Epidemiology, Clinical Data, and Management of Aseptic Abscess Syndrome: Review of Published Cases Outside France
Abstract
1. Introduction
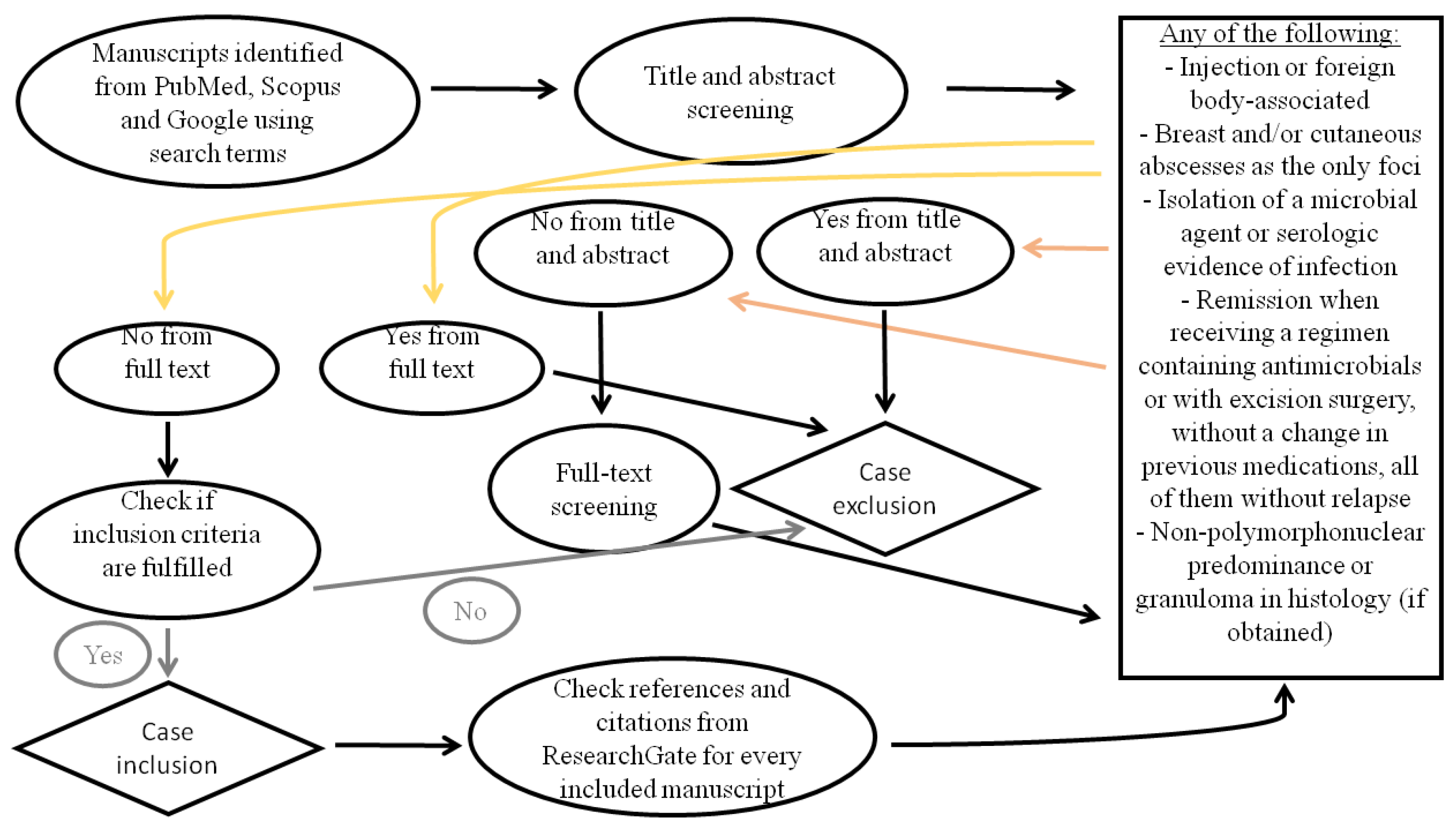
2. Methods
2.1. Eligibility Criteria
- (1)
- Deep abscess(es) on imaging, with a predominance of neutrophils if puncture or biopsy is performed;
- (2)
- Negative blood cultures, serological or molecular tests for pathogens, and, in the case of abscess puncture or biopsy, negative infectious workup of pus or biopsy specimen;
- (3)
- Antibiotic failure, if prescribed, after at least two weeks of treatment for typical pathogens and three months for mycobacteria;
- (4)
- Fast clinical improvement observed the day after administering corticosteroids (CSs) (at least 0.5 mg/kg of prednisolone equivalent), followed by radiological improvement after one month of corticosteroids, sometimes associated with immunosuppressants.
- (3)
- Failure of antibiotic or antimycobacterial regimens, if prescribed;
- (4)
- Clinical improvement after administering CS or other immunomodulators or granulocyte monocyte apheresis (GMA), followed by radiological improvement and/or resolution of symptoms and inflammatory syndrome with the aforementioned approach and without receiving antimicrobials.
2.2. Search Strategy
- PubMed and Scopus: (“aseptic” OR “sterile” OR “lupus” OR “polychondritis” OR “familial Mediterranean fever” OR “polyarteritis nodosa” OR “inflammatory bowel disease” OR “Crohn’s disease” OR “ulcerative colitis” OR “Behcet” OR “sarcoidosis” OR “pyoderma gangrenosum” OR “Sweet syndrome” OR “neutrophilic dermatosis” OR “rheumatoid arthritis” OR “Cogan”) AND “abscess”
- Google: “aseptic abscess”, “σύνδρομο άσηπτων αποστημάτων” (in Greek)
2.3. Study Selection
2.4. Data Extraction and Synthesis
3. Results
3.1. Clinical and Laboratory Data
3.2. Treatment and Follow-Up
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- André, M.; Aumaitre, O.; Souweine, B.; Deseubis, T.; Philippe, P.; Kauffman, P.; Piette, J.C.; Marcheix, J.C. Maladie de Crohn précédée pendant trois ans d’abcès multiples et aseptiques. La Rev. Médecine Interne 1992, 13, S531. [Google Scholar] [CrossRef]
- André, M.; Godeau, B.; Aumaitre, O.; Piette, J.C.; Rousset, H.; Vital Durand, D.; Marcheix, J.C. Abcès viscéraux aseptiques: Une manifestation systémique inhabituelle inaugurant la maladie de Crohn (5 observations). La Rev. Médecine Interne 1993, 14, 416. [Google Scholar] [CrossRef]
- André, M.; Aumaitre, O.; Marcheix, J.C.; Piette, J.C. Unexplained sterile systemic abscesses in Crohn’s disease: Aseptic abscesses as a new entity. Am. J. Gastroenterol. 1995, 90, 1183–1184. [Google Scholar]
- Georgin-Lavialle, S.; Rodrigues, F.; Hentgen, V.; Fayand, A.; Quartier, P.; Bader-Meunier, B.; Bachmeyer, C.; Savey, L.; Louvrier, C.; Sarrabay, G.; et al. Clinical overview of auto-inflammatory diseases. Rev. Med. Interne 2018, 39, 214–232. [Google Scholar] [CrossRef]
- Trefond, L.; Frances, C.; Costedoat-Chalumeau, N.; Piette, J.C.; Haroche, J.; Sailler, L.; Assaad, S.; Viallard, J.F.; Jego, P.; Hot, A.; et al. Aseptic Abscess Syndrome: Clinical Characteristics, Associated Diseases, and up to 30 Years’ Evolution Data on a 71-Patient Series. J. Clin. Med. 2022, 11, 3669. [Google Scholar] [CrossRef]
- André, M.F.J.; Piette, J.C.; Kémény, J.L.; Ninet, J.; Jego, P.; Delèvaux, I.; Wechsler, B.; Weiller, P.J.; Francès, C.; Blétry, O.; et al. Aseptic abscesses: A study of 30 patients with or without inflammatory bowel disease and review of the literature. Medicine 2007, 86, 145–161. [Google Scholar] [CrossRef]
- Weber, D.; Decker, M.; Schuster, M.; Folz, S.; Stürmer, C.J.; Lutz, M.P. Crizotinib: Aseptic abscesses in multiple organs during treatment of EML4-ALK-positive NSCLC. J. Cancer Res. Clin. Oncol. 2021, 147, 3769–3771. [Google Scholar] [CrossRef]
- Ndieugnou Djangang, N.; Peluso, L.; Talamonti, M.; Izzi, A.; Gevenois, P.A.; Garufi, A.; Goffard, J.C.; Henrard, S.; Severgnini, P.; Vincent, J.L.; et al. Eosinopenia in COVID-19 Patients: A Retrospective Analysis. Microorganisms 2020, 8, 1929. [Google Scholar] [CrossRef]
- Diamantopoulos, P.T.; Charakopoulos, E.; Symeonidis, A.; Kotsianidis, I.; Viniou, N.A.; Pappa, V.; Pontikoglou, C.; Tsokanas, D.; Drakos, G.; Kourakli, A.; et al. Real world data on the prognostic significance of monocytopenia in myelodysplastic syndrome. Sci. Rep. 2022, 12, 17914. [Google Scholar] [CrossRef]
- Lemley, D.E.; Chun, B.; Cupps, T.R. Sterile splenic abscesses in systemic Weber-Christian disease. Unique source of abdominal pain. Am. J. Med. 1987, 83, 567–570. [Google Scholar] [CrossRef]
- Olcott, E.W.; Openshaw, K.L. Polyarteritis nodosa mimicking hepatic candidiasis on postcontrast CT. J. Comput. Assist. Tomogr. 1994, 18, 305–307. [Google Scholar] [CrossRef] [PubMed]
- Kasuga, I.; Yanagisawa, N.; Takeo, C.; Koga, M.; Kiyokawa, H.; Yonemaru, M.; Ichinose, Y.; Toyama, K. Multiple pulmonary nodules in association with pyoderma gangrenosum. Respir. Med. 1997, 91, 493–495. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Fukuhara, K.; Urano, Y.; Kimura, S.; Hori, K.; Arase, S. Pyoderma gangrenosum with rheumatoid arthritis and pulmonary aseptic abscess responding to treatment with dapsone. Br. J. Dermatol. 1998, 139, 556–558. [Google Scholar] [CrossRef]
- Vadillo, M.; Jucgla, A.; Podzamczer, D.; Rufi, G.; Domingo, A. Pyoderma gangrenosum with liver, spleen and bone involvement in a patient with chronic myelomonocytic leukaemia. Br. J. Dermatol. 1999, 141, 541–543. [Google Scholar] [CrossRef]
- Brown, T.S.; Marshall, G.S.; Callen, J.P. Cavitating pulmonary infiltrate in an adolescent with pyoderma gangrenosum: A rarely recognized extracutaneous manifestation of a neutrophilic dermatosis. J. Am. Acad. Dermatol. 2000, 43, 108–112. [Google Scholar] [CrossRef]
- Miserocchi, E.; Modorati, G.; Foster, C.S.; Brancato, R. Ocular and extracutaneous involvement in pyoderma gangrenosum. Ophthalmology 2002, 109, 1941–1943. [Google Scholar] [CrossRef]
- Ochiai, T.; Hara, H.; Shimojima, H.; Fujitsuka, A.; Morishima, T.; Yamazaki, T.; Sawada, U. Articular and pancreatic involvement in pyoderma gangrenosum associated with myelodysplastic syndrome. Dermatology 2002, 205, 70–72. [Google Scholar] [CrossRef]
- Mijuşković, Z.P.; Zecević, R.D.; Pavlović, M.D. Pyoderma gangrenosum with spleen involvement and monoclonal IgA gammopathy. J. Eur. Acad. Dermatol. Venereol. 2004, 18, 697–699. [Google Scholar] [CrossRef]
- Sitjas, D.; Llistosella, E.; Peñarroja, G.; Castro, A.; Codina-Barreras, A. Pioderma gangrenoso con afectación hepatoesplénica y articular. Actas Dermo-Sifiliográficas 2004, 95, 641–643. [Google Scholar] [CrossRef]
- Hubbard, V.G.; Friedmann, A.C.; Goldsmith, P. Systemic pyoderma responding to infliximab and adalimumab. Br. J. Dermatol. 2005, 152, 1059–1061. [Google Scholar] [CrossRef]
- Holstein, A.; Egberts, E.H.; Von Herbay, A. Rheumatoid-like nodules in the spleen: New extraintestinal manifestation of Crohn’s disease? J. Gastroenterol. Hepatol. 2006, 21, 295–298. [Google Scholar] [CrossRef]
- Field, S.; Powell, F.C.; Young, V.; Barnes, L. Pyoderma gangrenosum manifesting as a cavitating lung lesion. Clin. Exp. Dermatol. 2008, 33, 418–421. [Google Scholar] [CrossRef] [PubMed]
- Mitrevski, M.; Granata, M.; Sedati, P.; Rota, F.; De Santis, A.; Remotti, D.; Callea, F.; Visentini, M. Sterile abscesses complicating monoclonal gammopathy of undetermined significance. Eur. J. Haematol. 2008, 81, 246. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, K.; Nanki, T.; Sugihara, T.; Miyasaka, N. A case of polyarteritis nodosa with periurethral aseptic abscesses and testicular lesions. Clin. Exp. Rheumatol. 2008, 26, 1113–1115. [Google Scholar]
- Klinger, S.; Mathis, N.; Jackson, S. Bullous Sweet syndrome associated with an aseptic splenic abscess. Cutis 2009, 84, 255–258. [Google Scholar]
- Renna, S.; Mocciaro, F.; Perricone, G.; Orlando, A.; Virdone, R.; Speciale, A.; Lima, G.; Stella, M.; Cottone, M. Is splenectomy a treatment option for aseptic abscesses in patients with Crohn’s disease? Eur. J. Gastroenterol. Hepatol. 2009, 21, 1314–1316. [Google Scholar] [CrossRef]
- Yosunkaya, S.; Toy, H.; Genc, E.; Akın, B.; Maden, E.; Özer, F. Pyoderma Gangrenosum Presenting with Pulmonary Cavitary Lesions. Eur. J. Gen. Med. 2009, 6, 131–135. [Google Scholar] [CrossRef]
- Zakout, R.; Fonseca, M.; Santos, J.M.; Marques, A.; Távora, I.; Oliveira, E.; Ferreira, C.; Victorino, R.M. Multiple aseptic liver abscesses as the initial manifestation of Crohn’s disease: Report of a case. Dis. Colon Rectum 2009, 52, 343–345. [Google Scholar] [CrossRef]
- Fortna, R.; Toporcer, M.; Elder, D.; Junkins-Hopkins, J. A Case of Sweet Syndrome with Spleen and Lymph Node Involvement Preceded by Parvovirus B19 infection, and a Review of the Literature on Extracutaneous Sweet Syndrome. Am. J. Dermatopathol. 2010, 32, 621–627. [Google Scholar] [CrossRef]
- Isik, M.; Çalgüneri, M.; Doğan, İ.; Yeşilkaya, Y.; Shourbagi, A. Aseptic Abscess: A Report of Two Cases. Eur. J. Inflamm. 2012, 10, 143–147. [Google Scholar] [CrossRef]
- Allen, C.; Hull, J.; Wilkison, N.; Burge, S. Pediatric Pyoderma Gangrenosum with Splenic and Pulmonary Involvement. Pediatr. Dermatol. 2013, 30, 497–499. [Google Scholar] [CrossRef]
- Brătucu, E.; Lazar, A.; Marincaş, M.; Daha, C.; Zurac, S. Aseptic mesenteric lymph node abscesses. In search of an answer. A new entity? Chirurgia 2013, 108, 152–160. [Google Scholar]
- Carvalho, L.R.; Zanuncio, V.V.; Gontijo, B. Pyoderma gangrenosum with renal and splenic impairment--case report. An. Bras. Dermatol. 2013, 88, 150–153. [Google Scholar] [CrossRef]
- Daloul, R.; Gupta, S.; Marin, M. Nodular kidney involvement in a patient with idiopathic Sweet’s syndrome. BMJ Case Rep. 2013, 2013, bcr2013010075. [Google Scholar] [CrossRef] [PubMed]
- Ito, T.; Sato, N.; Yamazaki, H.; Koike, T.; Emura, I.; Saeki, T. A case of aseptic abscesses syndrome treated with corticosteroids and TNF-alpha blockade. Mod. Rheumatol. 2013, 23, 195–199. [Google Scholar] [CrossRef] [PubMed]
- Maeshima, K.; Ishii, K.; Inoue, M.; Himeno, K.; Seike, M. Behçet’s disease complicated by multiple aseptic abscesses of the liver and spleen. World J. Gastroenterol. 2013, 19, 3165–3168. [Google Scholar] [CrossRef]
- Brooks, J.; Ghaffari, G. Aseptic splenic abscess as precursory extraintestinal manifestation of inflammatory bowel disease. Case Rep. Med. 2014, 2014, 684231. [Google Scholar] [CrossRef]
- Fukuda, S.; Nanki, T.; Morio, T.; Hasegawa, H.; Koike, R.; Miyasaka, N. Recurrent mitral valve regurgitation with neutrophil infiltration in a patient with multiple aseptic abscesses. Mod. Rheumatol. 2014, 24, 537–539. [Google Scholar] [CrossRef]
- Husein, D.; Staels, K.; Dewaele, F.; Ballaux, D.; T’Sjoen, G. Sterile pituitary abscess associated with hypophysitis and panhypopituitarism: Case report. Endocr. Abstr. 2014, 35, 867. [Google Scholar] [CrossRef]
- Li, X.; Chandra, S. A case of ulcerative colitis presenting as pyoderma gangrenosum and lung nodule. J. Community Hosp. Intern. Med. Perspect. 2014, 4, 23402. [Google Scholar] [CrossRef]
- Nomoto, H.; Hayashi, Y.; Shinozaki, S.; Yano, T.; Sunada, K.; Sasao, W.; Kitamura, A.; Ohashi, M.; Hiyama, S.; Lefor, A.; et al. Ulcerative colitis-associated pulmonary nodules with cavity formation successfully treated with mesalazine and granulocyte–monocyte apheresis. Clin. J. Gastroenterol. 2014, 7, 476–480. [Google Scholar] [CrossRef]
- Baline, K.; Khadir, K.; Chiheb, S.; Marnissi, F.; Benchikhi, H. Pyoderma gangrenosum granulomateux profond: Une forme originale de pyoderma gangrenosum? Ann. Dermatol. Vénéréologie 2015, 142, 340–345. [Google Scholar] [CrossRef]
- Gade, M.; Studstrup, F.; Andersen, A.K.; Hilberg, O.; Fogh, C.; Bendstrup, E. Pulmonary manifestations of pyoderma gangrenosum: 2 cases and a review of the literature. Respir. Med. 2015, 109, 443–450, We included only the second case in our study. [Google Scholar] [CrossRef] [PubMed]
- Be, M.; Cha, H.J.; Park, C.; Park, Y.; Jung, H.; Lee, Y.; Jegal, Y. Multiple pulmonary cavitary nodules with pyoderma gangrenosum in patient with rheumatoid arthritis. Ann. Transl. Med. 2016, 4, 39. [Google Scholar] [CrossRef] [PubMed]
- Cosgarea, R.; Senilă, S.C.; Badea, R.; Ungureanu, L. Pyoderma gangrenosum with spleen involvement. Review of the literature and case report. J. Dermatol. Case Rep. 2016, 10, 26–31. [Google Scholar] [CrossRef]
- Johnson, K.; Sadik, K. Aseptic Splenic Abscess and Sweet Syndrome. J. Am. Osteopath. Assoc. 2016, 116, 330. [Google Scholar] [CrossRef][Green Version]
- Panos, Z.; Giannopoulos, G.; Papangeli, E.; Antalis, E.; Pavli, A.; Spathis, A.; Poulakou, G.; Dimitriadis, G.; Panayiotides, I.; Boumpas, D.; et al. Aseptic abscess syndrome associated with traveler’s diarrhea after a trip to Malaysia. IDCases 2016, 6, 23–25. [Google Scholar] [CrossRef]
- Reynolds, C.; Schofer, N.; Zengin, E.; Lohse, A.; Faiss, S.; Schmiedel, S. Multiple Abszesse nach Südamerikakreuzfahrt. Der Internist 2016, 57, 284–288. [Google Scholar] [CrossRef]
- Sakata, K.K.; Penupolu, S.; Colby, T.V.; Gotway, M.B.; Wesselius, L.J. Pulmonary pyoderma gangrenosum without cutaneous manifestations. Clin. Respir. J. 2016, 10, 508–511. [Google Scholar] [CrossRef]
- Sakharpe, A.K.; Sakharpe, A.K.; Mirmanesh, M.; Dunn, H.; Wilhelm, J.; Badr, A.S.; Kohli, H. A case and review of aseptic liver abscess in Crohn’s disease. Int. J. Colorectal. Dis. 2016, 31, 787–788. [Google Scholar] [CrossRef]
- Shadafny, N.; Heyman, S.N.; Bursztyn, M.; Dinaburg, A.; Nir-Paz, R.; Ackerman, Z. Multiple Sterile Splenic and Lymph Node Abscesses in a Patient with Long-Standing Ulcerative Colitis. Isr. Med. Assoc. J. 2016, 18, 633–635. [Google Scholar]
- Wawrzycki, B.; Chodorowska, G.; Pietrzak, A.; Prystupa, A.; Krupski, W.; Majdan, M.; Mosiewicz, J.; Krasowska, D. Ulcerative colitis accompanied by aseptic abscesses syndrome and small-vessel vasculitis. J. Pre-Clin. Clin. Res. 2016, 10, 140–143. [Google Scholar] [CrossRef][Green Version]
- Bollegala, N.; Khan, R.; Scaffidi, M.A.; Al-Mazroui, A.; Tessolini, J.; Showler, A.; Colak, E.; Grover, S.C. Aseptic Abscesses and Inflammatory Bowel Disease: Two Cases and Review of Literature. Can. J. Gastroenterol. Hepatol. 2017, 2017, 5124354, We included only the first case in our study. [Google Scholar] [CrossRef]
- Fujikawa, T.; Suzuka, T. Rare case of pyoderma gangrenosum originating in the spleen. BMJ Case Rep. 2017, 2017, bcr2016216909. [Google Scholar] [CrossRef]
- Göbel, T.; Rauen-Vossloh, J.; Hotz, H.G.; Boldt, A.; Erhardt, A. Conservative treatment of an aseptic abscess syndrome with splenic abscesses in Crohn’s disease. Z. Gastroenterol. 2017, 55, 1313–1317. [Google Scholar] [CrossRef] [PubMed]
- Lim, K.I.; Yang, D.H.; Ryoo, E. Behçet’s disease with multiple splenic abscesses in a child. Intest. Res. 2017, 15, 422–428. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Takahashi, A.; Nakajima, H.; Sano, S. Iliopsoas and intraperitoneal abscesses associated with pyoderma gangrenosum. J. Dermatol. 2017, 44, e218–e219. [Google Scholar] [CrossRef]
- Yang, Y.; Chen, D. Treatment of Aseptic Liver Abscess Due to Crohn’s Disease Using Infliximab. Clin. Gastroenterol. Hepatol. 2017, 15, A27–A28. [Google Scholar] [CrossRef]
- Yildiz, H.; Munting, A.; Komuta, M.; Danse, E.; Lefebvre, C. Aseptic lung and liver abscesses: A diagnostic challenge. Acta Clin. Belg. 2017, 72, 259–263. [Google Scholar] [CrossRef]
- Doll, R.; Friedman, K.; Hostoffer, R. Aseptic Abscess Syndrome, a Case of Prolonged Remission Following Splenectomy. Am. J. Gastroenterol. 2018, 113, 1264–1265. [Google Scholar] [CrossRef]
- Kim, A.; Parker, N.; Daunov, M.; Khatib, J.; Sreekumar, B.; Ammori, J.; Wessell, K.; Hirsch, C.; Fulton, S. Aseptic abscesses—A missed diagnosis. In Proceedings of the 2018 ACP National Abstract Competition, New Orleans, LA, USA, 19–21 April 2018. [Google Scholar]
- Marzano, A.V.; Ortega-Loayza, A.G.; Ceccherini, I.; Cugno, M. LPIN2 gene mutation in a patient with overlapping neutrophilic disease (pyoderma gangrenosum and aseptic abscess syndrome). JAAD Case Rep. 2018, 4, 120–122. [Google Scholar] [CrossRef]
- Sutton, E.; Groff, R.; Sutton, L.M. Sweet syndrome with aseptic splenic abscesses and multiple myeloma. Cutis 2018, 101, E27–E29. [Google Scholar] [PubMed]
- Bavaro, D.F.; Ingravallo, G.; Signorile, F.; Fortarezza, F.; Di Gennaro, F.; Angarano, G.; Saracino, A. Splenic abscesses as a first manifestation of Crohn’s disease: A case report. BMC Gastroenterol. 2019, 19, 144. [Google Scholar] [CrossRef] [PubMed]
- Dinis de Freitas, J.; Costa, F.; Rovisco, J. Hepatic vasculitis mimicking multiple liver abscesses in Cogan’s Syndrome. Acta Reumatol. Port. 2019, 44, 9. [Google Scholar]
- Gamboa, J.; Benavides Arenas, R.; Perilla, O.; Velez, D.; Castaño, P.; Pemberthy, C. Abscesos esplénicos asépticos asociados a enfermedad de Crohn. Acta Médica Colomb. 2019, 43, 26–29. [Google Scholar] [CrossRef]
- Panagopoulos, D.; Themistocleous, M. Central nervous system manifestation of lupus erythematosus resembling brain abscess. Int. J. Pediatr. Adolesc. Med. 2019, 6, 29–37. [Google Scholar] [CrossRef]
- Xu, P.; Cai, Y.; Ying, X.; Shi, S.; Song, W. A case of persistent fever, cutaneous manifestations and pulmonary and splenic nodules: Clinical experience and a literature review. Intern. Med. J. 2019, 49, 247–251. [Google Scholar] [CrossRef]
- Agirgol, S.; Ustaoglu, E.; Demir, F.T.; Akbulut, T.O.; Turkoglu, Z.; Kaya, H.; Pehlivanoğlu, F. Aseptic Abscess Syndrome with Severe Skin Involvement: Case Report. Indian. J. Dermatol. 2020, 65, 434–436. [Google Scholar] [CrossRef]
- Fillman, H.; Riquelme, P.; Sullivan, P.D.; Mansoor, A.M. Aseptic abscess syndrome. BMJ Case Rep. 2020, 13, e236437. [Google Scholar] [CrossRef]
- Kędzierska, M.A.; Szmurło, A.; Szymańska, E.; Walecka, I. Pyoderma gangrenosum with visceral involvement: A severe, recurrent disease affecting pulmonary, splenic, mesorectal, and subcutaneous tissues. Pol. Arch. Intern. Med. 2020, 130, 688–690. [Google Scholar] [CrossRef]
- Mathapathi, S.; Preziosi, M. Multiple Hepatic Micro-Hypodensities as a Presenting Sign in Systemic Lupus Erythematosus- A Case Report. Open Rheumatol. J. 2020, 14, 22–27. [Google Scholar] [CrossRef]
- Tiwari, A.; Mithun, C.B. Sarcoidosis mimicking as liver & splenic abscess. Indian J. Med. Res. 2020, 152, S186–S187. [Google Scholar] [CrossRef]
- Weins, A.B.; Scharffetter-Kochanek, K.; Weiss, T.; Crisan, D.; Hehl, N.; Weiss, J.; Sindrilaru, A. Successful targeted cytokine blockade in a case of aseptic abscess syndrome. J. Dtsch. Dermatol. Ges. 2020, 18, 908–910. [Google Scholar] [CrossRef] [PubMed]
- Hafner, S.; Seufferlein, T.; Kleger, A.; Müller, M. Aseptic Liver Abscesses as an Exceptional Finding in Cogan’s Syndrome. Hepatology 2021, 73, 2067–2070. [Google Scholar] [CrossRef] [PubMed]
- Moafa, G.; Unis, G. S2420 Aseptic Systemic Abscess Syndrome: A Rare Syndromic Presentation of Crohn’s Disease. Am. J. Gastroenterol. 2021, 116, S1025–S1026. [Google Scholar] [CrossRef]
- Santa Lucia, G.; DeMaio, A.; Karlin, S.; Elston, D. A case of extracutaneous pyoderma gangrenosum in a patient with persistent cutaneous and systemic symptoms: Implications for differential diagnosis and treatment. JAAD Case Rep. 2021, 15, 85–87. [Google Scholar] [CrossRef]
- Sheehan, J.L.; Brandler, J.; Rice, M.D. A Case of Recurrent Hepatic Abscesses. Gastroenterology 2021, 161, 1393–1394. [Google Scholar] [CrossRef]
- Shimizu, M.S.; Matsuo, T.; Mori, N. A Rare Manifestation Associated with a Urinary Tract Infection in a Patient with Ulcerative Colitis. Gastroenterology 2021, 161, e14–e15. [Google Scholar] [CrossRef]
- Soffer, S.; Dahan, S.; Maklakovski, M.; Dagan, A. A Case of Aseptic Renal Abscesses Associated With IBD. Inflamm. Bowel Dis. 2021, 27, e28–e29. [Google Scholar] [CrossRef]
- Yamaguchi, Y.; Nakagawa, M.; Nakagawa, S.; Nagao, K.; Inoue, S.; Sugiyama, T.; Izawa, S.; Hijikata, Y.; Ebi, M.; Funaki, Y.; et al. Rapidly Progressing Aseptic Abscesses in a Patient with Ulcerative Colitis. Intern. Med. 2021, 60, 725–730. [Google Scholar] [CrossRef]
- Akagi, Y.; Yamagiwa, Y.; Shirai, H.; Suzuki, T.; Tsuru, I.; Ishikawa, A.; Akiyama, N.; Ogura, M.; Kobayashi, K.; Bae, Y.; et al. Aseptic Cavernosal Abscess: An Unrecognized Feature of Neutrophilic Dermatosis. Intern. Med. 2022, 61, 917–921. [Google Scholar] [CrossRef] [PubMed]
- Bansal, A.; Singla, A.; Singh, A.; Kaur, S. Pyoderma Gangrenosum with Splenic Abscess- A Rare Association. Indian Dermatol. Online J. 2022, 13, 244–247. [Google Scholar] [CrossRef] [PubMed]
- Chakrabarti, K.; Vreede, A. Case Report: Abscess as a Manifestation of Autoinflammatory Disease: American College of Rheumatology/Association of Rheumatology Professionals; 2022. The Rheumatologist®, 14 June 2022. Available online: https://www.the-rheumatologist.org/ (accessed on 25 April 2025). We included only the first case in our study.
- Dhruv, S.; Anwar, S.; Polavarapu, A.; Yousaf, F.; Mukherjee, I. Recurrent aseptic liver abscesses in a patient with Crohn’s disease: True infection or a Crohn’s flare? Arab. J. Gastroenterol. 2022, 23, 58–60. [Google Scholar] [CrossRef]
- Kita, A.; Hashimoto, Y.; Sato, K.; Itoi, Y.; Kasuga, K.; Tanaka, H.; Hosaka, H.; Kuribayashi, S.; Uraoka, T. Ulcerative colitis complicated by pyoderma gangrenosum and multiple aseptic abscesses. Nihon Shokakibyo Gakkai Zasshi 2022, 119, 1014–1021. [Google Scholar] [CrossRef]
- Owczarczyk-Saczonek, A.; Kasprowicz-Furmańczyk, M.; Kuna, J.; Klimek, P.; Krajewska-Włodarczyk, M. Aseptic Abscess Syndrome in Rheumatoid Arthritis Patient. Medicina 2022, 58, 1354. [Google Scholar] [CrossRef]
- Ozeki, K.; Tanida, S.; Kataoka, H. Aseptic Abscess Syndrome with Ulcerative Colitis and Pyoderma Gangrenosum. Clin. Gastroenterol. Hepatol. 2022, 20, A27. [Google Scholar] [CrossRef]
- Sato, N.; Yamaide, F.; Shibata, R.; Nakano, T.; Yamaide, A.; Saito, T.; Shimojo, N. Successful management of a case of intestinal Behçet’s disease with a splenic abscess by intensified immunosuppressive therapy without splenectomy. Mod. Rheumatol. Case Rep. 2022, 6, 266–269. [Google Scholar] [CrossRef]
- Tam, L.; Akhtar, D.; Salh, B. A155 Aseptic Abscess Syndrome in Inflammatory Bowel Disease: A Case Report. J. Can. Assoc. Gastroenterol. 2022, 5, 29–31. [Google Scholar] [CrossRef]
- Zhang, C.; Elmaoued, A.; Rincy, B.; Ploussard, B.; Saab-Chalhoub, M.; Alexander, A.J.; Allam, E. Sweet syndrome with osseous and splenic involvement: A case report. Radiol. Case Rep. 2022, 17, 194–200. [Google Scholar] [CrossRef]
- Zinngrebe, J.; Moepps, B.; Monecke, T.; Gierschik, P.; Schlichtig, F.; Barth, T.F.E.; Strauß, G.; Boldrin, E.; Posovszky, C.; Schulz, A.; et al. Compound heterozygous variants in OTULIN are associated with fulminant atypical late-onset ORAS. EMBO Mol. Med. 2022, 14, e14901. [Google Scholar] [CrossRef]
- Bilgin, S.; Uğurlu, S. FMF presented by aseptic abscesses. J. Turk Soc. Rheumatol. 2023, 15, 167–170. [Google Scholar] [CrossRef]
- Dai, C.; Huang, Y.H. Successful treatment of Crohn’s disease, aseptic liver abscess and psoriasis with ustekinumab. Rev. Esp. Enferm. Dig. 2023, 115, 155–156. [Google Scholar] [CrossRef]
- Eitan, M.; Benjaminov, F.; Zinger, C.; Kitay Cohen, Y.; Ringel, Y. Aseptic Liver Abscess in a Patient with Diversion Colitis. ACG Case Rep. J. 2023, 10, e01169. [Google Scholar] [CrossRef]
- González-Velásquez, M.; Velásquez-Franco, C.J.; Bernal-Macías, S.; Gutiérrez-Bolaños, J. Aseptic Abscess Syndrome in a Patient with Rheumatoid Arthritis: A Case Report. Iatreia 2023, 37, 97–105. [Google Scholar] [CrossRef]
- Kranidioti, H.K.E.; Pirounaki, M.; Kafiri, G.; Kontos, G.; Manolakopoulos, S.; Deutsch, M.; Vassilopoulos, D. Σύνδρομο άσηπτων ηπατικών και σπληνικών αποστημάτων (Aseptic abscess syndrome, AAS) σε υγιή 46χρονη γυναίκα. In Proceedings of the 21st Panhellenic Congress of Hepatology, Ioannina, Greece, 17–20 May 2023. (In Greek). [Google Scholar]
- Sritharan, S.; Lau, P.S.; Manan, K.; Mohan, A. Case report: Aseptic splenic abscesses in childhood-onset systemic lupus erythematosus. Front. Pediatr. 2023, 11, 1214551. [Google Scholar] [CrossRef]
- Toba, T.; Ikegami, R.; Nogami, A.; Watanabe, N.; Fujii, K.; Ogawa, Y.; Hojo, A.; Fujimoto, A.; Matsuda, T. Multiple ulcerative colitis-associated aseptic abscesses successfully treated with infliximab: A case report. Clin. J. Gastroenterol. 2023, 16, 537–542. [Google Scholar] [CrossRef] [PubMed]
- Dias, P.R.; Bolt, L.; Iking-Konert, C.; Arrigo, M.; Huber, L.C. Multiple Abscess Collections: Antibiotics or Steroids? Case Rep. Immunol. 2024, 2024, 3671685. [Google Scholar] [CrossRef] [PubMed]
- Eibschutz, L.; Lodyga, M.; Ofri, D.; Yerrabothala, S.; Lansigan, F. Aseptic Abscess Syndrome in Hematological Malignancies: Case Reports and Insights. Blood 2024, 144, 5981, We included only the first case in our study. [Google Scholar] [CrossRef]
- Karmacharya, S.; Shrestha, A.A.; Nakarmi, S.; Bhochhibhoya, M.; Vaidya, B. Aseptic pyomyositis in rheumatoid arthritis treated with corticosteroid and DMARDs. Oxf. Med. Case Rep. 2024, 2024, omae059. [Google Scholar] [CrossRef]
- McGrath, M.; Geng, C.; Rainho, A.; Figueroa, E. Aseptic Splenic Abscesses with Concomitant Sweet Syndrome as Extraintestinal Manifestations of New-Onset Crohn’s Disease. ACG Case Rep. J. 2024, 11, e01464. [Google Scholar] [CrossRef]
- Merola, J.F.; Cochran, R.L.; Kroshinsky, D.; Prabhu, M.; Kwan, M.C. Case 22-2024: A 30-Year-Old Woman with Postpartum Fever, Abdominal Pain, and Skin Ulcers. N. Engl. J. Med. 2024, 391, 260–271. [Google Scholar] [CrossRef] [PubMed]
- Ren, J.; Zhou, J.; Wang, Q.; Liu, L.; Liu, W.; Wang, S.; Zheng, Y.; Luo, L.; Yang, Q. Lung and Cutaneous Abscesses in a Patient with Ulcerative Colitis: A Case Report and Literature Review. Infect. Drug Resist. 2024, 17, 3483–3490. [Google Scholar] [CrossRef]
- Sasi, S.; Eltahir, M.; Ibrahim, E.; Padmakumari, A.; Kolleri, J.; Abdallah, T.; Shehatta, A.L.; Hadwan, N.; Jaouni, H.; Al-Maslamani, M. Hepatic micro-abscesses as an unusual initial presentation of systemic lupus erythematosus: A case report. Clin. Case Rep. 2024, 12, e8586. [Google Scholar] [CrossRef]
- Ucci, F.M.; Scrivo, R.; Alessandri, C.; Conti, F.; Priori, R. Aseptic abscess syndrome: A case report of a patient achieving remission with both infliximab originator and biosimilar administered at varied intervals. Front. Immunol. 2024, 15, 1454813. [Google Scholar] [CrossRef]
- Fragonikolaki, M.; Nousiopoulou, E.; Aggelakis, N.; Katsikas, T.; Tampaki, M.; Bouklas, D.; Kakiopoulos, G.; Argyraki, A. Σύνδρομο άσηπτων σπληνικών αποστημάτων (Aseptic abscess syndrome). Μια σπάνια φλεγμονώδης διαταραχή σε υγιή γυναίκα. In Proceedings of the 51st Panhellenic Medical Congress, Athens, Greece, 22–24 May 2025. (In Greek). [Google Scholar]
- Yılmaz, B.; Güzel Dirim, M.; Gültekin, B.; Şenkal, N.; Medetalibeyoğlu, A.; Yegen, G.; Köse, M.; Çağatay, A.A. Aseptic Abscess Syndrome: A Unique Case of Splenic Involvement and Systemic Inflammation. Turk. J. Haematol. 2025, 42, 72–73. [Google Scholar] [CrossRef]
- Kanno, T.; Ito, M.; Tsuji, H.; Kawase, N.; Taki, Y. A case of pyoderma gangrenosum involving the prostate gland after radiation therapy for prostate cancer. Hinyokika Kiyo 2002, 48, 565–568. [Google Scholar]
- AlDossary, S.J.; AlFawaz, T.S.; AlMutairi, A.K. Pyoderma gangrenosum with splenic involvement. Int. J. Pediatr. Adolesc. Med. 2016, 3, 78–80. [Google Scholar] [CrossRef]
- Maritsi, D.N.; Tavernaraki, K.; Vartzelis, G. Pyoderma gangrenosum with systemic and pulmonary involvement in a toddler. Pediatr. Int. 2015, 57, 505–506. [Google Scholar] [CrossRef]
- Bharti, A.; Meena, L.P. Systemic lupus erythematosus with hepatosplenic granuloma: A rare case. Case Rep. Immunol. 2014, 2014, 737453. [Google Scholar] [CrossRef]
- Takahashi, H.; Ogawa, M.; Hoshina, T.; Kusuhara, K. Multiple Nodules in the Kidney and Spleen Presenting as the Initial Manifestation of Crohn Disease. Inflamm. Bowel Dis. 2021, 27, e91–e92. [Google Scholar] [CrossRef]
- Asako, Y.; Miyamoto, S.; Fujikawa, N.; Furuki, S.; Yamashita, T. A Case of Aseptic Temporomandibular Joint Abscess Associated with Inflammatory Bowel Disease. Nippon. Jibiinkoka Gakkai Kaiho 2021, 124, 897–902. [Google Scholar] [CrossRef]
- Ohata, C.; Ozawa, K.; Itami, S.; Yoshikawa, K.; Inoue, M.; Maeda, H. Pyoderma Gangrenosum with Pulmonary Aseptic Abscess. Hifu No Kagaku 2005, 4, 542–547. [Google Scholar] [CrossRef]
- Yoshida, E.; Chiyomaru, K.; Fukuda, H.; Hamada, M.; Nomura, T. A Case of Pyoderma Gangrenosum with Aseptic Cervical and Mediastinal Abscesses in a Patient with MDS and Tolosa-Hunt Syndrome. Hifu No Kagaku 2006, 5, 133–138. [Google Scholar] [CrossRef]
- Lamport, R.D.; Cheskin, L.J.; Moscatello, S.A.; Nikoomanesh, P. Sterile epidural and bilateral psoas abscesses in a patient with Crohn’s disease. Am. J. Gastroenterol. 1994, 89, 1086–1089. [Google Scholar] [PubMed]
- Lacy, M. Review of inflammatory biomarkers in hospitalized adults with suspected infection. Southwest Respir. Crit. Care Chron. 2018, 6, 4. [Google Scholar] [CrossRef]
- Rahimian, J.; Wilson, T.; Oram, V.; Holzman, R.S. Pyogenic Liver Abscess: Recent Trends in Etiology and Mortality. Clin. Infect. Dis. 2004, 39, 1654–1659. [Google Scholar] [CrossRef]
- Lin, Y.-T.; Wang, Y.-F.; Yang, J.C.-H.; Yu, C.-J.; Wu, S.-G.; Shih, J.-Y.; Yang, P.-C. Development of Renal Cysts after Crizotinib Treatment in Advanced ALK-Positive Non–Small-Cell Lung Cancer. J. Thorac. Oncol. 2014, 9, 1720–1725. [Google Scholar] [CrossRef]
- Toyota, S.; Kato, T.; Ehara, H.; Sugie, S. Crizotinib Associated Renal Abscess—A Case Report. Hinyokika Kiyo 2024, 70, 283–287. [Google Scholar] [CrossRef]
- Oliveira-Silva, T.; Naegele-Bazzanella, C.; Zukin, M.; Cavalcante, N.; de-Oliveira-Siciliano, A.; Araújo-Oliveira-Neto, J.; Baldotto, C. Crizotinib-Related Aseptic Abscesses in Lung Adenocarcinoma and ROS1-Gene Rearrangement: A Case Report. Braz. J. Oncol. 2025, 21, s00451805098. [Google Scholar] [CrossRef]
- Trefond, L.; Billard, E.; Pereira, B.; Richard, D.; Vazeille, E.; Bonnet, R.; Barnich, N.; Andre, M. Host-microbiota relationship in the pathophysiology of aseptic abscess syndrome: Protocol for a multicentre case-control study (ABSCESSBIOT). BMJ Open 2023, 13, e073776. [Google Scholar] [CrossRef]
- Sève, P.; Turner, R.; Stankovic, K.; Perard, L.; Broussolle, C. Transient monoclonal gammopathy in a patient with Bartonella quintana endocarditis. Am. J. Hematol. 2006, 81, 115–117. [Google Scholar] [CrossRef]
- Alexandra, P.; Jean-Philippe, B.; Nathalie, S.; François-Xavier, M.; Laurent, R.; Jean-Baptiste, O. Une gammapathie monoclonale d’apparition brutale ? Ann. Biol. Clin. 2015, 73, 185–189. [Google Scholar] [CrossRef]
- Park, D.S.; Cho, J.H.; Lee, J.H.; Lee, K.E. Clinical course of monoclonal and oligoclonal gammopathies in patients infected with Orientia tsutsugamushi. Am. J. Trop. Med. Hyg. 2009, 81, 660–664. [Google Scholar] [CrossRef]
- Quintero, C.; Corona, J.; Ponce, M.; Avilés, A.; Gutierrez, O.; Candelaria, M. Transient monoclonal gammopathy and hypercalcemia in a male patient with Systemic Lupus Erythematosus. Rev. Del Lab. Clínico 2019, 12, 133–136. [Google Scholar] [CrossRef]
- Stoimenis, D.; Spyridonidou, C.; Papaioannou, N. Transient Monoclonal Gammopathy Induced by Disseminated Staphylococcus aureus Infection. Case Rep. Med. 2012, 2012, 607104. [Google Scholar] [CrossRef][Green Version]
- Strobel, S.L. Transient paraproteinemia: An intriguing immunological anomaly. Ann. Clin. Lab. Sci. 2003, 33, 265–270. [Google Scholar]
- Gómez, M.; Hermida, F.J. Transient monoclonal gammopathy: A single-center study. Rev. Clin. Esp. 2025, 225, 502286. [Google Scholar] [CrossRef]
- Choi, J.J.; McCarthy, M.W. Novel applications for serum procalcitonin testing in clinical practice. Expert Rev. Mol. Diagn. 2018, 18, 27–34. [Google Scholar] [CrossRef]
- Dahaba, A.A.; Rehak, P.H.; List, W.F. Procalcitonin and C-reactive protein plasma concentrations in nonseptic uremic patients undergoing hemodialysis. Intensive. Care Med. 2003, 29, 579–583. [Google Scholar] [CrossRef]
- Schmidt, M.; Burchardi, C.; Sitter, T.; Held, E.; Schiffl, H. Procalcitonin in patients undergoing chronic hemodialysis. Nephron 2000, 84, 187–188. [Google Scholar] [CrossRef]
- Ito, N.; Takahashi, M.; Miwa, Y.; Kagami, S.; Hayakawa, H.; Inaba, A.; Orimo, S. Adult-onset Still’s disease presenting with aseptic meningitis as the first symptom in an elderly patient. eNeurologicalSci 2019, 16, 100202. [Google Scholar] [CrossRef]
- Wang, J.; Niu, R.; Jiang, L.; Wang, Y.; Shao, X.; Wu, M.; Ma, Y. The diagnostic values of C-reactive protein and procalcitonin in identifying systemic lupus erythematosus infection and disease activity. Medicine 2019, 98, e16798. [Google Scholar] [CrossRef] [PubMed]
- Scirè, C.A.; Cavagna, L.; Perotti, C.; Bruschi, E.; Caporali, R.; Montecucco, C. Diagnostic value of procalcitonin measurement in febrile patients with systemic autoimmune diseases. Clin. Exp. Rheumatol. 2006, 24, 123–128. [Google Scholar] [PubMed]
- Ames, P.R.; Walker, E.; Aw, D.; Marshall, D.; de Villiers, F.; Staber, M. Multi-organ failure in adult onset Still’s disease: A septic disguise. Clin. Rheumatol. 2009, 28 (Suppl. S1), S3–S6. [Google Scholar] [CrossRef] [PubMed]
- Yıldırım, R.; Oğuzman, S.; Üsküdar Cansu, D.; Dinler, M.; Korkmaz, C. Procalcitonin can increase during activation of adult-onset Still’s disease (AOSD) in the absence of an ınfection focus. Rheumatology 2023, 62, e109–e110. [Google Scholar] [CrossRef]
- Vasishta, S.; Patel, S. Elevated Procalcitonin in Acute Pseudogout Flare: A Case Report. Cureus 2019, 11, e4853. [Google Scholar] [CrossRef]
- Darby, J.B.; Rees, C.A.; Bocchini, C.E.; Cruz, A.T.; Kellermayer, R.; Finegold, M.J.; Barlow, S.E. A Case of an 11-year-old With Cough, Diarrhea, and Findings of Concern in His Lungs and Spleen. Pediatrics 2016, 137, e20150155. [Google Scholar] [CrossRef]
- Navarro Moreno, E.; López González, J.; Lázaro Sáez, M. Metastatic Crohn’s disease with splenic affectation: A very rare case. Gastroenterol. Hepatol. 2024, 47, 180–182. [Google Scholar] [CrossRef]
- Castellani, L.; Sartini, A.; Marocchi, M.; Balbi, T.; Belluzzi, A. Splenic granulomas in a patient with severe Crohn’s disease associated with multiple extraintestinal manifestations. Inflamm. Bowel Dis. 2011, 17, E35–E37. [Google Scholar] [CrossRef]
- Puli, S.R.; Presti, M.E.; Alpert, M.A. Splenic granulomas in Crohn disease. Am. J. Med. Sci. 2003, 326, 141–144. [Google Scholar] [CrossRef]
- El-Kersh, K.; Fraig, M.; Cavallazzi, R.; Saad, M.; Perez, R.L. Pulmonary necrobiotic nodules in Crohn’s disease: A rare extra-intestinal manifestation. Respir. Care 2014, 59, e190–e192. [Google Scholar] [CrossRef]
- Myc, L.A.; Girton, M.R.; Stoler, M.H.; Davis, E.M. Necrobiotic Pulmonary Nodules of Crohn’s Disease in a Patient Receiving Vedolizumab. Am. J. Respir. Crit. Care Med. 2019, 199, e1–e2. [Google Scholar] [CrossRef]
- Pektas, S.D.; AltintaŞ, N.; Izdes, S.; YÜCel, S.; DoĞAn, H. A Fatal Case of Pyoderma Gangrenosum with Multiple Organ System Involvement: Unexpected Progression: Case Report. Turk. Klin. J. Dermatol. 2015, 25, 67–70. [Google Scholar] [CrossRef][Green Version]

| Characteristic | n = 108 |
|---|---|
| Mean age (years) during diagnosis–all cases | 39.1 ± 19.8 |
| Μedian age (years)–adult cases [IQR] | 43 [27–56] (n = 95) |
| Μean age (years) ± SD–pediatric cases | 8.8 ± 5.6 (n = 13) * |
| Female gender–no. (%) | 59 (54.6) |
| Symptoms directly attributed to AAS–no. (%) | |
| Fever | 86 (79.6) |
| Pain | 72 (66.7) |
| Weight loss | 17 (15.7) |
| Nausea and/or vomiting | 5 (4.6) |
| Dyspnea | 7 (6.5) |
| Cough | 13 (12) |
| Hemoptysis | 3 (2.8) |
| Mucocutaneous manifestations (n = 20) ◊ | 6 (30) |
| Others ¶ | 6 (5.6) |
| Median time (months) between initial symptoms and diagnosis [IQR] | 2 [1–5.5] (n = 92) |
| Abscess location–no. (%) | |
| Spleen | 56 (51.9) |
| Liver | 38 (35.2) |
| Deep-seated lymph nodes | 16 (14.8) |
| Pancreas | 4 (3.7) |
| Kidney | 8 (7.4) |
| Other intraabdominal | 6 (5.6) |
| Genitalia and/or prostate | 5 (4.6) |
| Muscle and/or deep soft tissues and/or bone–joint | 14 (13) |
| Lung | 25 (23.1) |
| Cardiovascular | 1 (0.9) |
| Brain or pituitary | 2 (1.9) |
| >1 focus during diagnosis | 37 (34.3) |
| Parameter | Value (n) |
|---|---|
| Median leukocytes–×10−9/L [IQR] | 16.2 [12.9–21.4] (n = 74) |
| Mean hemoglobin ± SD (g/dL) | 9.9 ± 2 (n = 51) |
| Median platelets–×10−9/L [IQR] | 438 [281–589] (n = 32) |
| Elevated aminotransferases–no. [%] ¶ | 14/35 [40] (n = 35) |
| Median ALP–U/L [IQR] | 186 [74–313] (n = 29) |
| Median γGT–U/L [IQR] | 123 [32–186] (n = 25) |
| Median CRP–mg/dL [IQR] | 15.5 [8.2–25] (n = 75) |
| Mean ESR ± SD (mm/h) | 79 ± 30 (n = 56) |
| Median ESR to ULN ratio [IQR] ◊ | 2.8 [2.3–3.9] (n = 56) |
| Associated Disorder Data | n = 108 |
|---|---|
| Associated disorder identified–no. (%) | 96 (88.9) |
| Inflammatory bowel disease | 34 (31.5) |
| Crohn’s disease | 20 (18.5) |
| Ulcerative colitis | 14 (13) |
| Neutrophilic dermatosis | 47 (43.5) |
| Pyoderma gangrenosum | 40 (37) |
| Sweet syndrome | 6 (5.6) |
| Systemic lupus erythematosus | 6 (5.6) |
| Rheumatoid arthritis | 6 (5.6) |
| Behçet disease | 4 (3.7) |
| Polyarteritis nodosa | 2 (1.9) |
| Cogan syndrome | 2 (1.9) |
| Sarcoidosis | 2 (1.9) |
| Familial Mediterranean fever | 1 (0.9) |
| OTULIN-related autoinflammatory syndrome | 1 (0.9) |
| Weber–Christian disease | 2 (1.9) |
| Diversion colitis | 1 (0.9) |
| Sclerosing mesenteritis | 1 (0.9) |
| Multiple myeloma | 1 (0.9) |
| Acute myelogenous leukemia | 1 (0.9) |
| Chronic myelomonocytic leukemia | 1 (0.9) |
| Myelodysplastic syndrome | 1 (0.9) |
| Status of associated disorder during AAS diagnosis | n = 95 |
| Concomitant diagnosis | 50 (52.6) |
| Disorder diagnosed before, active at that moment | 21 (22.1) |
| Disorder diagnosed before, on remission at that moment | 21 (22.1) |
| Disorder diagnosed after | 3 (3.2) |
| Parameter | n = 108 |
|---|---|
| Abscess puncture or biopsy–no. (%) | 77 (71.3) |
| Management–no. (%) | |
| CS | 101 (93.5) |
| CS only | 27 (25) |
| Anti-TNF | 29 (26.9) |
| Azathioprine | 19 (7.6) |
| Methotrexate | 6 (5.6) |
| Hydroxychloroquine | 6 (5.6) |
| Dapsone | 5 (4.6) |
| Colchicine | 5 (4.6) |
| Calcineurin inhibitors | 11 (10.2) |
| Granulocyte–monocyte apheresis | 4 (3.7) |
| Others * | 14 (13) |
| Excision surgery | 23 (21.3) |
| Max CS dosage (prednisolone equivalent) | n = 76 |
| Medium (≤0.5 mg/kg/day) | 11 (14.5) |
| High (>0.5 mg/kg/day and ≤1.5 mg/kg/day) | 47 (61.8) |
| Pulse (>1.5 mg/kg/day) | 18 (23.7) |
| Reason for prescribing other immunomodulator(s) (added to CS)-no. (%) | n = 66 |
| Administered before AAS diagnosis for associated disorder | 6 (9.1) |
| Administered during or after AAS diagnosis but before relapse | 40 (60.6) |
| Administered due to the lack of response to CS during AAS diagnosis | 2 (3) |
| Administered due to the lack of response to CS during AAS relapse | 2 (3) |
| Administered due to AAS relapse during CS tapering | 11 (16.7) |
| Administered due to relapse of associated disease | 5 (7.6) |
| Relapse–no. (%) | 36 (42.4) [n = 85] |
| Relapse including different organ(s) no. (%) | 18 (62.1) [n = 29] ¶ |
| Median follow-up time (months) [IQR] | 12 [5.8–26.3] (n = 70) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Eleftheriotis, G.; Fragonikolaki, M.; Karelaki, C.; Syrigou, E.; Georgiadis, S.; Georgiadi, K.; Skopelitis, E. Epidemiology, Clinical Data, and Management of Aseptic Abscess Syndrome: Review of Published Cases Outside France. Epidemiologia 2025, 6, 44. https://doi.org/10.3390/epidemiologia6030044
Eleftheriotis G, Fragonikolaki M, Karelaki C, Syrigou E, Georgiadis S, Georgiadi K, Skopelitis E. Epidemiology, Clinical Data, and Management of Aseptic Abscess Syndrome: Review of Published Cases Outside France. Epidemiologia. 2025; 6(3):44. https://doi.org/10.3390/epidemiologia6030044
Chicago/Turabian StyleEleftheriotis, Gerasimos, Michaela Fragonikolaki, Chrysi Karelaki, Ergina Syrigou, Spyridon Georgiadis, Kyriaki Georgiadi, and Elias Skopelitis. 2025. "Epidemiology, Clinical Data, and Management of Aseptic Abscess Syndrome: Review of Published Cases Outside France" Epidemiologia 6, no. 3: 44. https://doi.org/10.3390/epidemiologia6030044
APA StyleEleftheriotis, G., Fragonikolaki, M., Karelaki, C., Syrigou, E., Georgiadis, S., Georgiadi, K., & Skopelitis, E. (2025). Epidemiology, Clinical Data, and Management of Aseptic Abscess Syndrome: Review of Published Cases Outside France. Epidemiologia, 6(3), 44. https://doi.org/10.3390/epidemiologia6030044






