Study of Spray Behaviors to Correlate with Engine Performance and Emissions of a Diesel Engine Using Canola-Based Biodiesel
Abstract
1. Introduction
2. Modelling
3. Model Validation
4. Simulation Results of Diesel and Biodiesel
4.1. Spray Behaviors
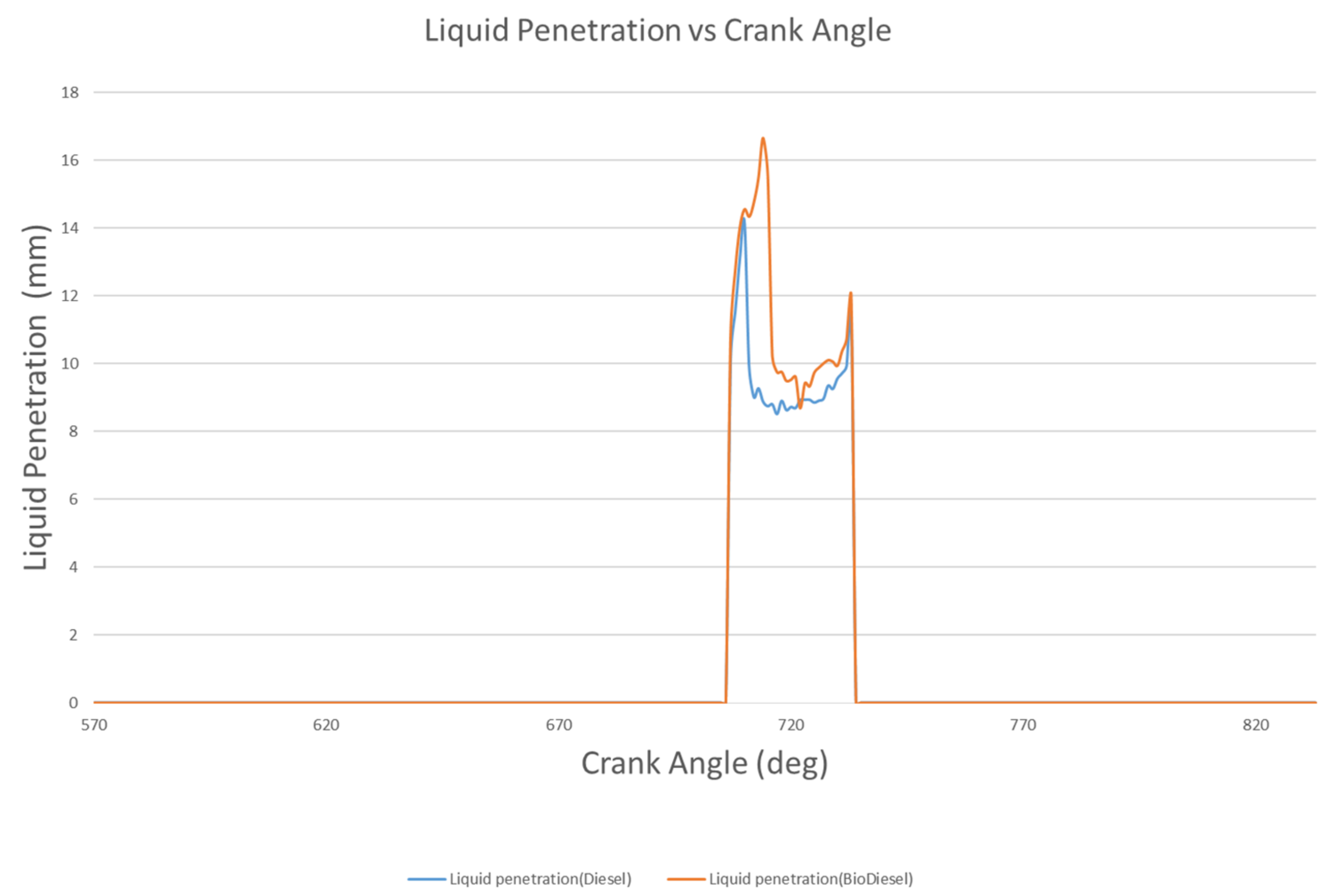
4.1.1. Liquid Penetration Length
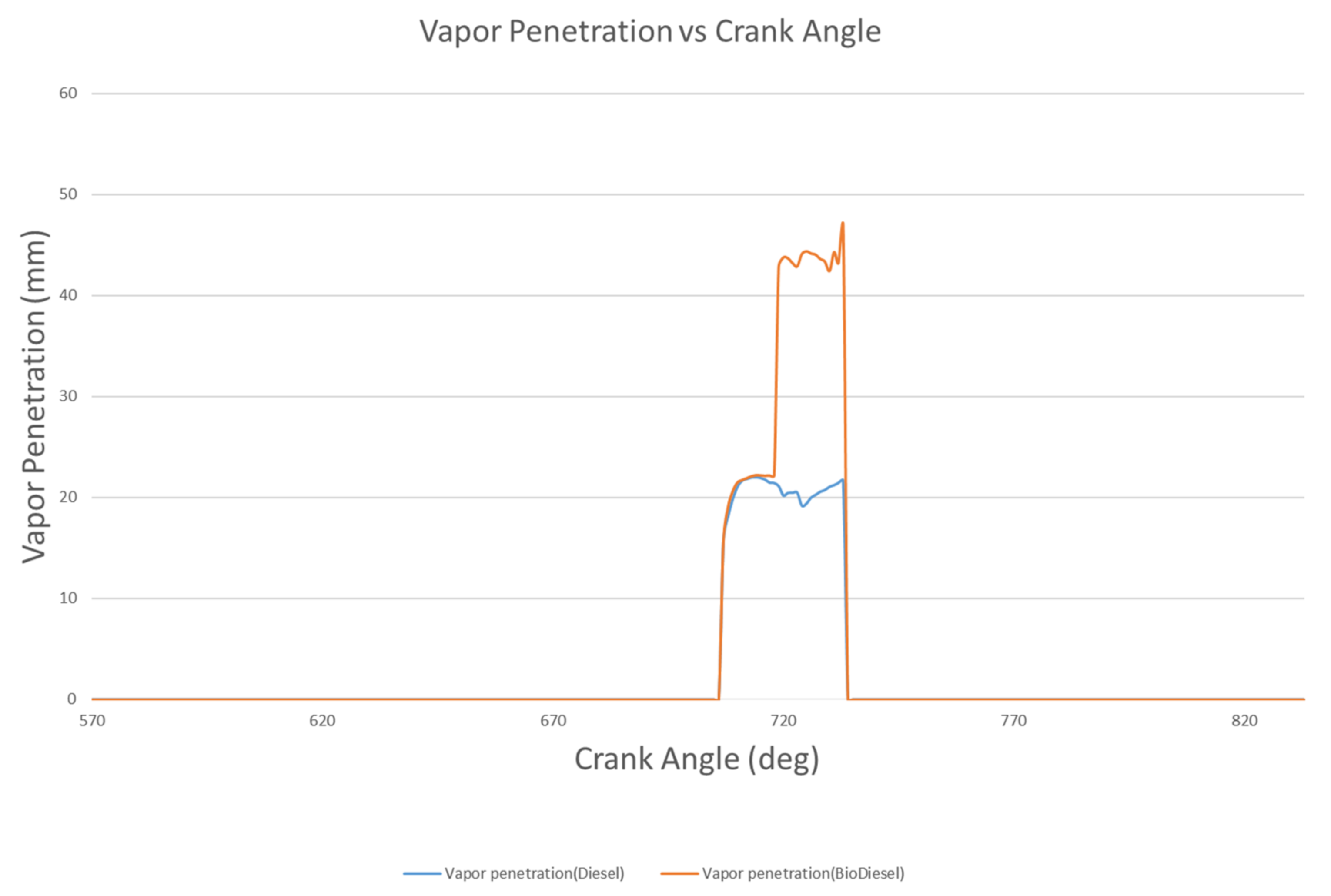
4.1.2. Vapor Penetration Length
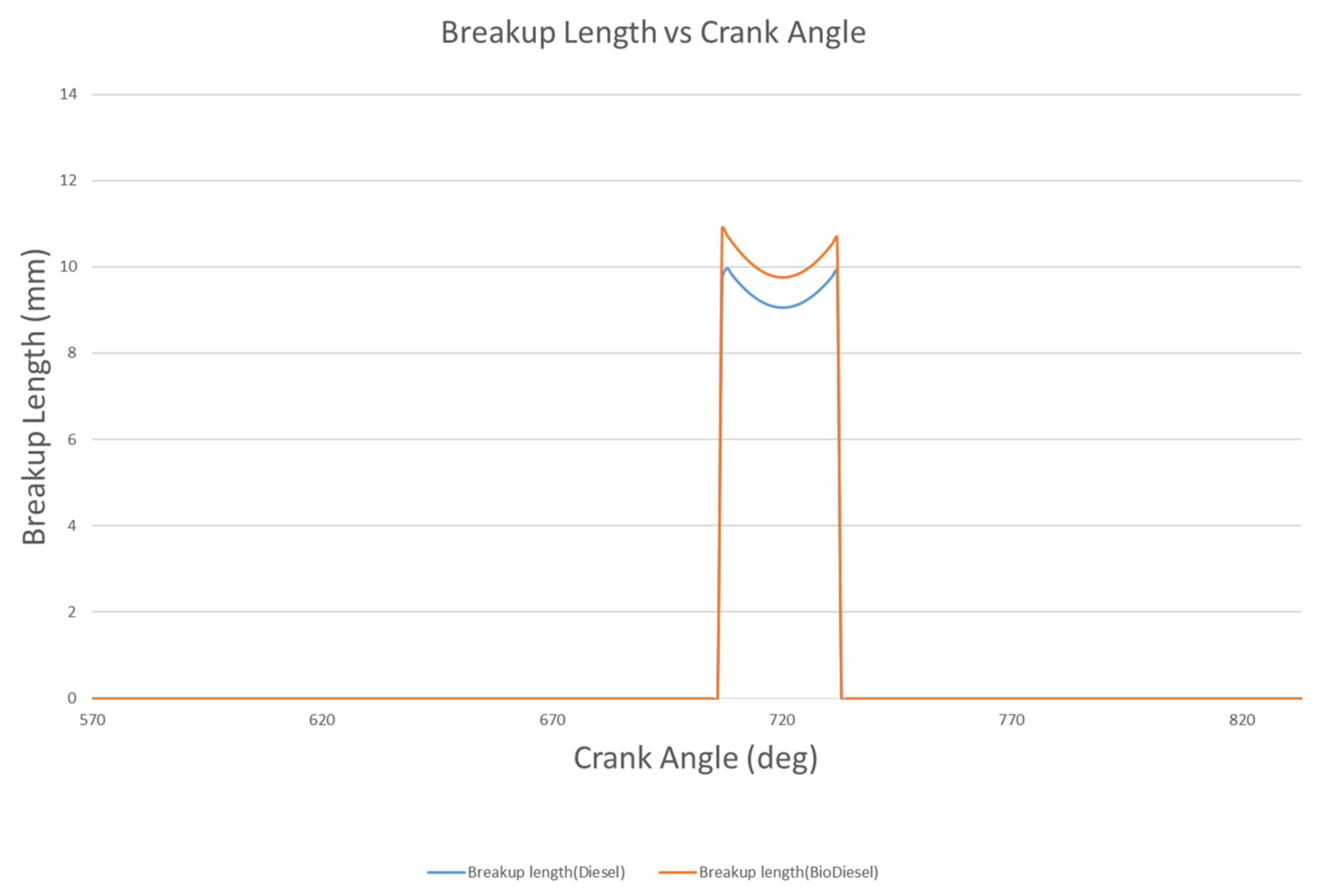
4.1.3. Spray Breakup Length
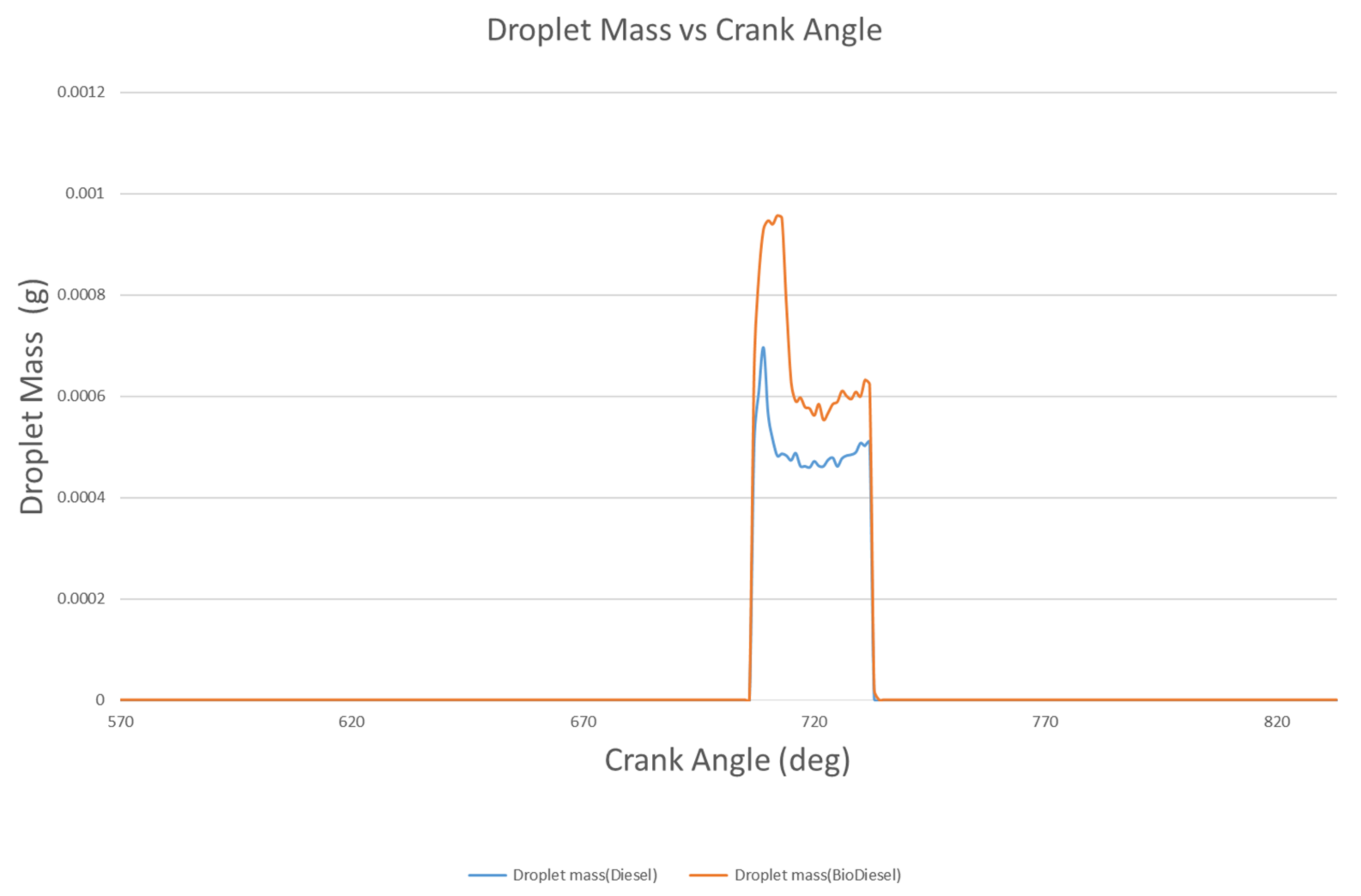
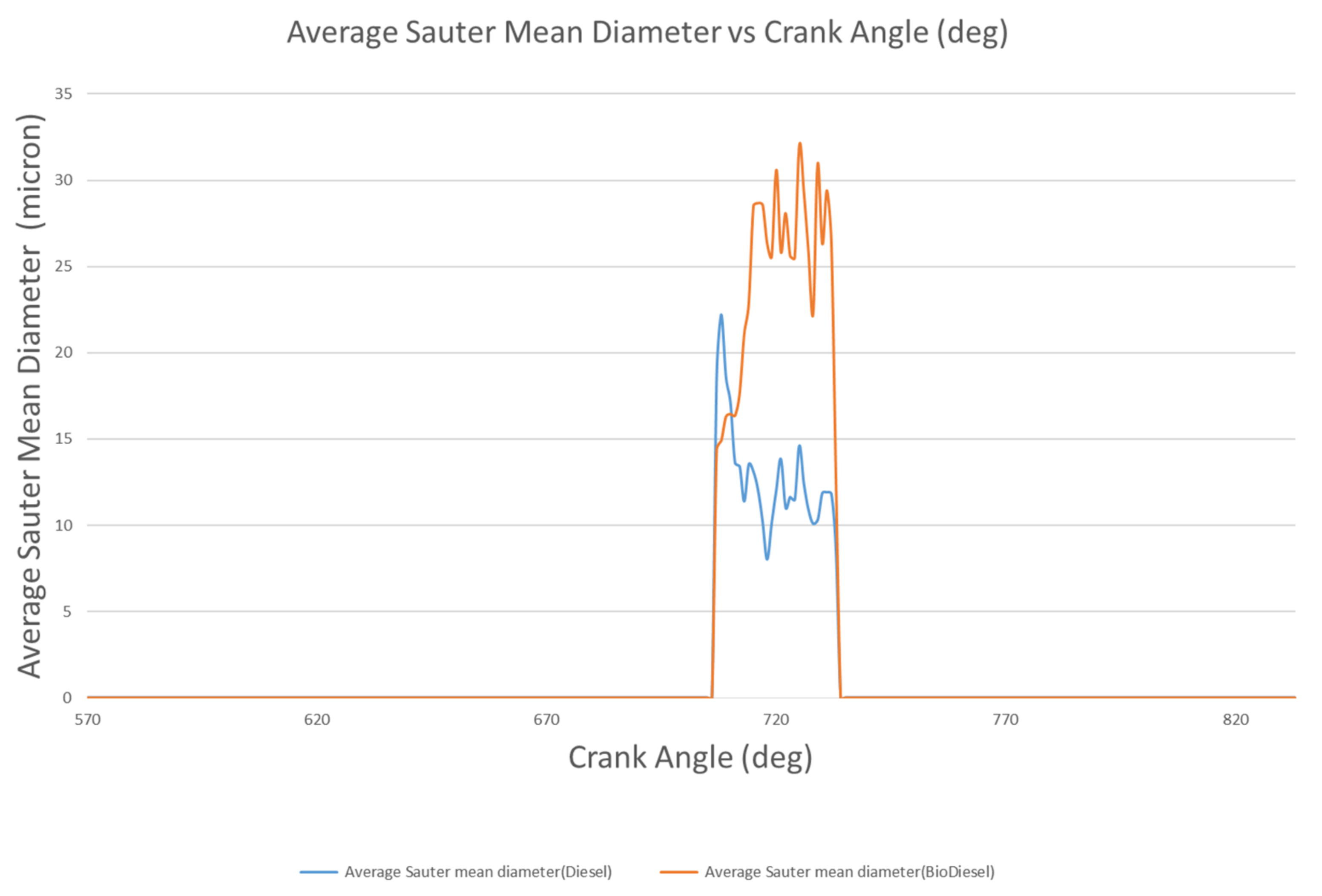
4.1.4. Droplet Mass and Diameter
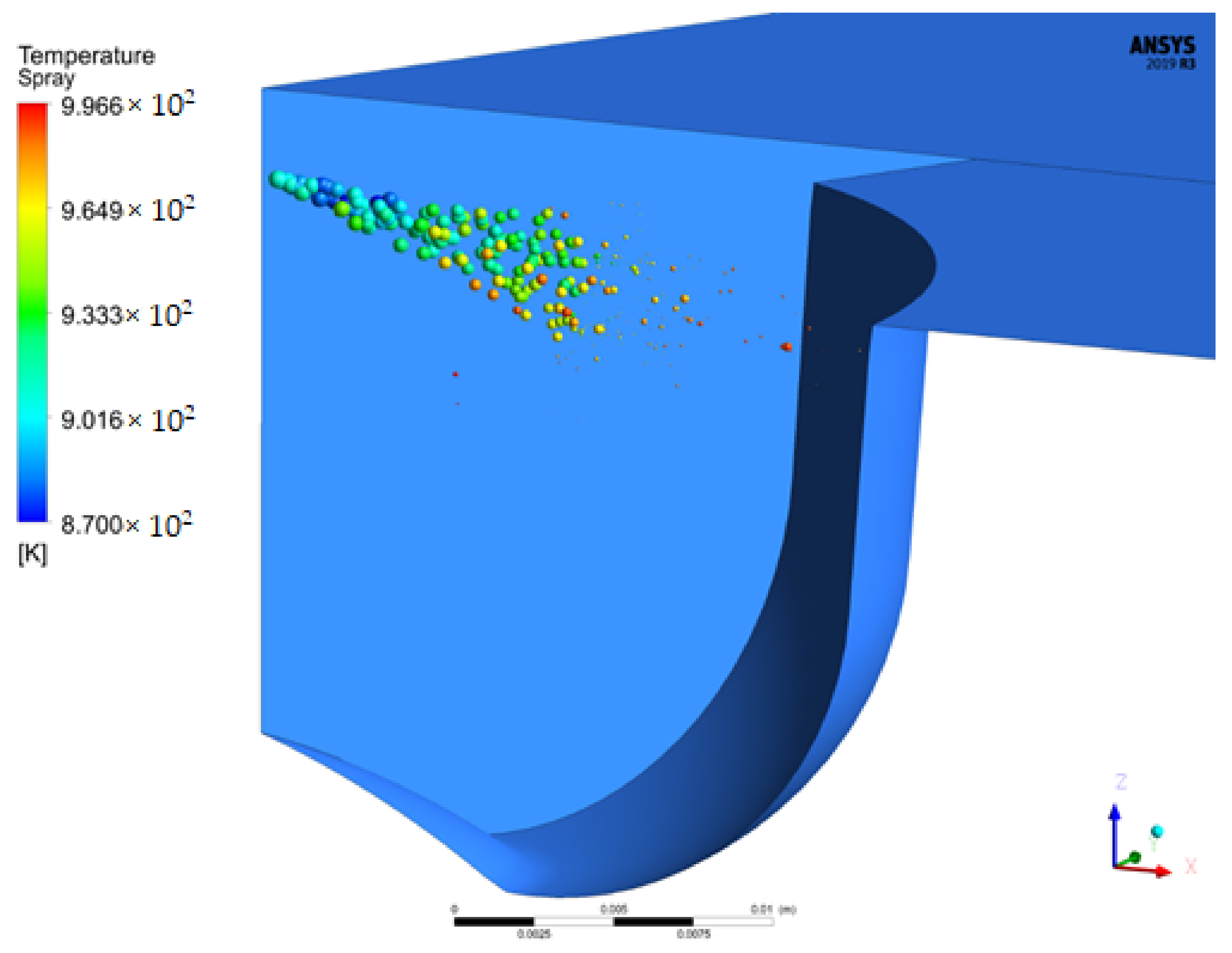
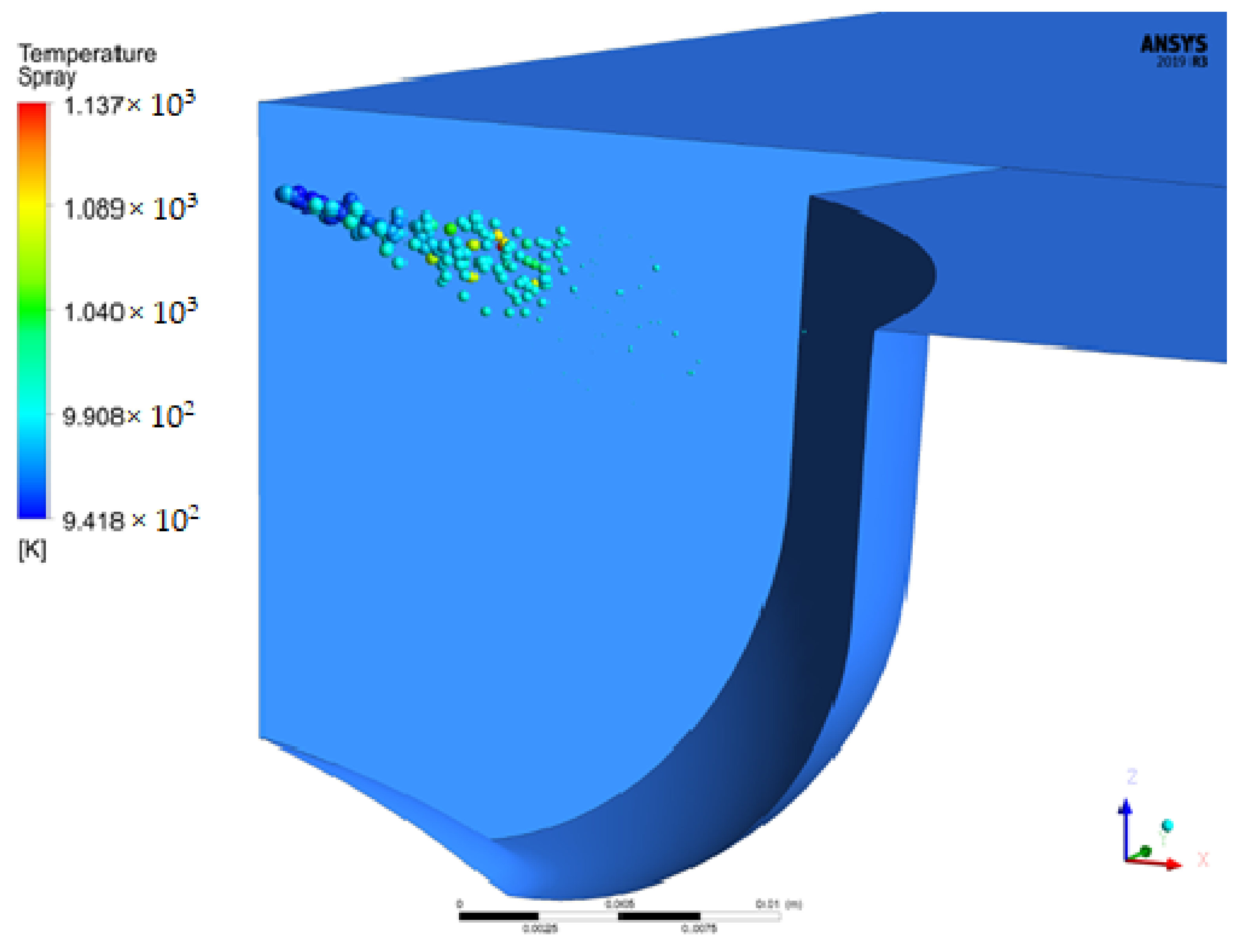
4.1.5. Molecular Images of Droplets
4.2. Combustion Analysis
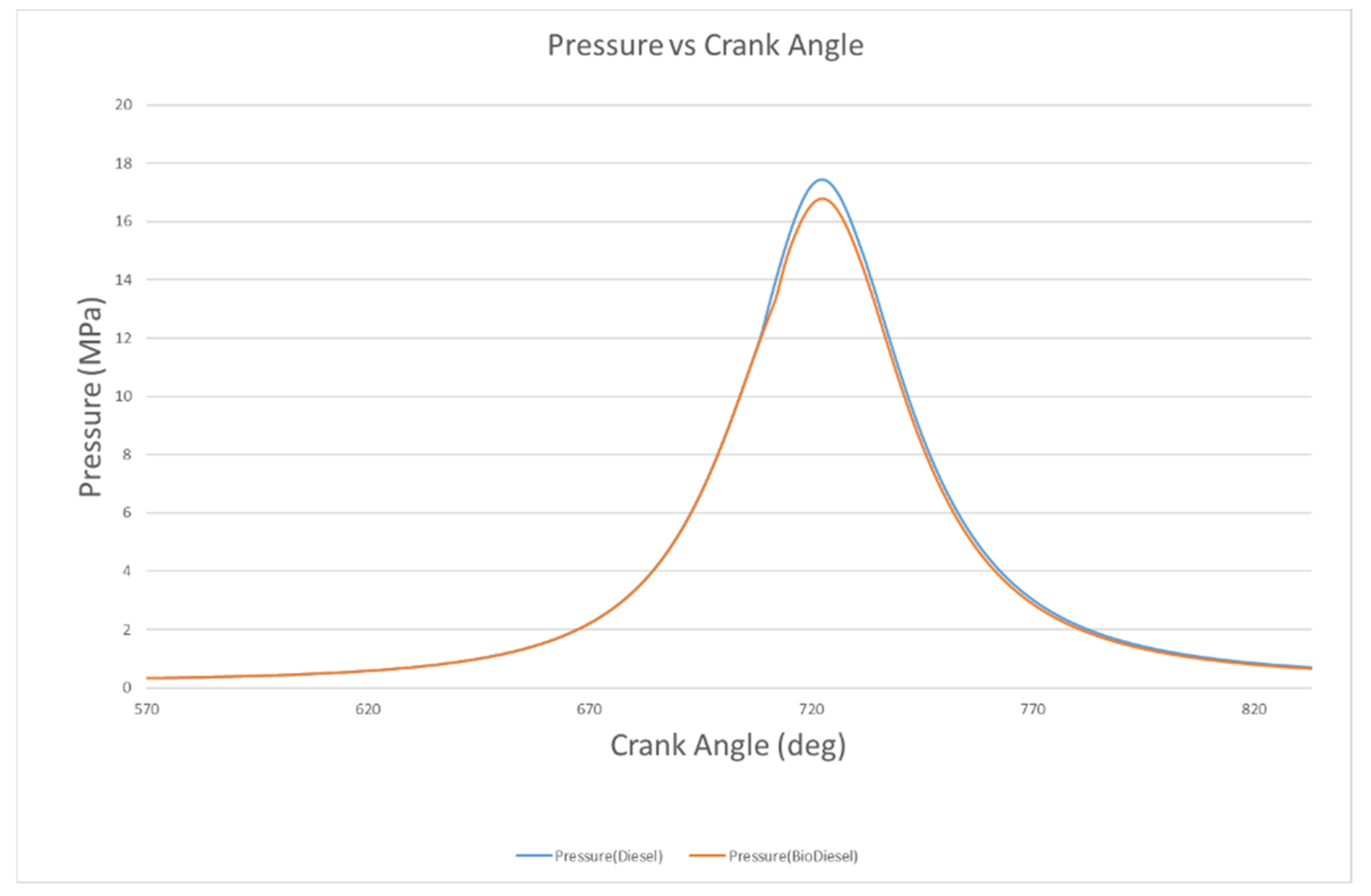
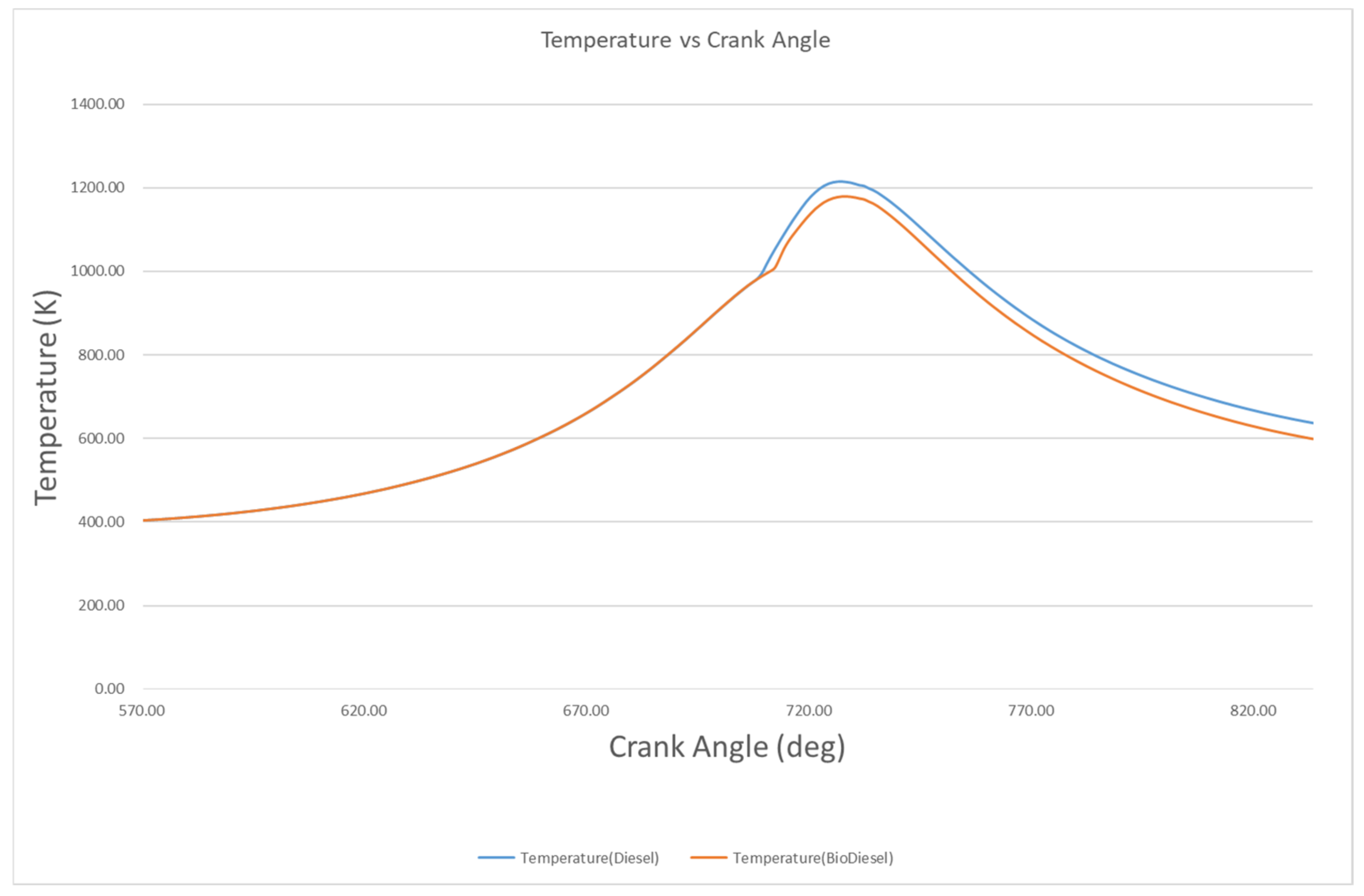
4.2.1. Pressure and Temperature
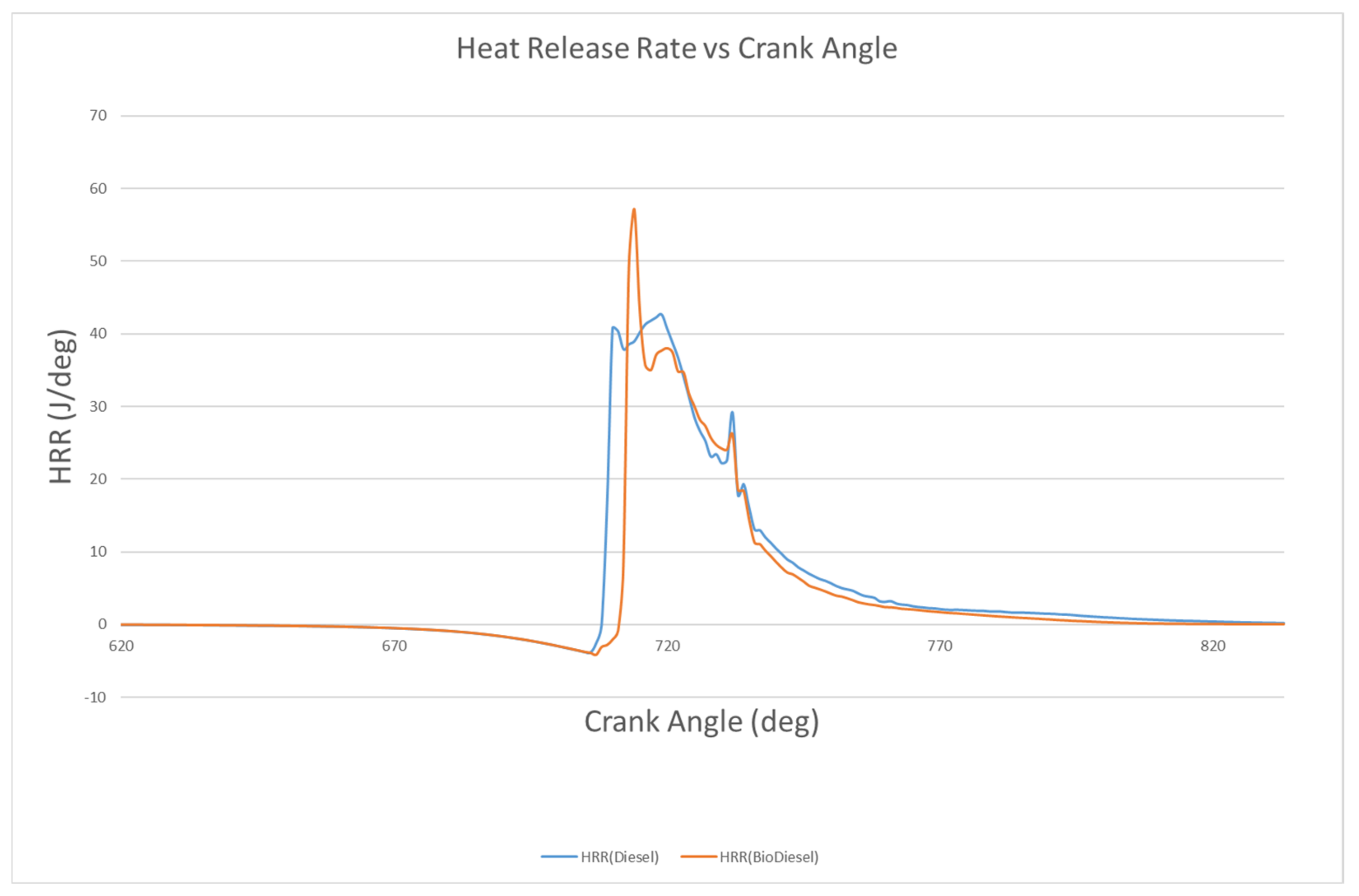
4.2.2. Heat Release Rate
4.2.3. Cumulative Heat Release
4.3. Performance
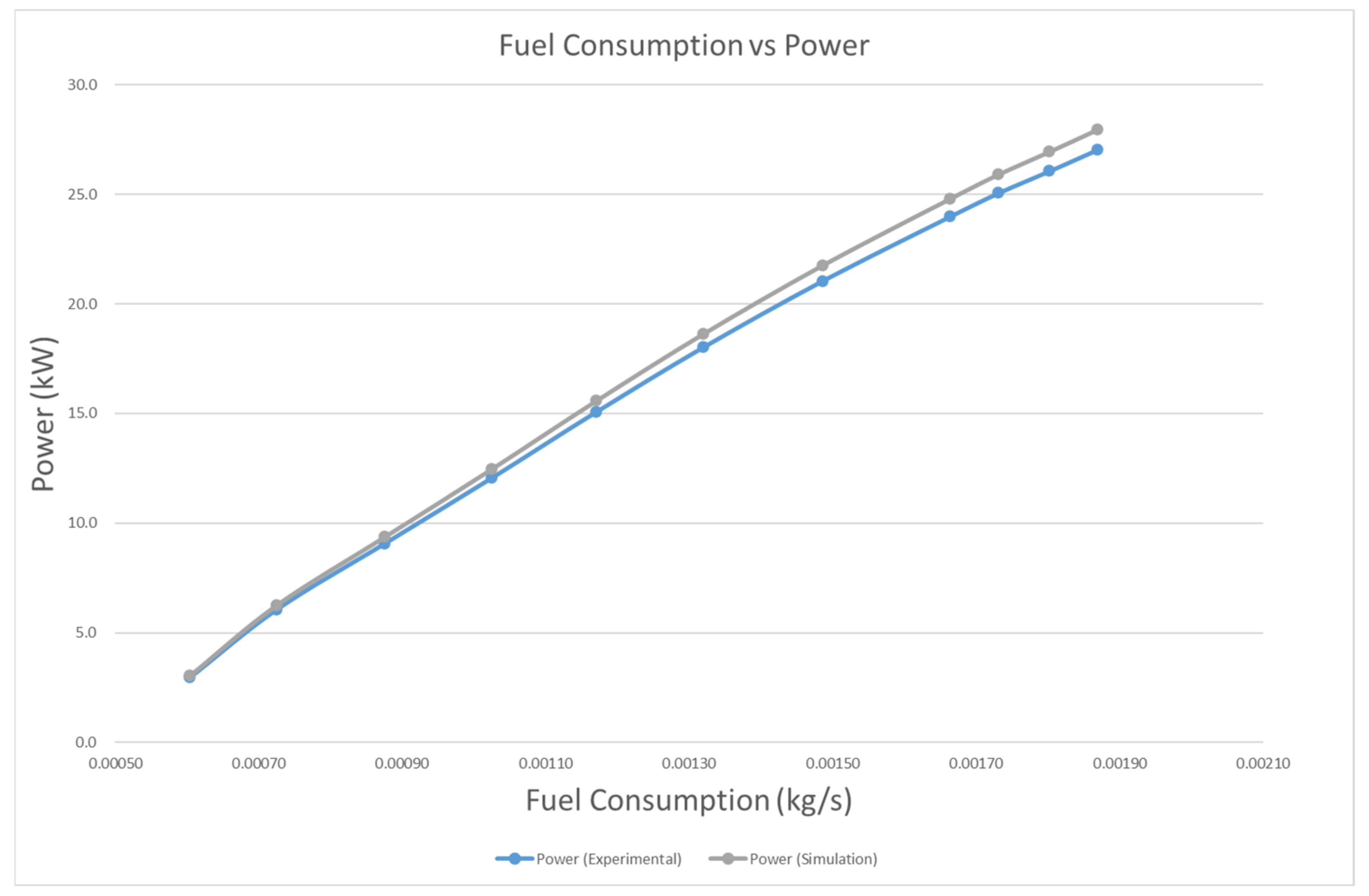
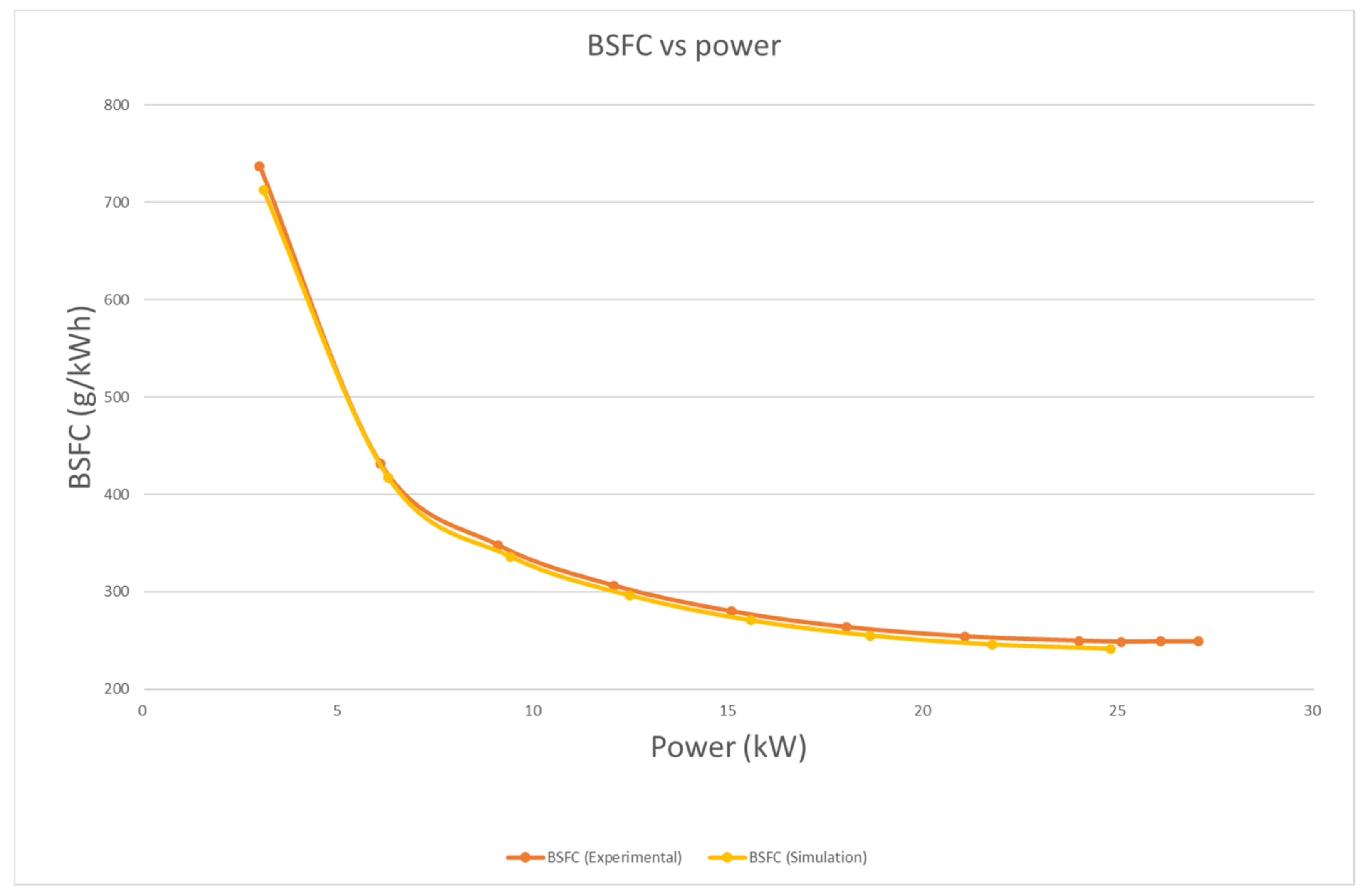
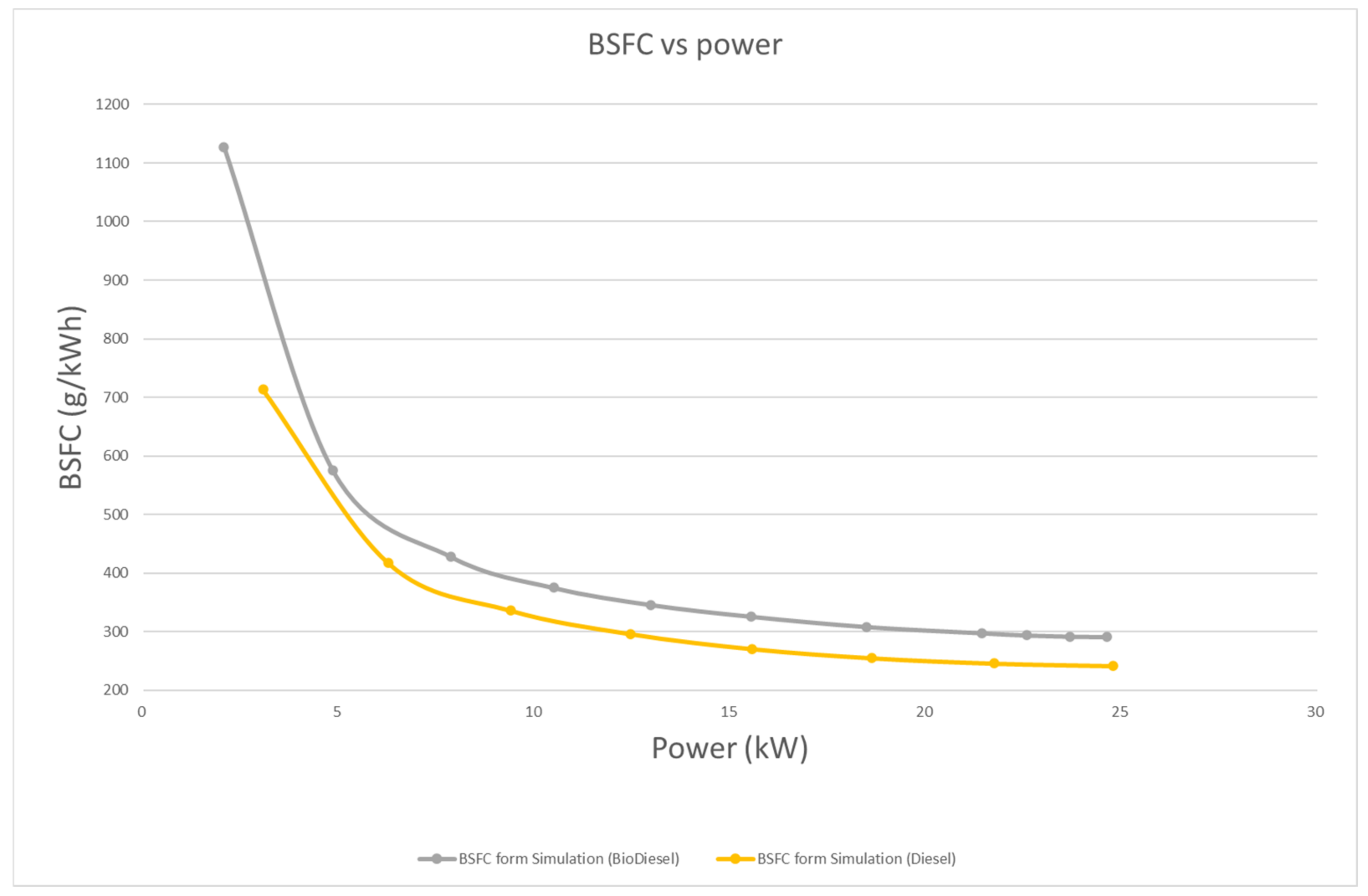
4.3.1. Brake Power and BSFC
4.3.2. Brake Thermal Efficiency vs. Power
4.4. Emission Results of Biodiesel and Diesel
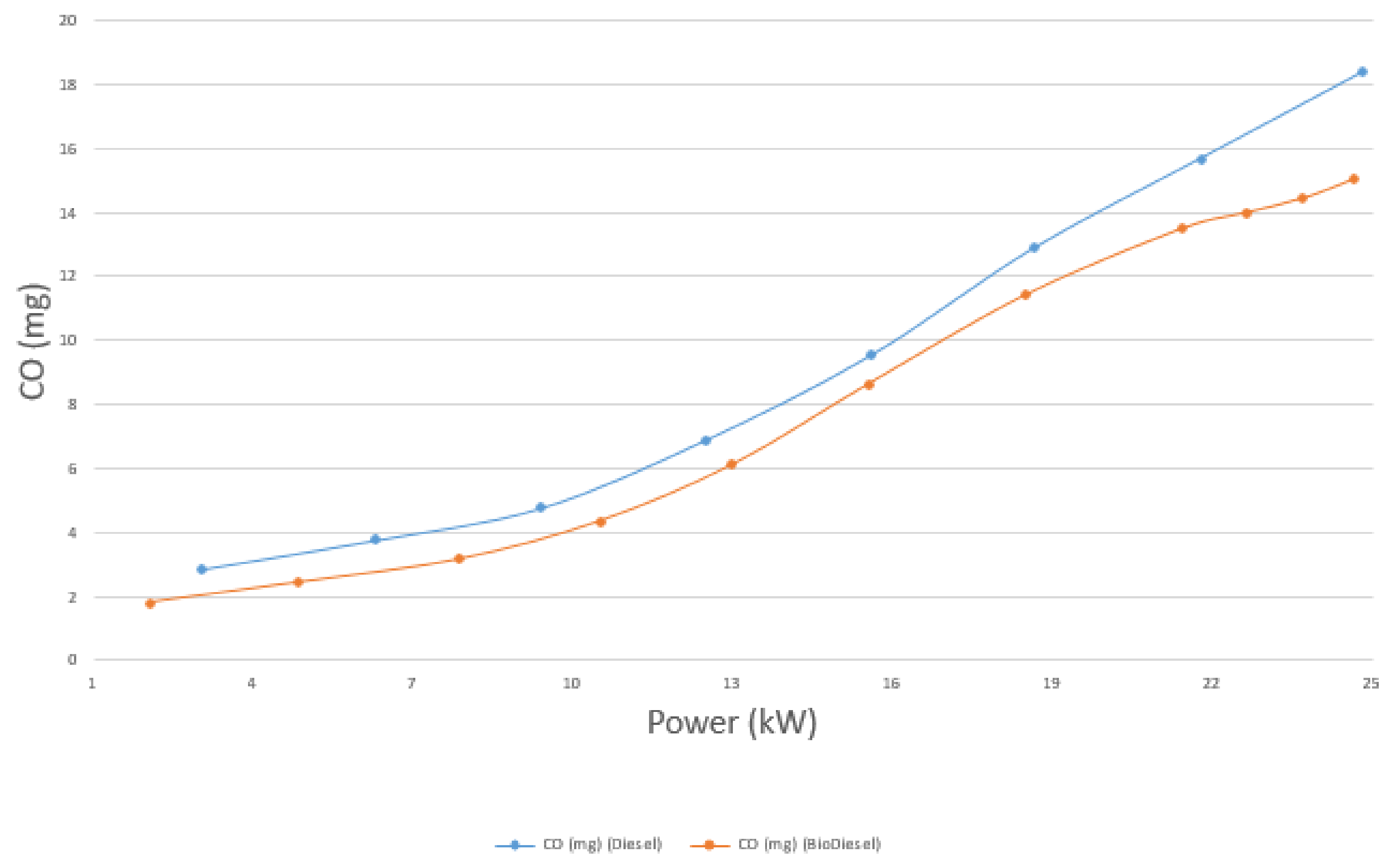
4.4.1. CO Emissions
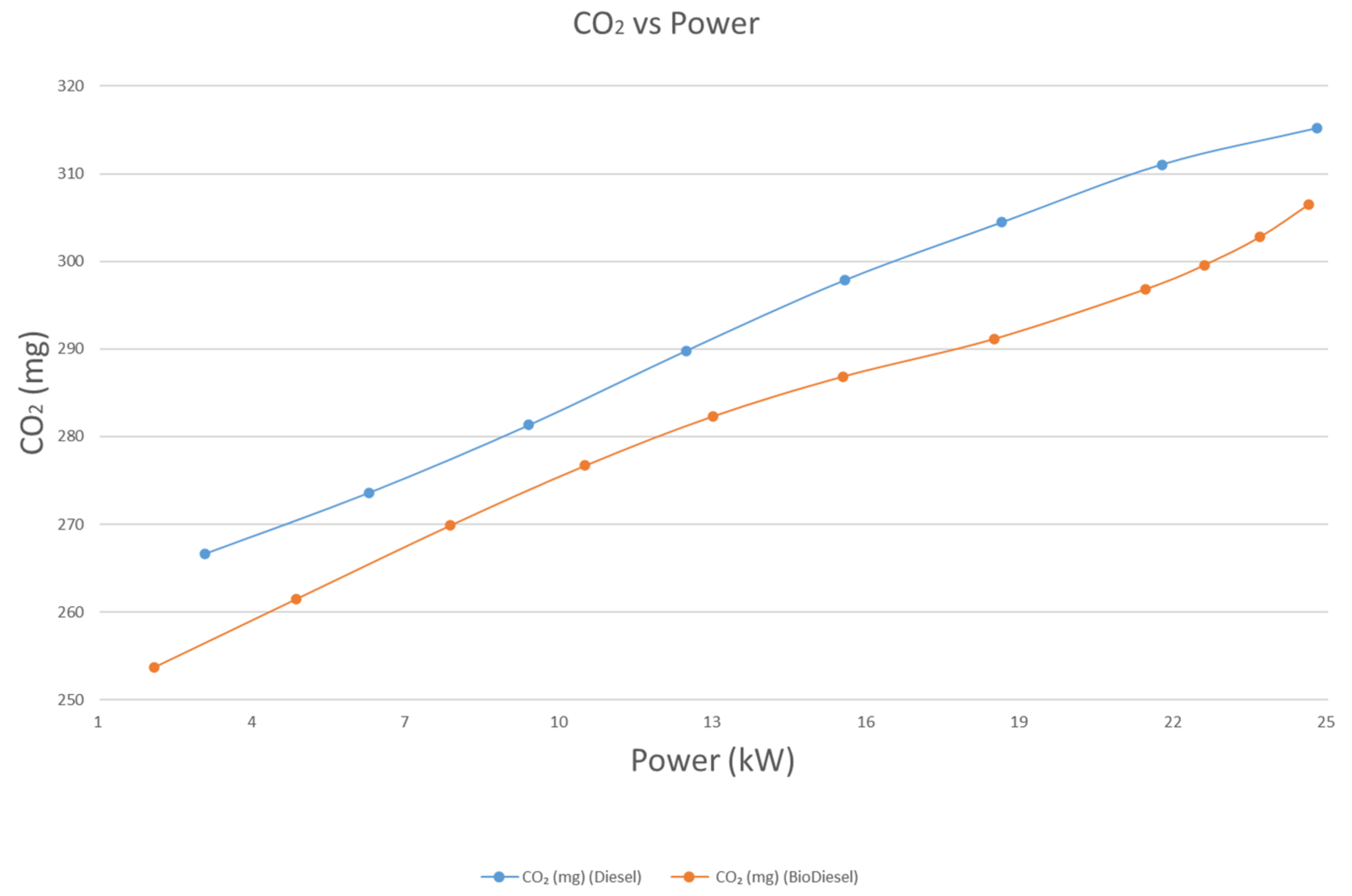
4.4.2. CO2 Emissions
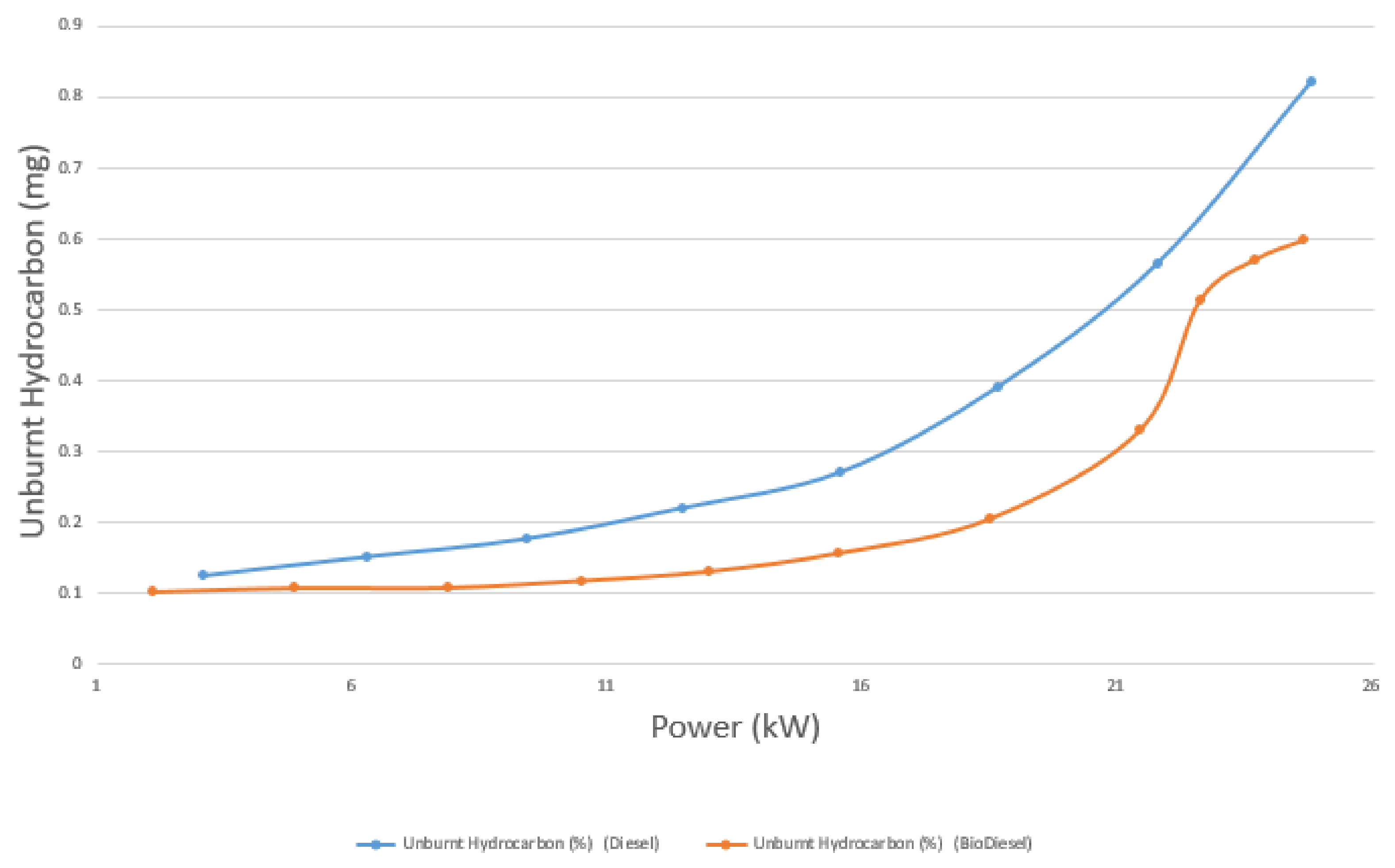
4.4.3. HC Emissions
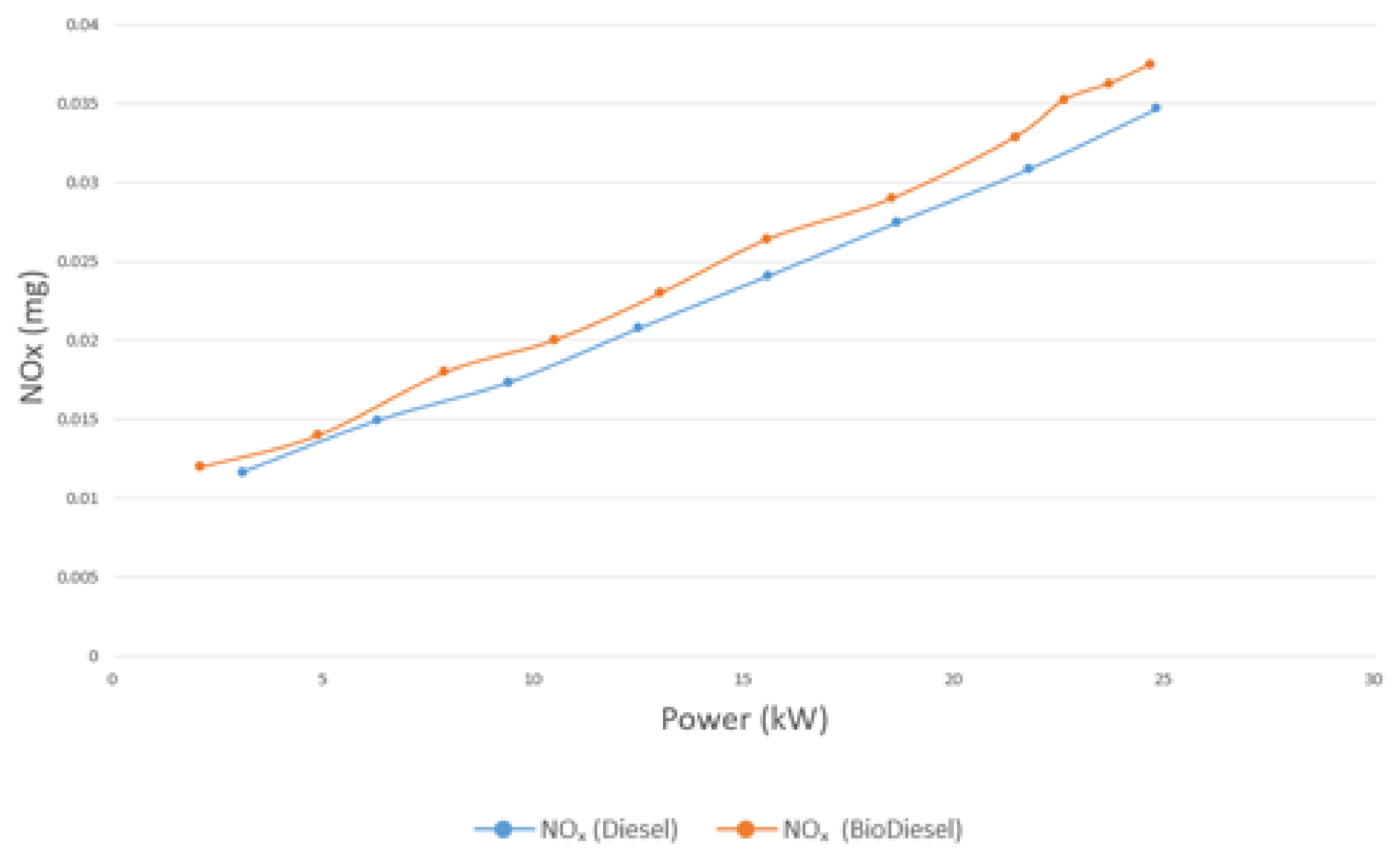
4.4.4. NOx Emissions
4.5. Relationship of Injection Process of Fuel with the Performances and Emissions
5. Strategies to Improve the Performance of Biodiesel in Diesel Engine in Terms of Injection and Combustion Processes
- Injecting at a higher pressure to reduce the size of liquid molecules, which will also reduce the liquid and vapor penetration lengths. This will allow the biodiesel to evaporate more quickly and mix properly with air within the limited time available to complete the combustion, thus improving the performance of the biodiesel.
- Injecting at a higher temperature will reduce the viscosity and density, which will decrease the size of liquid molecules and reduce the penetration lengths. As shown earlier, biodiesel molecules are around 141 °C lower than those of diesel, which hinders the evaporation process. Therefore, heating will increase the temperature of biodiesel molecules and, thus, will improve the evaporation process, and thereby improve the performance of the diesel engine when biodiesel is used.
- With biodiesel, the occurrence of peak pressure is later (further away from TDC) and the combustion duration is longer than that of diesel. Therefore, advancing the injection timing will allow biodiesel to have more time to complete combustion. This will improve the engine performance when biodiesel is used. However, injection timing needs further research to find the optimum injection timing.
- Blending biodiesel with diesel or a lighter fuel such as kerosene will reduce the viscosity and density. Researchers applied this technique and found improvement. However, the recommended blend is up to 20% biodiesel with 80% diesel or kerosene (B20). This technique combined with the previously mentioned techniques is expected to allow a greater percentage of biodiesel to be present in the blend. This again needs further investigation.
6. Conclusions
- (1)
- The liquid penetration length of biodiesel is 17.7 mm at 714 °CA, but rapidly decreases to 9.74 mm at 717 °CA. However, the liquid penetration length of diesel at 714 °CA is 9.02 mm, and rapidly decreases to 9.02 mm at 717 °CA. Biodiesel has a vapor penetration length that fluctuates between 42.86 mm to 44.26 mm, whereas the vapor penetration length of diesel fluctuates between 19 and 22 mm.
- (2)
- The liquid breakup lengths of biodiesel vary between 9.75 mm and 10.74 mm, while these values vary between 9.06 mm and 10.99 mm for diesel. The droplet masses of biodiesel and diesel are 9.47 mg and 6.97 mg at 712 °CA and 709 °CA, respectively. At 709 °CA, the molecular images of biodiesel and diesel show that the maximum temperatures of the fuel molecules are 996 K and 1137 K, respectively.
- (3)
- The effects of points 1 and 2 reduce the evaporation rate of biodiesel as compared to diesel, which results in inferior mixing with air; this ultimately reduces the performance of biodiesel relative to diesel.
- (4)
- The peak pressures of biodiesel and diesel are 16.9 MPa and 17.4 MPa, with these occurring at 722 °CA and 123 °CA, respectively. However, the in-cylinder temperatures of biodiesel and biodiesel are 1179 K and 1216 K at 728 °CA and 726 °CA, respectively.
- (5)
- The longer delay of biodiesel than diesel results in more vapor mixing with air, which, when ignited, produces higher heat release rate as compared to diesel. The heat release rate of biodiesel and diesel are 57.23 J/deg. and 40.18 J/deg., with these occurring at 714 °CA and 710 °CA, respectively. The cumulative heat release graph shows that biodiesel and diesel have combustion durations of 42.98 °CA and 37.02 °CA, respectively. From the graph, it is also found that the ignition delay of biodiesel and diesel are 8 °CA and 4 °CA, respectively.
- (6)
- In terms of performance, power with biodiesel is, on average, 16.9% lower than that of diesel. Overall, the BSFC of biodiesel is 19.9% higher than that of diesel. This is mainly because of the lower calorific value of biodiesel.
- (7)
- The emissions of CO, CO2, and unburnt HC, on average, are 17.8%, 3.4%, and 23.5% lower for biodiesel than diesel, respectively. On the other hand, the emission of NOX is 14.39% higher for biodiesel than diesel.
Author Contributions
Funding
Conflicts of Interest
References
- IEA. World Energy Balances 2021 Overview. Available online: https://www.iea.org/reports/world-energy-balances-overview (accessed on 17 June 2021).
- IEA. CO₂ Emissions from Fuel Combustion 2021. Available online: https://www.iea.org/reports/co2-emissions-from-fuel-combustion-overview (accessed on 17 June 2021).
- Mahlia, T.; Syazmi, Z.A.H.S.; Mofijur, M.; Pg Abas, A.E.; Bilad, M.R.; Ong, H.C.; Silitonga, A.S. Patent landscape review on biodiesel production: Technology updates. Renew. Sustain. Energy Rev. 2020, 118, 109526. [Google Scholar] [CrossRef]
- IEA. Renewables Information 2020 Overview. Available online: https://www.iea.org/reports/renewables-information-overview (accessed on 25 November 2020).
- Mahmudul, H.M.; Hagos, F.Y.; Mamat, R.; Adam, A.A.; Ishak, W.F.W.; Alenezi, R. Production, characterization and performance of biodiesel as an alternative fuel in diesel engines–A review. Renew. Sustain. Energy Rev. 2017, 72, 497–509. [Google Scholar] [CrossRef]
- Bari, S.; Saad, I. Optimization of vane numbers through simulation and experiment, and investigation of the effect on the performance and emissions of a CI (compression ignition) engine run with biodiesel. Energy 2015, 79, 248–263. [Google Scholar] [CrossRef]
- Bari, S.; Hossain, S.N.; Saad, I. A Review on Techniques to Improve Performance and Reduce Emissions of Diesel Engine Running with Higher Viscous Fuels (HVFs). Fuel 2019, 264. [Google Scholar] [CrossRef]
- Khoobbakht, G.; Kheiralipour, K.; Rasouli, H.; Rafiee, M.; Hadipour, M.; Karimi, M. Experimental exergy analysis of transesterification in biodiesel production. Energy 2020, 196, 117092. [Google Scholar] [CrossRef]
- FAO. FAOSTAT. Available online: https://www.fao.org/faostat/en/#data/QC/visualize (accessed on 26 November 2021).
- AEGIC. Australian Canola. Available online: https://www.aegic.org.au/wp-content/uploads/2018/02/AEGIC-Grain-Note-canola_LR.pdf (accessed on 25 November 2020).
- Kirkegaard, J.A.; Lilley, J.M.; Morrison, M.J. Drivers of trends in Australian canola productivity and future prospects. Crop Pasture Sci. 2016, 67, 1–9. [Google Scholar] [CrossRef]
- FAO. World Agriculture: Towards 2015/2030. Earthscan. Available online: www.fao.org/docrep/005/y4252e/y4252e00.htm (accessed on 26 November 2021).
- Bari, S.; Saad, I. Effect of guide vane height on the performance and emissions of a compression ignition (CI) engine run with biodiesel through simulation and experiment. Appl. Energy 2014, 136, 431–444. [Google Scholar] [CrossRef]
- Manigandan, S.; Gunasekar, P.; Praveenkumar, T.R.; Sabir, J.S.; Mathimani, T.; Pugazhendhi, A.; Brindhadevi, K. Performance, noise and emission characteristics of DI engine using canola and Moringa oleifera biodiesel blends using soluble multiwalled carbon nanotubes. Fuel 2021, 289, 119829. [Google Scholar] [CrossRef]
- Simsek, S. Effects of biodiesel obtained from Canola, sefflower oils and waste oils on the engine performance and exhaust emissions. Fuel 2020, 265, 117026. [Google Scholar] [CrossRef]
- Temizer, İ.; Eskici, B. Investigation on the combustion characteristics and lubrication of biodiesel and diesel fuel used in a diesel engine. Fuel 2020, 278, 118363. [Google Scholar] [CrossRef]
- Saad, I.; Bari, S. Guide vane swirl and tumble device to improve in-cylinder air flow of CI engine using vegetable oil. Procedia Eng. 2014, 90, 425–430. [Google Scholar] [CrossRef][Green Version]
- Lim, T.; Bari, S.; Yu, C. Using crude palm oil (CPO) as diesel engine fuel. ASEAN J. Sci. Technol. Dev. 2002, 19, 1–13. [Google Scholar] [CrossRef]
- Bari, S.; Hossain, S.; Saad, I. A review on improving airflow characteristics inside the combustion chamber of CI engines to improve the performance with higher viscous biofuels. Fuel 2020, 264, 116769. [Google Scholar] [CrossRef]
- Cong, G.J.; Ki, Y.S.; Soo, K.M.; Jung, C.N. Application of Canola Oil Biodiesel/Diesel Blends in a Common Rail Diesel Engine. Appl. Sci. 2016, 7, 34. [Google Scholar] [CrossRef]
- Can, Ö.; Öztürk, E.; Yücesu, H.S. Combustion and exhaust emissions of canola biodiesel blends in a single cylinder DI diesel engine. Renew. Energy 2017, 109, 73–82. [Google Scholar] [CrossRef]
- Anbarasu, A.; Karthikeyan, A. Diesel engine performance and emission evaluation using Canola biodiesel emulsion fuel. Aust. J. Mech. Eng. 2016, 14, 174–181. [Google Scholar] [CrossRef]
- Nguyen, T.; Pham, M.; Anh, T.L. Spray, combustion, performance and emission characteristics of a common rail diesel engine fueled by fish-oil biodiesel blends. Fuel 2020, 269, 117108. [Google Scholar] [CrossRef]
- Bari, S.; Zhang, C. Analysis of Performance and Emission of Diesel Engines Operating on Palm Oil Biodiesel. SAE Int. J. Adv. Curr. Pract. Mobil. 2020, 2, 1862–1869. [Google Scholar]
- Bari, S. Durability issues of running diesel engine with crude palm oil. In Proceedings of the 2009 1st International Conference on the Developements in Renewable Energy Technology (ICDRET), Dhaka, Bangladesh, 17-19 December 2009; 2009; pp. 1–4. [Google Scholar] [CrossRef]
- Algayyim, S.J.M.; Wandel, A.P. Macroscopic and microscopic characteristics of biofuel spray (biodiesel and alcohols) in CI engines: A review. Fuel 2021, 292, 120303. [Google Scholar] [CrossRef]
- Yu, S.; Yin, B.; Deng, W.; Jia, H.; Ye, Z.; Xu, B.; Xu, H. Experimental study on the spray and mixing characteristics for equilateral triangular and circular nozzles with diesel and biodiesel under high injection pressures. Fuel 2019, 239, 97–107. [Google Scholar] [CrossRef]
- Kuti, O.A.; Sarathy, S.M.; Nishida, K. Spray combustion simulation study of waste cooking oil biodiesel and diesel under direct injection diesel engine conditions. Fuel 2020, 267, 11724. [Google Scholar] [CrossRef]
- Chong, C.T.; Hochgreb, S. Flame structure, spectroscopy and emissions quantification of rapeseed biodiesel under model gas turbine conditions. Appl. Energy 2017, 185, 1383–1392. [Google Scholar] [CrossRef]
- Yin, B.; Yu, S.; Jia, H.; Yu, J. Numerical research of diesel spray and atomization coupled cavitation by Large Eddy Simulation (LES) under high injection pressure. Int. J. Heat Fluid Flow 2016, 59, 1–9. [Google Scholar] [CrossRef]
- Agarwal, A.K.; Som, S.; Shukla, P.C.; Goyal, H.; Longman, D. In-nozzle flow and spray characteristics for mineral diesel, Karanja, and Jatropha biodiesels. Appl. Energy 2015, 156, 138–148. [Google Scholar] [CrossRef]
- Wang, Z.; Li, Y.; Wang, C.; Xu, H.; Wyszynski, M.L. Experimental study on primary breakup of diesel spray under cold start conditions. Fuel 2016, 183, 617–626. [Google Scholar] [CrossRef]
- Xue, F.; Luo, F.; Cui, H.; Moro, A.; Zhou, L. Numerical analyses of transient flow characteristics within each nozzle hole of an asymmetric diesel injector. Int. J. Heat Mass Transf. 2017, 104, 18–27. [Google Scholar] [CrossRef]
- Amsden, A.A.; Findley, M. KIVA-3V: A Block-Structured KIVA Program for Engines With Vertical or Canted Valves; Lawrence Livermore National Lab. (LLNL): Livermore, CA, USA, 1997.
- Yakhot, V.; Orszag, S.A. Renormalization group analysis of turbulence. I. Basic theory. J. Sci. Comput. 1986, 1, 3–51. [Google Scholar] [CrossRef]
- Tan, Z. Multi-Dimensional Modeling Of Ignition And Combustion In Premixed And DIS/CI (Direct Injection Spark/Compression Ignition) Engines; University of Wisconsin-Madison: Madison, WI, USA, 2004. [Google Scholar]
- Beale, J.C.; Reitz, R.D. Modeling spray atomization with the Kelvin-Helmholtz/Rayleigh-Taylor hybrid model. At. Sprays 1999, 9, 623–650. [Google Scholar]
- Su, T.; Patterson, M.; Reitz, R.D.; Farrell, P. Experimental and Numerical Studies of High Pressure Multiple Injection Sprays; SAE Transactions: Warrendale, PA, USA, 1996; Volume 105, pp. 1281–1292. [Google Scholar]
- Wang, Y.; Ge, H.-W.; Reitz, R.D. Validation of mesh-and timestep-independent spray models for multi-dimensional engine CFD simulation. SAE Int. J. Fuels Lubr. 2010, 3, 277–302. [Google Scholar] [CrossRef]
- Reitz, R.D.; Diwakar, R. Effect of Drop Breakup On Fuel Sprays; SAE Transactions: Warrendale, PA, USA, 1986; pp. 218–227. [Google Scholar]
- Bellman, R.; Pennington, R.H. Effects of surface tension and viscosity on Taylor instability. Q. Appl. Math. 1954, 12, 151–162. [Google Scholar] [CrossRef]
- Ibrahim, A.; Bari, S.; Bruno, F. A Study on EGR Utilization In Natural Gas SI Engines Using A Two-Zone Combustion Model; SAE Technical Paper; SAE Transactions: Warrendale, PA, USA, 2007; ISSN 0148-7191. [Google Scholar]
- Bari, S.; Yu, C.; Lim, T. Performance deterioration and durability issues while running a diesel engine with crude palm oil. Proc. Inst. Mech. Eng. Part D J. Automob. Eng. 2002, 216, 785–792. [Google Scholar] [CrossRef]
- Som, S.; Longman, D.; Ramírez, A.; Aggarwal, S. A comparison of injector flow and spray characteristics of biodiesel with petrodiesel. Fuel 2010, 89, 4014–4024. [Google Scholar] [CrossRef]
- Subramanian, K.; Lahane, S. Comparative evaluations of injection and spray characteristics of a diesel engine using karanja biodiesel–diesel blends. Int. J. Energy Res. 2013, 37, 582–597. [Google Scholar] [CrossRef]
- Sivakumar, D.; Vankeswaram, S.; Sakthikumar, R.; Raghunandan, B. Analysis on the atomization characteristics of aviation biofuel discharging from simplex swirl atomizer. Int. J. Multiph. Flow 2015, 72, 88–96. [Google Scholar] [CrossRef]
- Park, S.H.; Kim, H.J.; Suh, H.K.; Lee, C.S. Experimental and numerical analysis of spray-atomization characteristics of biodiesel fuel in various fuel and ambient temperatures conditions. Int. J. Heat Fluid Flow 2009, 30, 960–970. [Google Scholar] [CrossRef]
- Chong, C.T.; Hochgreb, S. Spray combustion characteristics of palm biodiesel. Combust. Sci. Technol. 2012, 184, 1093–1107. [Google Scholar] [CrossRef]
- Kim, H.J.; Park, S.H.; Lee, C.S. A study on the macroscopic spray behavior and atomization characteristics of biodiesel and dimethyl ether sprays under increased ambient pressure. Fuel Process. Technol. 2010, 91, 354–363. [Google Scholar] [CrossRef]
- Liu, H.-P.; Strank, S.; Werst, M.; Hebner, R.; Osara, J. Combustion Emissions Modeling and Testing of Neat Biodiesel Fuels, University of Texas at Austin. Available online: https://repositories.lib.utexas.edu/bitstream/handle/2152/30746/PR_499.pdf?sequence=1 (accessed on 17 June 2021).
- Praveen, A.; Rao, G.L.N.; Balakrishna, B.; Rao, K.S. Comparative analysis of the performance and emission characteristics of a diesel engine by using canola and sesame biodiesel-diesel blends. AIP Conf. Proc. 2019, 2161, 020029. [Google Scholar]
- Raghavulu, K.V.; Uppalapati, S.; Jani, S.; Rajalingam, A.; Rasu, N.G. Effect on performance and emission of canola oil and snake gourd oil biodiesel blended in fossil Diesel-Biodiesel blend. Mater. Today Proc. 2021, 37, 1091–1095. [Google Scholar] [CrossRef]
- Mehmet, Ş. The influence of canola oil biodiesel on performance, combustion characteristics and exhaust emissions of a small diesel engine. Sak. Univ. J. Sci. 2019, 23, 121–128. [Google Scholar]
- Hawi, M.; Kosaka, H.; Sato, S.; Nagasawa, T.; Elwardany, A.; Ahmed, M. Effect of injection pressure and ambient density on spray characteristics of diesel and biodiesel surrogate fuels. Fuel 2019, 254, 115674. [Google Scholar] [CrossRef]
- Geng, L.; Wang, Y.; Wang, Y.; Li, H. Effect of the injection pressure and orifice diameter on the spray characteristics of biodiesel. J. Traffic Transp. Eng. 2020, 7, 331–339. [Google Scholar] [CrossRef]
- Anis, S.; Budiandono, G.N. Investigation of the effects of preheating temperature of biodiesel-diesel fuel blends on spray characteristics and injection pump performances. Renew. Energy 2019, 140, 274–280. [Google Scholar] [CrossRef]
- Yilmaz, N. Performance and emission characteristics of a diesel engine fuelled with biodiesel–ethanol and biodiesel–methanol blends at elevated air temperatures. Fuel 2012, 94, 440–443. [Google Scholar] [CrossRef]
- Balasubramanian, D.; Hoang, A.T.; Venugopal, I.P.; Shanmugam, A.; Gao, J.; Wongwuttanasatian, T. Numerical and experimental evaluation on the pooled effect of waste cooking oil biodiesel/diesel blends and exhaust gas recirculation in a twin-cylinder diesel engine. Fuel 2021, 287, 119815. [Google Scholar] [CrossRef]
- Matbouei, M.; Weston, D.; Liang, X.; Hainsworth, S. An investigation of the effect of temperature on the oxidation processes of metallic diesel engine fuel system materials and B100 biodiesel from used cooking oil in exposure testing. Fuel 2021, 285, 119063. [Google Scholar] [CrossRef] [PubMed]
- Reddy, S.R.; Murali, G.; Shaik, A.A.; Raju, V.D.; Reddy, M.S. Experimental evaluation of diesel engine powered with waste mango seed biodiesel at different injection timings and EGR rates. Fuel 2021, 285, 119047. [Google Scholar] [CrossRef]
- Santhosh, K.; Kumar, G. Effect of injection time on combustion, performance and emission characteristics of direct injection CI engine fuelled with equi-volume of 1-hexanol/diesel blends. Energy 2021, 214, 118984. [Google Scholar] [CrossRef]
- Saad, I.; Bari, S. CFD investigation of in-cylinder air flow to optimize number of guide vanes to improve CI engine performance using higher viscous fuel. Int. J. Automot. Mech. Eng. 2013, 8, 1096–1107. [Google Scholar] [CrossRef]
- Sawant, P.; Bari, S. Effects of variable intake valve timings and valve lift on the performance and fuel efficiency of an internal combustion engine. SAE Tech. Pap. 2018. [Google Scholar] [CrossRef]




| Technical Specification | Value/Type |
|---|---|
| Bore | 104 mm |
| Stroke | 108 mm |
| Number of cylinders | 4 |
| Compression ratio | 17.9 |
| Engine speed | 1500 rpm |
| Engine displacement | 4000 cc |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bari, S.; Zhang, C.; Kafrawi, F.; Lee, K.H. Study of Spray Behaviors to Correlate with Engine Performance and Emissions of a Diesel Engine Using Canola-Based Biodiesel. Fuels 2022, 3, 87-112. https://doi.org/10.3390/fuels3010007
Bari S, Zhang C, Kafrawi F, Lee KH. Study of Spray Behaviors to Correlate with Engine Performance and Emissions of a Diesel Engine Using Canola-Based Biodiesel. Fuels. 2022; 3(1):87-112. https://doi.org/10.3390/fuels3010007
Chicago/Turabian StyleBari, Saiful, Chi Zhang, Fahad Kafrawi, and Kang Hei Lee. 2022. "Study of Spray Behaviors to Correlate with Engine Performance and Emissions of a Diesel Engine Using Canola-Based Biodiesel" Fuels 3, no. 1: 87-112. https://doi.org/10.3390/fuels3010007
APA StyleBari, S., Zhang, C., Kafrawi, F., & Lee, K. H. (2022). Study of Spray Behaviors to Correlate with Engine Performance and Emissions of a Diesel Engine Using Canola-Based Biodiesel. Fuels, 3(1), 87-112. https://doi.org/10.3390/fuels3010007





