature profile. Part I: Reactivities of dimethyl carbonate, ethyl methyl carbonate and diethyl carbonate. Combust. Flame 2022, 237, 111810. [Google Scholar] [CrossRef]
- Marinov, N.M. A Detailed Chemical Kinetic Model for High Temperature Ethanol Oxidation. Int. J. Chem. Kinet. 1999, 31, 183–220. [Google Scholar] [CrossRef]
- Glaude, P.A.; Pitz, W.J.; Thomson, M.J. Chemical kinetic modeling of dimethyl carbonate in an opposed-flow diffusion flame. Proc. Combust. Inst. 2005, 30, 1111–1118. [Google Scholar] [CrossRef]
- Rau, F.; Hartl, S.; Voss, S.; Still, M.; Hasse, C.; Trimis, D. Laminar burning velocity measurements using the Heat Flux method and numerical predictions of iso-octane/ethanol blends for different preheat temperatures. Fuel 2015, 140, 10–16. [Google Scholar] [CrossRef]
- Mannaa, O.A.; Mansour, M.S.; Roberts, W.L.; Chung, S.H. Laminar Burning Velocities of Fuels for Advanced Combustion Engines (FACE) Gasoline and Gasoline Surrogates with and without Ethanol Blending Associated with Octane Rating. Combust. Sci. Technol. 2016, 188, 692–706. [Google Scholar] [CrossRef]
- Burluka, A.A.; Gaughan, R.G.; Griffiths, J.F.; Mandilas, C.; Sheppard, C.G.W.; Woolley, R. Turbulent burning rates of gasoline components, Part 1—Effect of fuel structure of C6 hydrocarbons. Fuel 2016, 167, 347–356. [Google Scholar] [CrossRef]
- Li, Z.; Han, W.; Liu, D.; Chen, Z. Laminar flame propagation and ignition properties of premixed iso-octane/air with hydrogen addition. Fuel 2015, 158, 443–450. [Google Scholar] [CrossRef]
- Baloo, M.; Dariani, B.M.; Akhlaghi, M.; Chitsaz, I. Effect of iso-octane/methane blend on laminar burning velocity and flame instability. Fuel 2015, 144, 264–273. [Google Scholar] [CrossRef]
- Petrakides, S.; Chen, R.; Gao, D.; Wei, H. Experimental Investigation on the Laminar Burning Velocities and Markstein Lengths of Methane and PRF95 Dual Fuels. Energy Fuels 2016, 30, 6777–6789. [Google Scholar] [CrossRef][Green Version]

| Bore and stroke | 100 mm and 120 mm |
| Corresponding piston speed | 1200 rpm |
| Compression ratio | 7.5 |
| Piston compression velocity | 5 m/s |
| Piston shape | Flat top |
| Fuel | Gasoline/DMC (DMC: 0, 20, 40, 60, 100 vol.%) Gasoline/ethanol (ethanol: 0, 20, 40, 60, 100 vol.%) |
| Equivalence ratio | 0.7 and 1.0 |
| Initial temperature of chamber | 340 K |
| Initial pressure of chamber | 0.05 and 0.10 MPa |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Suzuki, S.; Takahashi, E.; Oguma, M.; Akihama, K. Effect of Blending Dimethyl Carbonate and Ethanol with Gasoline on Combustion Characteristics. Fuels 2023, 4, 441-453. https://doi.org/10.3390/fuels4040027
Suzuki S, Takahashi E, Oguma M, Akihama K. Effect of Blending Dimethyl Carbonate and Ethanol with Gasoline on Combustion Characteristics. Fuels. 2023; 4(4):441-453. https://doi.org/10.3390/fuels4040027
Chicago/Turabian StyleSuzuki, Shunsuke, Eiichi Takahashi, Mitsuharu Oguma, and Kazuhiro Akihama. 2023. "Effect of Blending Dimethyl Carbonate and Ethanol with Gasoline on Combustion Characteristics" Fuels 4, no. 4: 441-453. https://doi.org/10.3390/fuels4040027
APA StyleSuzuki, S., Takahashi, E., Oguma, M., & Akihama, K. (2023). Effect of Blending Dimethyl Carbonate and Ethanol with Gasoline on Combustion Characteristics. Fuels, 4(4), 441-453. https://doi.org/10.3390/fuels4040027






