Epidemiological, Socioeconomic, and Health Service Factors Associated with Tuberculosis Treatment Interruption in Brazil
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Population Base
2.2. Data Sources and Variables
2.3. Data Collection and Statistical Analysis Procedures
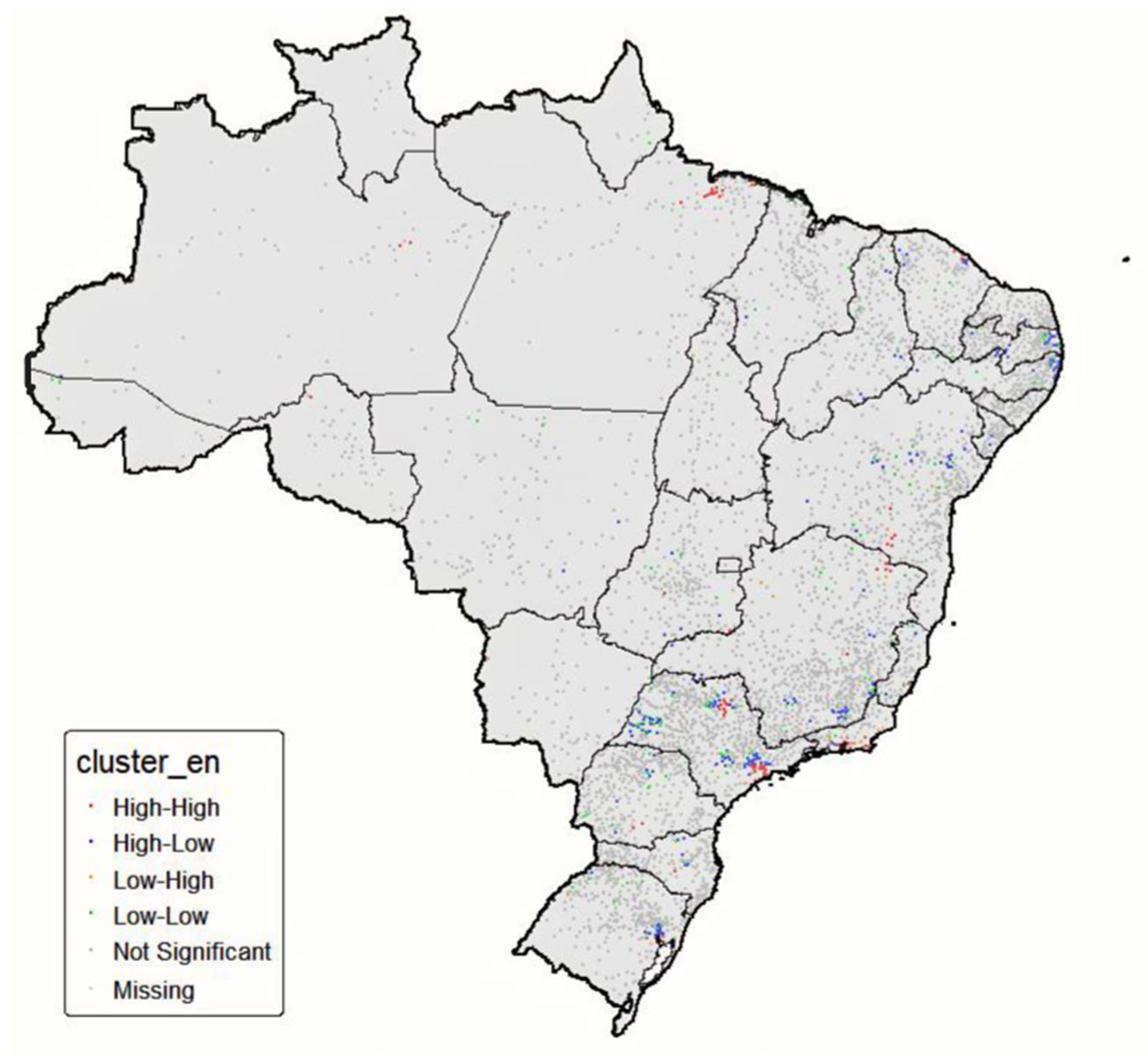
2.4. Spatial Analysis
2.5. Statistical Modeling
2.6. Model Evaluation
2.7. Ethical Considerations
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Organização Mundial da Saúde. Relatório Global Sobre Tuberculose: 2024; Organização Mundial da Saúde: Geneva, Switzerland, 2024; Available online: https://www.who.int/teams/global-tuberculosis-programme/tb-reports/global-tuberculosis-report-2024 (accessed on 29 June 2025).
- Organização Mundial da Saúde. Relatório Global Sobre Tuberculose 2020; Organização Mundial da Saúde: Geneva, Switzerland, 2020; Available online: https://iris.who.int/bitstream/handle/10665/336069/9789240013131-eng.pdf?sequence=1 (accessed on 29 June 2025).
- Departamento de Doenças de Condições Crônicas e Infecções Sexualmente Transmissíveis, Secretaria de Vigilância em Saúde, Ministério da Saúde. Manual de Recomendações para o Controle da Tuberculose no Brasil; Ministério da Saúde: Brasília, Brazil, 2019. Available online: https://www.gov.br/saude/pt-br/centrais-de-conteudo/publicacoes/svsa/tuberculose/manual-de-recomendacoes-e-controle-da-tuberculose-no-brasil-2a-ed.pdf/view (accessed on 29 June 2025).
- Departamento de HIV/AIDS, Tuberculose, Hepatites Virais e Infecções Sexualmente Transmissíveis, Secretaria de Vigilância em Saúde e Ambiente, Ministério da Saúde. Nota Informativa no 5/2024-CGTM/.DATHI/SVSA/MS. Implementação do tratamento encurtado datuberculose sensível não grave em crianças eadolescentes (2RHZ (E)/ 2RH); Ministério da Saúde: Brasília, Brazil, 2024. Available online: https://www.gov.br/aids/pt-br/central-de-conteudo/notas-informativas/2024/nota-informativa-no-5-2024-cgtm.pdf/view (accessed on 22 August 2025).
- Departamento de HIV, Aids, Tuberculose, Hepatites Virais e Infecções Sexualmente Transmissíveis, Secretaria de Vigilância em Saúde e Ambiente, Ministério da Saúde. Boletim Epidemiológico: Tuberculose 2025; Ministério da Saúde: Brasília, Brazil, 2025. Available online: https://www.gov.br/aids/pt-br/central-de-conteudo/boletins-epidemiologicos/2025/boletim-epidemiologico-tuberculose-2025/view (accessed on 29 June 2025).
- Departamento de Doenças de Condições Crônicas e Infecções Sexualmente Transmissíveis, Secretaria de Vigilância em Saúde, Ministério da Saúde. Brasil Livre da Tuberculose: Plano Nacional pelo Fim da Tuberculose como Problema de Saúde Pública; Ministério da Saúde: Brasília, Brazil, 2017. Available online: https://bvsms.saude.gov.br/bvs/publicacoes/brasil_livre_tuberculose_plano_nacional.pdf (accessed on 29 June 2025).
- Organização Mundial da Saúde. Relatório global sobre tuberculose de 2016; Organização Mundial da Saúde: Geneva, Switzerland, 2016; Available online: https://iris.who.int/bitstream/handle/10665/250441/9789241565394-eng.pdf?sequence=1 (accessed on 29 June 2025).
- Harling, G.; Neto, A.S.L.; Sousa, G.S.; Machado, M.M.T.; Castro, M.C. Determinantes da transmissão da tuberculose e abandono do tratamento em Fortaleza, Brasil. BMC Saúde Pública 2017, 17, 508. [Google Scholar] [CrossRef][Green Version]
- Pelissari, D.M.; Rocha, M.S.; Bartholomay, P.; Sanchez, M.N.; Duarte, E.C.; Arakaki-Sanchez, D.; Dantas, C.O.; Jacobs, M.G.; Andrade, K.B.; Codenotti, S.B.; et al. Identificando cenários socioeconômicos, epidemiológicos e operacionais para o controle da tuberculose no Brasil: Um estudo ecológico. BMJ Open 2018, 8, e018545. [Google Scholar] [CrossRef]
- Pelissari, D.M.; Diaz-Quijano, F.A. Aglomeração domiciliar como potencial mediador dos determinantes socioeconômicos da incidência de tuberculose no Brasil. PLoS ONE 2017, 12, e0176116. [Google Scholar] [CrossRef]
- Rigby, R.A.; Stasinopoulos, D.M. Generalized additive models for location, scale and shape (with discussion). J. R. Stat. Soc. Ser. C Appl. Stat. 2005, 54, 507–554. [Google Scholar] [CrossRef]
- Conselho Nacional de Saúde. Resolução n.º 510, de 07 de abril de 2016. 2016. Available online: https://www.gov.br/conselho-nacional-de-saude/pt-br/acesso-a-informacao/legislacao/resolucoes/2016/resolucao-no-510.pdf/view (accessed on 29 June 2025).
- Cortez, A.O.; Melo, A.C.; Neves, L.O.; Resende, K.A.; Camargos, P. Tuberculose no Brasil: Um país, múltiplas realidades. J Bras Pneumol. 2021, 47, 2, e20200119. [Google Scholar] [CrossRef]
- San Pedro, A.; Oliveira, R.M. Tuberculosis and socioeconomic indicators: Systematic review of the literature. Rev. Panam. Salud Publica 2013, 33, 294–301. [Google Scholar] [CrossRef]
- Giacomet, C.L.; Ramos, A.C.; Moura, H.S.; Berra, T.Z.; Alves, Y.M.; Delpino, F.M.; Farley, J.E.; Reynolds, N.R.; Alonso, J.B.; Teibo, T.K.; et al. A distributional regression approach to modeling the impact of structural and intermediary social determinants on communities burdened by tuberculosis in Eastern Amazonia–Brazil. Arch. Public Health. 2023, 81, 135. [Google Scholar] [CrossRef]
- Maciel, E.L.; Reis-Santos, B. Determinants of tuberculosis in Brazil: From conceptual framework to practical application. Rev. Panam. Salud Publica. 2015, 38, 28–34. Available online: https://pubmed.ncbi.nlm.nih.gov/26506318/ (accessed on 29 June 2025). PMID: 26506318.
- Prado Junior, J.C.; Medronho, R.d. Análise espacial da cura da tuberculose na atenção primária no Rio de Janeiro, Brasil. BMC Saúde Pública 2021, 21, 1841. [Google Scholar] [CrossRef]
- Emani, S.; Alves, K.; Alves, L.C.; da Silva, D.A.; Oliveira, P.B.; Castro, M.C.; Cohen, T.; Couto, R.D.; Sanchez, M.; Menzies, N.A. Quantifying gaps in the tuberculosis care cascade in Brazil: A mathematical model study using national program data. PLoS Medicine. 2024, 21, e1004361. [Google Scholar] [CrossRef]
- Oliveira GP, Torrens AW, Bartholomay P, Barreira D. Tuberculosis in Brazil: Last ten years analysis—2001–2010. Braz. J. Infect. Dis. 2013, 17, 218–233. [Google Scholar] [CrossRef]
- Sousa, G.J.B.; Maranhão, T.A.; Leitão, T.M.J.S.; Souza, J.T.; Moreira, T.M.M.; Pereira, M.L.D. Prevalência e fatores associados ao abandono do tratamento da tuberculose. Rev. Esc. Enferm. USP 2021, 55, e03767. [Google Scholar] [CrossRef]
- Alves, K.K.A.F.; Borralho, L.M.; Araújo, A.J.; Bernardino, I.M.; Figueiredo, T.M.R.M. Fatores associados à recuperação e ao abandono do tratamento da tuberculose na população carcerária. Revista Brasileira de Epidemiologia 2020, 23, e200079. [Google Scholar] [CrossRef]
- Creswell, J.; Rai, B.; Wali, R.; Sudrungrot, S.; Adhikari, L.M.; Pant, R.; Pyakurel, S.; Uranw, D.; Codlin, A.J. Introducing new tuberculosis diagnostics: The impact of Xpert® MTB/RIF testing on case notifications in Nepal. Int. J. Tuberc. Lung Dis. 2015, 19, 545–551. [Google Scholar] [CrossRef]
- Albert, H.; Nathavitharana, R.R.; Isaacs, C.; Pai, M.; Denkinger, C.M.; Boehme, C.C. Development, roll-out and impact of Xpert MTB/RIF for tuberculosis: What lessons have we learnt and how can we do better? Eur. Respir. J. 2016, 48, 516–525. [Google Scholar] [CrossRef]
- Storla, D.G.; Yimer, S.; Bjune, G.A. A systematic review of delay in the diagnosis and treatment of tuberculosis. BMC Public Health 2008, 8, 15. [Google Scholar] [CrossRef]
- Creswell, J.; Codlin, A.J.; Andre, E.; Micek, M.A.; Bedru, A.; Carter, E.J.; Yadav, R.P.; Mosneaga, A.; Rai, B.; Banu, S.; et al. Results from early programmatic implementation of Xpert MTB/RIF testing in nine countries. BMC Infect Dis. 2014, 14, 2. [Google Scholar] [CrossRef]
- Fundação Oswaldo Cruz. Laboratório de Referência Nacional do Hélio Fraga Capacita Monitores em Teste Rápido Molecular Para Tuberculose; Fundação Oswaldo Cruz: Rio de Janeiro, Brazil, 2022; Available online: https://informe.ensp.fiocruz.br/noticias/53241 (accessed on 29 June 2025).
- Maciel, E.L.N.; Sales, C.M.M.; Bertolde, A.I.; Reis-Santos, B.; Fregona, G.; Zandonade, E. Implementação do teste rápido molecular para tuberculose em unidades de saúde: Desafios e perspectivas no Brasil. BMC Health Serv Res. 2015, 15, 356. [Google Scholar] [CrossRef]
- Baluku, J.B.; Kabamooli, R. A; Kajumba, N; Nabwana, M,; Kateete, D; Kiguli, S; Andia-Biraro, I. Contact tracing is associated with treatment success of index tuberculosis cases in Uganda. Int. J. Infect. Dis. 2021, 109, 129–136. [Google Scholar] [CrossRef]
- Matias, G.L.; Sales, M.V.; Andrade, G.S.; Teixeira, B.D.; Tenorio, M.E.; Palácio, M.A.; Correia, M.L.; Takenami, I. Diagnosis and treatment of latent tuberculosis infection among household contacts in inland Bahia, Brazil: A cross-sectional follow-up study. Sao Paulo Med J. 2024, 143, 1, e2023339. [Google Scholar] [CrossRef]
- Lima, L.V.; Pavinati, G.; Palmieri, I.G.S.; Vieira, J.P.; Blasque, J.C.; Higarashi, I.H.; Fernandes, C.A.M.; Magnabosco, G.T. Fatores associados à perda de seguimento no tratamento da tuberculose no Brasil: Um estudo de coorte retrospectivo. Rev. Gaúcha Enferm. 2023, 44, e20230077. [Google Scholar] [CrossRef] [PubMed]
- Cola, J.P.; Pinto, A.S.; Souza, J.S.; Hertel, J.F.H.F.; Galavote, H.S.; do Prado, T.N.; Maciel, E.L.N. Fatores associados ao abandono do tratamento da tuberculose: Um estudo transversal entre 2014 e 2019. J. Hum. Growth Dev. 2024, 34, 286–295. [Google Scholar] [CrossRef]
- Santos, D.A.S.; Marques, A.L.A.; Goulart, L.S.; Mattos, M.; Olinda, R.A. Fatores associados ao abandono do tratamento da tuberculose pulmonar. Cogitare Enferm. 2021, 26, e72794. [Google Scholar] [CrossRef]
- Perlaza, C.L.; Mosquera, F.E.C.; Murillo, L.M.R.; Sepulveda, V.B.; Arenas, C.D.C. Fatores de abandono do tratamento da tuberculose na rede pública de saúde. Rev Saúde Pública 2023, 57, 8. [Google Scholar] [CrossRef]
- Ferreira, M.R.L.; Bonfim, R.O.; Siqueira, T.C.; Orfão, N.H. Abandono do tratamento da tuberculose: Uma revisão integrativa. Rev. Enferm. Contemp. 2018, 7, 63–71. [Google Scholar] [CrossRef]
- Departamento de HIV/Aids, Tuberculose, Hepatites Virais e Infecções Sexualmente Transmissíveis, Secretaria de Vigilância em Saúde e Ambiente, Ministério da Saúde. Boletim Epidemiológico: Coinfecção TB-HIV 2022; Ministério da Saúde: Brasília, Brazil, 2023. Available online: https://www.gov.br/aids/pt-br/central-de-conteudo/boletins-epidemiologicos/2022/coinfeccao-tb-hiv/boletim_coinfeccao_tb_hiv_2022.pdf/view (accessed on 29 June 2025).
- Quintero, M.C.F.; Vendramini, S.H.F.; Santos, M.L.S.G.; Rocha dos Santos, M.; Gazetta, C.E.; Garcia Lourenção, L.; Sperli Geraldes Soler, Z.A.; da Cruz Oliveira, S.A.; Geraldes Marin dos Santos Sasaki, N.; Zanon Ponce, M.A.; et al. Acesso ao diagnóstico de tuberculose em municípios brasileiros de médio porte. Rev. Salud Pública 2018, 20, 103–109. [Google Scholar] [CrossRef]
- Lucena, L.A.; Dantas, G.B.S.; Carneiro, T.V.; Lacerda, H.G. Fatores Associados ao Abandono do Tratamento da Tuberculose no Brasil: Uma Revisão Sistemática. Rev. Soc. Bras. Med. Trop. 2023, 56, e0155–e2022. [Google Scholar] [CrossRef]
- Souza, M.S.P.L.; Aquino, R.; Pereira, S.M.; Costa, M.D.C.N.; Barreto, M.L.; Natividade, M.; Ximenes, R.; Souza, W.; Dantas, O.M.; Braga, J.U. Fatores associados ao acesso geográfico aos serviços de saúde para pessoas com Tuberculose em três capitais do Nordeste brasileiro. Cad. Saúde Pública 2015, 31, 111–120. [Google Scholar] [CrossRef]
- Fonseca, E.P.; Melo, L.B.; Pereira, A.C.; Mendes, K.L.C.; Verdi, M.R.D.M.; Meneghim, M.C. Não Adesão ao Tratamento da Tuberculose no Estado de São Paulo: Reflexões sobre Gestão em Saúde e Enfermagem. 2023. Available online: https://preprints.scielo.org/index.php/scielo/preprint/view/7106/version/7517 (accessed on 16 December 2024).
- Ferreira, H.S.; Mascarello, K.C.; Cola, J.P.; Vieira, A.C.B.C.; Carlesso, G.F.; Sales, C.M.M.; Maciel, E.L.N. Fatores sociais preditores de cura da Tuberculose em capitais brasileiras. Rev. Bras. Pesq. Saúde. 2022, 24, 26–37. [Google Scholar] [CrossRef]
- Mutembo, S.; Mutanga, J.N.; Musokotwane, K.; Kanene, C.; Dobbin, K.; Yao, X.; Li, C.; Marconi, V.C.; Whalen, C.C. Urban-rural disparities in treatment outcomes among recurrent TB cases in Southern Province, Zambia. BMC Infect. Dis. 2019, 19, 1087. [Google Scholar] [CrossRef]
- Ahmed, M.; Mohan, R. A comparative study of factors for interruption of antitubercular treatment among defaulters in urban and rural areas of Kamrup District, Assam. J. Family Med. Primary Care 2021, 10, 127–131. [Google Scholar] [CrossRef]
- Jesus, G.S.; Pescarini, J.M.; Silva, A.F.; Torrens, A.; Carvalho, W.M.; Junior, E.P.; Ichihara, M.Y.; Barreto, M.L.; Rebouças, P.; Macinko, J.; et al. The effect of primary health care on tuberculosis in a nationwide cohort of 7· 3 million Brazilian people: A quasi-experimental study. Lancet Global Health 2022, 10, e390–e397. [Google Scholar] [CrossRef] [PubMed]
- Pinto, F.G.; Garcia, W.M.B.; Silva-Junior, R.G.P.; Ferro, G.B.; da Costa, A.G.; de Carvalho Zavarise, M.; da Silva Morais, C.A.; Mendes, E.A.R.; Gaia, S.L.; Lobato, M.Y.F. Adesão ao tratamento da tuberculose na Atenção Primária à Saúde: Fatores favoráveis e desfavoráveis para esse processo. Res. Soc. Dev. 2022, 11, e3011426962. [Google Scholar] [CrossRef]
- Lima, S.V.M.A.; Araújo, K.C.G.M.; Nunes, M.A.P.; Nunes, C. Identificação precoce de indivíduos em risco de perda de seguimento do tratamento da tuberculose: Uma análise hierárquica generalizada. Heliyon 2021, 7, e06788. [Google Scholar] [CrossRef]
- Aragão, F.B.A.; Calori, M.Y.; Berra, T.Z.; Ramos, A.C.V.; Maciel, E.L.N.; Cunha, J.H.D.S.; Souza, L.B.D.; Santos Neto, M.; Arcêncio, R.A.; Fiorati, R.C. Proteção social em áreas vulneráveis à tuberculose: Um estudo de métodos mistos em São Luís, Maranhão. Rev. Brás Enferm. 2024, 77, e20230428. [Google Scholar] [CrossRef]
- Orlandi, G.M.; Pereira, E.G.; Biagolini, R.E.M.; França, F.O.S.; Bertolozzi, M.R. Incentivos sociais para adesão ao tratamento da tuberculose. Rev. Brás Enferm. 2019, 72, 1182–1190. [Google Scholar] [CrossRef]
- Furlan, M.C.R.; Oliveira SPde Marcon, S.S. Fatores associados ao abandono do tratamento de tuberculose no estado do Paraná. Acta Paul. Enferm. 2012, 25, 108–114. [Google Scholar] [CrossRef]
- Rocha, G.S.S.; Lima, M.G.; Moreira, J.L.; Ribeiro, K.C.; Ceccato, M.D.G.B.; Carvalho, W.D.S.; Silveira, M.R. Conhecimento dos agentes comunitários de saúde sobre a tuberculose, suas medidas de controle e tratamento diretamente distribuído. Cad. Saúde Pública. 2015, 31, 1483–1496. [Google Scholar] [CrossRef]
- Vieira-Meyer, A.P.G.F.; Morais, A.P.P.; Campelo, I.L.B.; Guimarães, J.M.X. Violência e vulnerabilidade no território do agente comunitário de saúde: Implicações no enfrentamento da COVID-19. Ciên. Saúde Colet. 2025, 26, 657–668. [Google Scholar] [CrossRef]
- Morgenstern, H. Ecologic studies in epidemiology: Concepts, principles, and methods. Annu. Rev. Public. Health 1995, 16, 61–81. [Google Scholar] [CrossRef]
- Diez-Roux, A.V. Multilevel analysis in public health research. Annu. Rev. Public. Health 2000, 21, 171–192. [Google Scholar] [CrossRef]
- Rothman, K.J.; Greenland, S.; Lash, T.L. Modern Epidemiology, 3rd ed.Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2008. [Google Scholar]
- Hino, P.; Yamamoto, T.T.; Magnabosco, G.T.; Bertolozzi, M.R.; Taminato, M.; Fornari, L.F. Impacto da COVID-19 no controle e reorganização da atenção à tuberculose. Acta Paul. Enferm. 2021, 34, eAPE002115. [Google Scholar] [CrossRef]
- Ribeiro, R.R.; Andrade, R.L.D.P.; Silva DCda Sthal, H.C.; Oliveira, J.A.; Regis, I.M.; Gonzalez, R.I.C. Repercussão da pandemia da COVID-19 nas ações de controle da tuberculose na atenção primária à saúde: Revisão de escopo. Ciênc. Saúde Coletiva 2025, 30, e00992024. [Google Scholar] [CrossRef]
- Silva JAda Rufino, E.N.M.; Sampaio, B.F.; Silva, D.M. Impacto da pandemia de covid-19 no número de casos e na mortalidade da tuberculose. Rev. Ibero-Am. De Humanidades Ciências E Educ. 2023, 9, 1964–1973. Available online: https://periodicorease.pro.br/rease/article/view/12500 (accessed on 29 June 2025).
| Epidemiological Variables of the Tuberculosis Control Program | Year | Source |
|---|---|---|
| Incidence rate of new pulmonary TB cases * | 2018–022 | DATASUS |
| TB mortality rate * | 2018–2022 | DATASUS |
| Proportion of sputum smear microscopy performed among new pulmonary TB cases * | 2018–2022 | DATASUS |
| Proportion of molecular rapid tests for TB performed among new pulmonary TB cases* | 2018–2022 | DATASUS |
| Proportion of drug susceptibility tests performed among new pulmonary TB cases* | 2018–2022 | DATASUS |
| Proportion of new pulmonary TB cases confirmed by laboratory tests * | 2018–2022 | DATASUS |
| Proportion of new pulmonary TB cases tested for HIV * | 2018–2022 | DATASUS |
| Proportion of TB-HIV coinfection among new pulmonary TB cases * | 2018–2022 | DATASUS |
| Proportion of new pulmonary TB cases among special population groups (incarcerated individuals, people experiencing homelessness, healthcare workers, immigrants, and Indigenous peoples) * | 2018–2022 | DATASUS |
| Proportion of contacts examined among new pulmonary TB cases * | 2018–2022 | DATASUS |
| Proportion of new pulmonary TB cases receiving Directly Observed Therapy (DOT) * | 2018–2022 | DATASUS |
| AIDS case detection rate | 2019 | DATHI |
| Variables related to health service coverage | ||
| Number of SUS hospital beds per 1000 inhabitants | 2019 | DATASUS |
| Number of medical consultations per inhabitant per year | 2019 | DATASUS |
| Percentage of homogeneity in vaccine coverage across vaccines | 2019 | DATASUS |
| Coverage rate of the Family Health Strategy (FHS) | 2019 | Site e-GESTOR |
| Coverage rate of Community Health Agents (CHA) | 2019 | Site e-GESTOR |
| Socioeconomic variables | ||
| Resident population | 2022 | IBGE |
| Population density | 2022 | IBGE |
| Municipal Human Development Index (MHDI) | 2010 | IBGE |
| Household per capita income | 2010 | Atlas Brasil |
| Gross Domestic Product (GDP) per capita | 2021 | IBGE |
| Gini Index | 2010 | Atlas Brasil |
| Unemployment rate | 2010 | Atlas Brasil |
| Illiteracy rate (≥15 years old) | 2022 | IBGE |
| Household crowding | 2010 | Atlas Brasil |
| Life expectancy at birth | 2010 | Atlas Brasil |
| Infant mortality rate | 2019 | DATASUS |
| Proportion of the population living in poverty | 2010 | Atlas Brasil |
| Proportion of the population residing in rural areas | 2022 | IBGE |
| Variable | Mean (SD) | Median (25th–75th Percentile IQR) |
|---|---|---|
| Treatment Interruption Rate (2018–2022) | 8.0 (13.96) | 0.00 (0.00–12.00) |
| Epidemiological and Operational Variables Related to the Tuberculosis Control Program | ||
| Incidence rate of new pulmonary TB cases | 92.251 (129.422) | 67.760 (40.040–107.840) |
| TB mortality rate | 8.37 (10.54) | 5.83 (0.00–12.97) |
| Proportion of new pulmonary TB cases undergoing sputum smear microscopy | 76.27 (24.23) | 81.25 (66.67–100.00) |
| Proportion of new pulmonary TB cases tested with rapid molecular TB tests | 27.16 (29.36) | 16.77 (0.00–45.45) |
| Proportion of drug susceptibility tests performed among new pulmonary TB cases (2019) | 8.69 (17.59) | 0.00 (0.00–9.38) |
| Proportion of new pulmonary TB cases confirmed by laboratory tests | 69.65 (25.30) | 73.53 (55.56–88.89) |
| Proportion of new pulmonary TB cases tested for HIV | 79.08 (25.53) | 87.68 (66.67–100.00) |
| Proportion of TB-HIV coinfection among new pulmonary TB cases | 5.87 (12.06) | 0.00 (0.00–7.89) |
| Proportion of new pulmonary TB cases in special populations (incarcerated individuals, people experiencing homelessness, healthcare workers, immigrants, and Indigenous peoples) | 10.11 (20.01) | 0.00 (0.00–12.50) |
| Proportion of contacts examined among new pulmonary TB cases | 80.20 (54.55) | 88.89 (63.89–100.00) |
| Proportion of new pulmonary TB cases receiving Directly Observed Therapy (DOT) | 47.87 (35.03) | 50.00 (14.29–77.78) |
| AIDS case detection rate | 9.79 (12.28) | 6.44 (0.00–15.46) |
| Variables Related to Health Service Coverage | ||
| Number of SUS hospital beds per 1000 inhabitants | 1.37 (1.62) | 1.11 (0.00–2.01) |
| Number of medical consultations per inhabitant per year | 6.38 (5.39) | 4.90 (2.67–8.67) |
| Homogeneity in vaccine coverage across vaccines (%) | 35.10 (30.05) | 30.00 (10.00–60.00) |
| Coverage rate of the Family Health Strategy (FHS) | 88.25 (22.01) | 100.00 (84.99–100.00) |
| Coverage rate of Community Health Agents (CHA) | 90.23 (21.36) | 100.00 (96.30–100.00) |
| Socioeconomic Variables | ||
| Resident population (IBGE) | 40,091 (217,521) | 13,016 (6347–26,881) |
| Population density (inhabitants/km2) | 126.89 (626.59) | 25.82 (11.93–60.17) |
| Gini Index | 0.50 (0.07) | 0.50 (0.45–0.54) |
| Municipal Human Development Index (MHDI) | 0.658 (0.073) | 0.664 (0.597–0.718) |
| Per capita Gross Domestic Product (GDP, R$) | 33,458.27 (42,955.50) | 22,951.75 (12,588.25–39,829.41) |
| Household per capita income (R$) | 490.82 (245.07) | 461·47 (276.94–651.41) |
| Unemployment rate | 6.96 (3.76) | 6.46 (4.41–8.78) |
| Household crowding | 25.93 (13.08) | 24.01 (16.09–33.33) |
| Life expectancy at birth | 73.04 (2.70) | 73.40 (71.08–75.14) |
| Infant mortality rate | 25.98 (23.90) | 23.05 (10.36–35.71) |
| Illiteracy rate (≥15 years old) | 11.11 (7.06) | 9.14 (5.09–16.72) |
| Proportion of the population living in poverty | 23.66 (18.11) | 18.97 (7.07–39.28) |
| Proportion of the population residing in rural areas | 29.83(19.98) | 27.44(12.97–44.49) |
| Variables (Proportion and Zero-Inflated) | Crude Analysis | |
|---|---|---|
| Coef. B | p-Value | |
| Epidemiological and Operational Variables Related to the Tuberculosis Control Program | ||
| Incidence rate of new pulmonary TB cases | ||
| Proportion | −0.002 | <0.001 |
| Zero-inflated | −0.015 | <0.001 |
| TB mortality rate | ||
| Proportion | −0.012 | <0.001 |
| Zero-inflated | −0.025 | <0.001 |
| Proportion of sputum smear microscopy performed among new pulmonary TB cases | ||
| Proportion | −0.005 | <0.001 |
| Zero-inflated | 0.0005 | 0.692 |
| Proportion of molecular rapid tests for TB performed among new pulmonary TB cases | ||
| Proportion | −0.002 | 0.006 |
| Zero-inflated | 0.001 | 0.581 |
| Proportion of drug susceptibility tests performed among new pulmonary TB cases | ||
| Proportion | −0.003 | 0.052 |
| Zero-inflated | −0.004 | 0.022 |
| Proportion of new pulmonary TB cases confirmed by laboratory tests | ||
| Proportion | −0.001 | 0.655 |
| Zero-inflated | −0.004 | <0.001 |
| Proportion of new pulmonary TB cases tested for HIV | ||
| Proportion | −0.011 | <0.001 |
| Zero-inflated | −0.006 | <0.001 |
| Proportion of TB-HIV coinfection among new pulmonary TB cases | ||
| Proportion | 0.011 | <0.001 |
| Zero-inflated | −0.008 | 0.001 |
| Proportion of new pulmonary TB cases among special population groups (incarcerated individuals, people experiencing homelessness, healthcare workers, immigrants, and Indigenous peoples) | ||
| Proportion | −0.004 | 0.004 |
| Zero-inflated | −0.013 | <0.001 |
| Proportion of contacts examined among new pulmonary TB cases | ||
| Proportion | −0.004 | <0.001 |
| Zero-inflated | 0.001 | 0.127 |
| Proportion of new pulmonary TB cases receiving Directly Observed Therapy (DOT) | ||
| Proportion | −0.006 | <0.001 |
| Zero-inflated | 0.010 | <0.001 |
| AIDS case detection rate | ||
| Proportion | −0.010 | <0.001 |
| Zero-inflated | −0.040 | <0.001 |
| Variables Related to Health Service Coverage | ||
| Number of SUS hospital beds per 1000 inhabitants | ||
| Proportion | −0.014 | 0.402 |
| Zero-inflated | −0.039 | 0.025 |
| Number of medical consultations per inhabitant per year | ||
| Proportion | 0.019 | <0.001 |
| Zero-inflated | 0.033 | <0.001 |
| Percentage of homogeneity in vaccine coverage across vaccines | ||
| Proportion | 0.003 | <0.001 |
| Zero-inflated | 0.011 | <0.001 |
| Coverage rate of the Family Health Strategy (FHS) | ||
| Proportion | 0.003 | 0.003 |
| Zero-inflated | 0.027 | <0.001 |
| Coverage rate of Community Health Agents (CHA) | ||
| Proportion | 0.002 | 0.023 |
| Zero-inflated | 0.027 | <0.001 |
| Socioeconomic Variables | ||
| Resident population (IBGE) | ||
| Proportion | −0.0000001 | 0.987 |
| Zero-inflated | −0.00008 | <0.001 |
| Population density (inhabitants/km2) | ||
| Proportion | −0.00003 | 0.504 |
| Zero-inflated | −0.009 | <0.001 |
| Gini Index | ||
| Proportion | −2.302 | <0.001 |
| Zero-inflated | −4.369 | <0.001 |
| Municipal Human Development Index (MHDI) | ||
| Proportion | 0.585 | 0.030 |
| Zero-inflated | −3.258 | <0.001 |
| Gross Domestic Product (GDP) per capita (R$) | ||
| Proportion | 0.0000007 | 0.916 |
| Zero-inflated | −0.000001 | 0.741 |
| Household per capita income (R$) | ||
| Proportion | 0.0001 | 0.091 |
| Zero-inflated | −0.001 | <0.001 |
| Unemployment rate | ||
| Proportion | −0.043 | <0.001 |
| Zero-inflated | −0.086 | <0.001 |
| Household crowding | ||
| Proportion | −0.013 | <0.001 |
| Zero-inflated | −0.032 | <0.001 |
| Life expectancy at birth | ||
| Proportion | 0.028 | <0.001 |
| Zero-inflated | −0.057 | <0.001 |
| Infant mortality rate | ||
| Proportion | 0.001 | 0.299 |
| Zero-inflated | 0.0001 | 0.929 |
| Illiteracy rate (≥15 years old) | ||
| Proportion | −0.009 | 0.003 |
| Zero-inflated | 0.040 | <0.001 |
| Proportion of the population living in poverty | ||
| Proportion | −0.005 | <0.001 |
| Zero-inflated | 0.005 | <0.001 |
| Proportion of the population residing in rural areas | ||
| Proportion | 0.005 | <0.001 |
| Zero-inflated | 0.028 | <0.001 |
| Variables (Proportion and Zero-Inflated) | Adjusted Analysis | |||||||
|---|---|---|---|---|---|---|---|---|
| Model 1—TB Variables | Model 2—TB + Health Variables | Model 3—TB + Health + Sociodemographic Variables | Model 4—TB + Health + Sociodemographic + Geographic Variables | |||||
| Coef. B | p-Value | Coef. B | p-Value | Coef. B | p-Value | Coef. B | p-Value | |
| Epidemiological and Operational Variables Related to the Tuberculosis Control Program | ||||||||
| Incidence rate of new pulmonary TB cases | ||||||||
| Proportion | −0.002 | <0.001 | −0.006 | <0.001 | −0.001 | <0.001 | −0.001 | <0.001 |
| Zero-inflated | −0.014 | <0.001 | −0.013 | <0.001 | −0.010 | <0.001 | −0.010 | <0.001 |
| Proportion of sputum smear microscopy performed among new pulmonary TB cases | ||||||||
| Proportion | −0.011 | <0.001 | −0.011 | <0.001 | −0.011 | <0.001 | −0.011 | <0.001 |
| Zero-inflated | - | - | - | - | - | - | - | - |
| Proportion of molecular rapid tests for TB performed among new pulmonary TB cases | ||||||||
| Proportion | −0.008 | <0.001 | −0.008 | <0.001 | −0.008 | <0.001 | −0.009 | <0.001 |
| Zero-inflated | - | - | - | - | - | - | - | - |
| Proportion of drug susceptibility tests performed among new pulmonary TB cases | ||||||||
| Proportion | 0.005 | 0.002 | 0.005 | 0.001 | 0.003 | 0.027 | 0.003 | 0.078 |
| Zero-inflated | - | - | - | - | - | - | - | - |
| Proportion of new pulmonary TB cases confirmed by laboratory tests | ||||||||
| Proportion | 0.012 | <0.001 | 0.012 | <0.001 | 0.013 | <0.001 | 0.014 | <0.001 |
| Zero-inflated | −0.004 | 0.006 | −0.003 | 0.024 | - | - | - | - |
| Proportion of new pulmonary TB cases tested for HIV | ||||||||
| Proportion | - | - | - | - | - | - | - | - |
| Zero-inflated | −0.003 | 0.048 | - | - | - | - | - | - |
| Proportion of TB-HIV coinfection among new pulmonary TB cases | ||||||||
| Proportion | - | - | - | - | - | - | - | - |
| Zero-inflated | −0.007 | 0.017 | −0.008 | 0.009 | - | - | - | - |
| Proportion of new pulmonary TB cases among special population groups (incarcerated individuals, people experiencing homelessness, healthcare professionals, immigrants, and Indigenous peoples) | ||||||||
| Proportion | - | - | - | - | - | - | - | - |
| Zero-inflated | −0.003 | 0.092 | - | - | - | - | - | - |
| Proportion of contacts examined among new pulmonary TB cases | ||||||||
| Proportion | −0.003 | <0.001 | −0.003 | <0.001 | −0.003 | < 0.001 | −0.003 | <0.001 |
| Zero-inflated | - | - | - | - | - | - | - | - |
| Proportion of new pulmonary TB cases receiving Directly Observed Therapy (DOT) | ||||||||
| Proportion | −0.004 | <0.001 | −0.005 | <0.001 | −0.005 | <0.001 | −0.005 | <0.001 |
| Zero-inflated | 0.012 | <0.001 | 0.009 | <0.001 | 0.010 | <0.001 | 0.010 | <0.001 |
| AIDS case detection rate | ||||||||
| Proportion | −0.006 | 0.001 | −0.004 | 0.011 | −0.004 | 0.049 | −0.004 | 0.007 |
| Zero-inflated | −0.023 | <0.001 | −0.022 | <0.001 | −0.006 | 0.040 | −0.006 | 0.039 |
| Variables Related to Health Service Coverage | ||||||||
| Number of SUS hospital beds per 1000 inhabitants | ||||||||
| Proportion | - | - | - | - | - | - | - | - |
| Zero-inflated | - | - | −0.067 | 0.001 | - | - | - | - |
| Number of medical consultations per inhabitant per year | ||||||||
| Proportion | - | - | 0.012 | 0.004 | - | - | - | - |
| Zero-inflated | - | - | 0.055 | <0.001 | 0.043 | <0.001 | 0.043 | <0.001 |
| Percentage of homogeneity in vaccine coverage across vaccines | ||||||||
| Proportion | - | - | 0.002 | 0.013 | - | - | - | - |
| Zero-inflated | - | - | 0.005 | <0.001 | - | - | - | - |
| Coverage rate of the Family Health Strategy (FHS) | ||||||||
| Proportion | - | - | 0.005 | <0.001 | 0.004 | <0.001 | 0.004 | <0.001 |
| Zero-inflated | - | - | 0.025 | <0.001 | - | - | - | - |
| Socioeconomic Variables | ||||||||
| Resident population (IBGE) | ||||||||
| Proportion | - | - | - | - | - | - | - | - |
| Zero-inflated | - | - | - | - | −0.00007 | <0.001 | −0.00007 | <0.001 |
| Gini Index | ||||||||
| Proportion | - | - | - | - | −1.784 | <0.001 | −1.851 | <0.001 |
| Zero-inflated | - | - | - | - | - | - | - | - |
| Household crowding | ||||||||
| Proportion | - | - | - | - | −0.007 | <0.001 | −0.008 | <0.001 |
| Zero-inflated | - | - | - | - | −0.019 | <0.001 | −0.019 | <0.001 |
| Illiteracy rate (≥15 years) | ||||||||
| Proportion | - | - | - | - | −0.018 | <0.001 | −0.011 | 0.028 |
| Zero-inflated | - | - | - | - | 0.036 | <0.001 | 0.038 | <0.001 |
| Proportion of the population residing in rural areas | ||||||||
| Proportion | - | - | - | - | 0.009 | <0.001 | 0.010 | <0.001 |
| Zero-inflated | - | - | - | - | 0.008 | <0.001 | 0.008 | <0.001 |
| Geographic Variables | ||||||||
| pb(X) | ||||||||
| Proportion | - | - | - | - | - | - | −0.017 | <0.001 |
| Zero-inflated | - | - | - | - | - | - | −0.0008 | 0.912 |
| pb(Y) | ||||||||
| Proportion | - | - | - | - | - | - | 0.0005 | 0.891 |
| Zero-inflated | - | - | - | - | - | - | −0.003 | 0.729 |
| Deviance | 2555.469 | 2224.58 | 1158.533 | 1035.138 | ||||
| AIC | 2591.469 | 2270.58 | 1206.533 | 1122.579 | ||||
| BIC | 2708.04 | 2419.441 | 1361.876 | 1405.562 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mendonça, J.S.; Silveira, F.S.A.; Colodette, R.M.; de Oliveira, D.M.; de Mendonça, É.T.; Cotta, R.M.M.; de Barros Junior, A.A.; Andrade, J.V.; Moreira, T.R. Epidemiological, Socioeconomic, and Health Service Factors Associated with Tuberculosis Treatment Interruption in Brazil. Epidemiologia 2025, 6, 81. https://doi.org/10.3390/epidemiologia6040081
Mendonça JS, Silveira FSA, Colodette RM, de Oliveira DM, de Mendonça ÉT, Cotta RMM, de Barros Junior AA, Andrade JV, Moreira TR. Epidemiological, Socioeconomic, and Health Service Factors Associated with Tuberculosis Treatment Interruption in Brazil. Epidemiologia. 2025; 6(4):81. https://doi.org/10.3390/epidemiologia6040081
Chicago/Turabian StyleMendonça, Jéssica Simões, Fabrício Sette Abrantes Silveira, Renata Maria Colodette, Deíse Moura de Oliveira, Érica Toledo de Mendonça, Rosângela Minardi Mitre Cotta, Antônio Almeida de Barros Junior, João Vitor Andrade, and Tiago Ricardo Moreira. 2025. "Epidemiological, Socioeconomic, and Health Service Factors Associated with Tuberculosis Treatment Interruption in Brazil" Epidemiologia 6, no. 4: 81. https://doi.org/10.3390/epidemiologia6040081
APA StyleMendonça, J. S., Silveira, F. S. A., Colodette, R. M., de Oliveira, D. M., de Mendonça, É. T., Cotta, R. M. M., de Barros Junior, A. A., Andrade, J. V., & Moreira, T. R. (2025). Epidemiological, Socioeconomic, and Health Service Factors Associated with Tuberculosis Treatment Interruption in Brazil. Epidemiologia, 6(4), 81. https://doi.org/10.3390/epidemiologia6040081







