Understanding the Role of Irisin in Longevity and Aging: A Narrative Review
Abstract
:1. Introduction
2. Methods
3. Results
3.1. Serum Irisin Levels in Healthy Older Adults
3.1.1. Irisin in Centenarians
3.1.2. Serum Irisin Levels in Healthy Older Adults with Ongoing Physical Activity
3.2. Serum Irisin Levels in Aging-Related Diseases
3.2.1. Serum Irisin Levels in Obese Older Adults
3.2.2. Irisin and Brain Diseases
3.2.3. Serum Irisin Levels in Chronic Obstructive Pulmonary Disease in Older Adults
3.2.4. Serum Irisin Levels in Older Patients with Sarcopenia
3.2.5. Serum Irisin Levels in Patients with Fractures
3.2.6. Serum Irisin Levels in Patients with Vascular Diseases
3.2.7. Serum Irisin Levels in Patients with Other Diseases
4. Discussion
5. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Eurostat. Population Structure and Ageing. 2022. Available online: https://ec.europa.eu/eurostat/statistics-explained/index.php?title=Population_structure_and_ageing (accessed on 5 March 2024).
- World Health Organization. Decade of Healthy Ageing: Baseline Report; World Health Organization: Geneva, Switzerland, 2020. [Google Scholar]
- Li, Z.; Zhang, Z.; Ren, Y.; Wang, Y.; Fang, J.; Yue, H.; Ma, S.; Guan, F. Aging and age-related diseases: From mechanisms to therapeutic strategies. Biogerontology 2021, 22, 165–187. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. World Health Statistics 2023: Monitoring Health for the SDGs, Sustainable Development Goals; World Health Organization: Geneva, Switzerland, 2023. [Google Scholar]
- Sanchez, B.; Munoz-Pinto, M.F.; Cano, M. Irisin enhances longevity by boosting SIRT1, AMPK, autophagy and telomerase. Expert. Rev. Mol. Med. 2022, 25, e4. [Google Scholar] [CrossRef]
- Calton, E.K.; Soares, M.J.; James, A.P.; Woodman, R.J. The potential role of irisin in the thermoregulatory responses to mild cold exposure in adults. Am. J. Hum. Biol. 2016, 28, 699–704. [Google Scholar] [CrossRef] [PubMed]
- Bostrom, P.; Wu, J.; Jedrychowski, M.P.; Korde, A.; Ye, L.; Lo, J.C.; Rasbach, K.A.; Boström, E.A.; Choi, J.H.; Long, J.Z.; et al. A PGC1-alpha-dependent myokine that drives brown-fat-like development of white fat and thermogenesis. Nature 2012, 481, 463–468. [Google Scholar] [CrossRef]
- Ferrer-Martinez, A.; Ruiz-Lozano, P.; Chien, K.R. Mouse PeP: A novel peroxisomal protein linked to myoblast differentiation and development. Dev. Dyn. 2002, 224, 154–167. [Google Scholar] [CrossRef]
- Teufel, A.; Malik, N.; Mukhopadhyay, M.; Westphal, H. Frcp1 and Frcp2, two novel fibronectin type III repeat containing genes. Gene 2002, 297, 79–83. [Google Scholar] [CrossRef]
- Aydin, S.; Kuloglu, T.; Aydin, S.; Eren, M.N.; Celik, A.; Yilmaz, M.; Kalayci, M.; Sahin, İ.; Gungor, O.; Gurel, A.; et al. Cardiac, skeletal muscle and serum irisin responses to with or without water exercise in young and old male rats: Cardiac muscle produces more irisin than skeletal muscle. Peptides 2014, 52, 68–73. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.K.; Jeong, Y.J.; Song, I.S.; Noh, Y.H.; Seo, K.W.; Kim, M.; Han, J. Glucocorticoid receptor positively regulates transcription of FNDC5 in the liver. Sci. Rep. 2017, 7, 43296. [Google Scholar] [CrossRef]
- Lima-Filho, R.; Fortuna, J.S.; Cozachenco, D.; Isaac, A.R.; Lyra, E.S.N.; Saldanha, A.; Santos, L.E.; Ferreira, S.T.; Lourenco, M.V.; De Felice, F.G. Brain FNDC5/Irisin Expression in Patients and Mouse Models of Major Depression. eNeuro 2023, 10, ENEURO.0256-22.2023. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Mao, L.; Li, C.; Hui, Y.; Yu, Z.; Sun, M.; Li, Y.; Guo, G.; Yang, W.; Cui, B.; et al. The potential role of FNDC5/irisin in various liver diseases: Awakening the sleeping beauties. Expert. Rev. Mol. Med. 2022, 24, e23. [Google Scholar] [CrossRef]
- Raschke, S.; Elsen, M.; Gassenhuber, H.; Sommerfeld, M.; Brockmann, B.; Schwahn, U.; Brockmann, B.; Jung, R.; Wisløff, U.; Tjønna, A.E.; et al. Evidence against a beneficial effect of irisin in humans. PLoS ONE 2013, 8, e73680. [Google Scholar] [CrossRef]
- Jedrychowski, M.P.; Wrann, C.D.; Paulo, J.A.; Gerber, K.K.; Szpyt, J.; Robinson, M.M.; Nair, K.S.; Gygi, S.P.; Spiegelman, B.M. Detection and Quantitation of Circulating Human Irisin by Tandem Mass Spectrometry. Cell Metab. 2015, 22, 734–740. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. WHO Consortium on Metrics and Evidence for Healthy Ageing Report of the Fourth Annual Meeting Held Virtually, 2–3 December 2020; World Health Organization: Geneva, Switzerland, 2023. [Google Scholar]
- Rodziewicz-Flis, E.A.; Kawa, M.; Kaczor, J.J.; Szaro-Truchan, M.; Flis, D.J.; Lombardi, G.; Ziemann, E. Changes in selected exerkines concentration post folk-dance training are accompanied by glucose homeostasis and physical performance improvement in older adults. Sci. Rep. 2023, 13, 8596. [Google Scholar]
- Gmiat, A.; Jaworska, J.; Micielska, K.; Kortas, J.; Prusik, K.; Prusik, K.; Lipowski, M.; Radulska, A.; Szupryczyńska, N.; Antosiewicz, J.; et al. Improvement of cognitive functions in response to a regular Nordic walking training in elderly women—A change dependent on the training experience. Exp. Gerontol. 2018, 104, 105–112. [Google Scholar] [CrossRef]
- Solianik, R.; Brazaitis, M.; Cekanauskaite-Krusnauskiene, A. Tai chi effects on balance in older adults: The role of sustained attention and myokines. J. Sports Med. Phys. Fitness 2022, 62, 1512–1518. [Google Scholar] [CrossRef]
- Kujawski, S.; Kujawska, A.; Kozakiewicz, M.; Jakovljevic, D.G.; Stankiewicz, B.; Newton, J.L.; Kędziora-Kornatowska, K.; Zalewski, P. Effects of Sitting Callisthenic Balance and Resistance Exercise Programs on Cognitive Function in Older Participants. Int. J. Environ. Res. Public Health 2022, 19, 14925. [Google Scholar] [CrossRef] [PubMed]
- Pazokian, F.; Amani-Shalamzari, S.; Rajabi, H. Effects of functional training with blood occlusion on the irisin, follistatin, and myostatin myokines in elderly men. Eur. Rev. Aging Phys. Act. 2022, 19, 22. [Google Scholar] [CrossRef]
- Rioux, B.V.; Brunt, K.R.; Eadie, A.L.; Bouchard, D.R.; Fox, J.; Senechal, M. Impact of acute circuit training on irisin in younger and older overweight adults. Appl. Physiol. Nutr. Metab. 2021, 46, 1248–1256. [Google Scholar] [CrossRef] [PubMed]
- Bizjak, D.A.; Zügel, M.; Schumann, U.; Tully, M.A.; Dallmeier, D.; Denkinger, M.; Steinacker, J.M. Do skeletal muscle composition and gene expression as well as acute exercise-induced serum adaptations in older adults depend on fitness status? BMC Geriatr. 2021, 21, 697. [Google Scholar] [CrossRef] [PubMed]
- Planella-Farrugia, C.; Comas, F.; Sabater-Masdeu, M.; Moreno, M.; Moreno-Navarrete, J.M.; Rovira, O.; Ricart, W.; Fernández-Real, J.M. Circulating Irisin and Myostatin as Markers of Muscle Strength and Physical Condition in Elderly Subjects. Front. Physiol. 2019, 10, 871. [Google Scholar] [CrossRef]
- Sanchis-Gomar, F.; Garatachea, N.; He, Z.H.; Pareja-Galeano, H.; Fuku, N.; Tian, Y.; Arai, Y.; Abe, Y.; Murakami, H.; Miyachi, M.; et al. FNDC5 (irisin) gene and exceptional longevity: A functional replication study with rs16835198 and rs726344 SNPs. Age 2014, 36, 9733. [Google Scholar] [CrossRef]
- Gmiat, A.; Mieszkowski, J.; Prusik, K.; Prusik, K.; Kortas, J.; Kochanowicz, A.; Radulska, A.; Lipiński, M.; Tomczyk, M.; Jaworska, J.; et al. Changes in pro-inflammatory markers and leucine concentrations in response to Nordic Walking training combined with vitamin D supplementation in elderly women. Biogerontology 2017, 18, 535–548. [Google Scholar] [CrossRef] [PubMed]
- Miyamoto-Mikami, E.; Sato, K.; Kurihara, T.; Hasegawa, N.; Fujie, S.; Fujita, S.; Sanada, K.; Hamaoka, T.; Tabata, I.; Iemitsu, M. Endurance training-induced increase in circulating irisin levels is associated with reduction of abdominal visceral fat in middle-aged and older adults. PLoS ONE 2015, 10, e0120354. [Google Scholar] [CrossRef] [PubMed]
- Prestes, J.; da Cunha Nascimento, D.; Tibana, R.A.; Teixeira, T.G.; Vieira, D.C.; Tajra, V.; de Farias, D.L.; Silva, A.O.; Funghetto, S.S.; de Souza, V.C.; et al. Understanding the individual responsiveness to resistance training periodization. Age 2015, 37, 9793. [Google Scholar] [CrossRef]
- Emanuele, E.; Minoretti, P.; Pareja-Galeano, H.; Sanchis-Gomar, F.; Garatachea, N.; Lucia, A. Serum irisin levels, precocious myocardial infarction, and healthy exceptional longevity. Am. J. Med. 2014, 127, 888–890. [Google Scholar] [CrossRef] [PubMed]
- Lima-Filho, R.A.S.; Benedet, A.L.; De Bastiani, M.A.; Povala, G.; Cozachenco, D.; Ferreira, S.T.; De Felice, F.G.; Rosa-Neto, P.; Zimmer, E.R.; Lourenco, M.V. Association of the fibronectin type III domain-containing protein 5 rs1746661 single nucleotide polymorphism with reduced brain glucose metabolism in elderly humans. Brain Commun. 2023, 5, fcad216. [Google Scholar] [CrossRef] [PubMed]
- Mucher, P.; Batmyagmar, D.; Perkmann, T.; Repl, M.; Radakovics, A.; Ponocny-Seliger, E.; Lukas, I.; Fritzer-Szekeres, M.; Lehrner, J.; Knogler, T.; et al. Basal myokine levels are associated with quality of life and depressed mood in older adults. Psychophysiology 2021, 58, e13799. [Google Scholar] [CrossRef] [PubMed]
- Conti, E.; Grana, D.; Stefanoni, G.; Corsini, A.; Botta, M.; Magni, P.; Aliprandi, A.; Lunetta, C.; Appollonio, I.; Ferrarese, C.; et al. Irisin and BDNF serum levels and behavioral disturbances in Alzheimer’s disease. Neurol. Sci. 2019, 40, 1145–1150. [Google Scholar] [CrossRef]
- Damirchi, A.; Hosseini, F.; Babaei, P. Mental Training Enhances Cognitive Function and BDNF More Than Either Physical or Combined Training in Elderly Women With MCI: A Small-Scale Study. Am. J. Alzheimer’s Dis. Other Dementias 2018, 33, 20–29. [Google Scholar] [CrossRef] [PubMed]
- Küster, O.; Laptinskaya, D.; Fissler, P.; Schnack, C.; Zügel, M.; Nold, V.; Thurm, F.; Pleiner, S.; Karabatsiakis, A.; von Einem, B.; et al. Novel Blood-Based Biomarkers of Cognition, Stress, and Physical or Cognitive Training in Older Adults at Risk of Dementia: Preliminary Evidence for a Role of BDNF, Irisin, and the Kynurenine Pathway. J. Alzheimer’s Dis. 2017, 59, 1097–1111. [Google Scholar] [CrossRef] [PubMed]
- Lage, V.; de Paula, F.A.; Lima, L.P.; Santos, J.N.V.; Dos Santos, J.M.; Viegas, A.A.; da Silva, G.P.; de Almeida, H.C.; Rodrigues, A.L.; Leopoldino, A.; et al. Plasma levels of myokines and inflammatory markers are related with functional and respiratory performance in older adults with COPD and sarcopenia. Exp. Gerontol. 2022, 164, 111834. [Google Scholar] [CrossRef] [PubMed]
- Sugiyama, Y.; Asai, K.; Yamada, K.; Kureya, Y.; Ijiri, N.; Watanabe, T.; Kanazawa, H.; Hirata, K. Decreased levels of irisin, a skeletal muscle cell-derived myokine, are related to emphysema associated with chronic obstructive pulmonary disease. Int. J. Chronic Obstr. Pulm. Dis. 2017, 12, 765–772. [Google Scholar] [CrossRef] [PubMed]
- Kureya, Y.; Kanazawa, H.; Ijiri, N.; Tochino, Y.; Watanabe, T.; Asai, K.; Hirata, K. Down-Regulation of Soluble α-Klotho is Associated with Reduction in Serum Irisin Levels in Chronic Obstructive Pulmonary Disease. Lung 2016, 194, 345–351. [Google Scholar] [CrossRef]
- Ijiri, N.; Kanazawa, H.; Asai, K.; Watanabe, T.; Hirata, K. Irisin, a newly discovered myokine, is a novel biomarker associated with physical activity in patients with chronic obstructive pulmonary disease. Respirology 2015, 20, 612–617. [Google Scholar] [CrossRef]
- Yan, J.; Liu, H.J.; Guo, W.C.; Yang, J. Low serum concentrations of Irisin are associated with increased risk of hip fracture in Chinese older women. Jt. Bone Spine 2018, 85, 353–358. [Google Scholar] [CrossRef]
- Ruan, Q.; Zhang, L.; Ruan, J.; Zhang, X.; Chen, J.; Ma, C.; Yu, Z. Detection and quantitation of irisin in human cerebrospinal fluid by tandem mass spectrometry. Peptides 2018, 103, 60–64. [Google Scholar] [CrossRef] [PubMed]
- Weber-Rajek, M.; Radziminska, A.; Straczynska, A.; Strojek, K.; Piekorz, Z.; Kozakiewicz, M.; Styczyńska, H. A Randomized-Controlled Trial Pilot Study Examining the Effect of Pelvic Floor Muscle Training on the Irisin Concentration in Overweight or Obese Elderly Women with Stress Urinary Incontinence. Biomed Res. Int. 2019, 2019, 7356187. [Google Scholar] [CrossRef]
- Sahin-Efe, A.; Upadhyay, J.; Ko, B.J.; Dincer, F.; Park, K.H.; Migdal, A.; Vokonas, P.; Mantzoros, C. Irisin and leptin concentrations in relation to obesity and developing type 2 diabetes: A cross sectional and a prospective case-control study nested in the Normative Aging Study. Metabolism 2018, 79, 24–32. [Google Scholar] [CrossRef]
- Tibana, R.A.; da Cunha Nascimento, D.; Frade de Souza, N.M.; de Souza, V.C.; de Sousa Neto, I.V.; Voltarelli, F.A.; Pereira, G.B.; Navalta, J.W.; Prestes, J. Irisin Levels Are not Associated to Resistance Training-Induced Alterations in Body Mass Composition in Older Untrained Women with and without Obesity. J. Nutr. Health Aging 2017, 21, 241–246. [Google Scholar] [CrossRef]
- Alsaawi, T.A.; Aldisi, D.; Abulmeaty, M.M.A.; Khattak, M.N.K.; Alnaami, A.M.; Sabico, S.; Al-Daghri, N.M. Screening for Sarcopenia among Elderly Arab Females: Influence of Body Composition, Lifestyle, Irisin, and Vitamin, D. Nutrients 2022, 14, 1855. [Google Scholar] [CrossRef] [PubMed]
- Baek, J.Y.; Jang, I.Y.; Jung, H.W.; Park, S.J.; Lee, J.Y.; Choi, E.; Lee, Y.S.; Lee, E.; Kim, B.J. Serum irisin level is independent of sarcopenia and related muscle parameters in older adults. Exp. Gerontol. 2022, 162, 111744. [Google Scholar] [CrossRef]
- Tsai, J.S.; Wang, S.Y.; Chang, C.H.; Chen, C.Y.; Wen, C.J.; Chen, G.Y.; Kuo, C.H.; Tseng, Y.J.; Chen, C.Y. Identification of traumatic acid as a potential plasma biomarker for sarcopenia using a metabolomics-based approach. J. Cachexia Sarcopenia Muscle 2022, 13, 276–286. [Google Scholar] [CrossRef]
- Park, H.S.; Kim, H.C.; Zhang, D.; Yeom, H.; Lim, S.K. The novel myokine irisin: Clinical implications and potential role as a biomarker for sarcopenia in postmenopausal women. Endocrine 2019, 64, 341–348. [Google Scholar] [CrossRef] [PubMed]
- Bosanac, J.; Straus, L.; Novakovic, M.; Kosuta, D.; Bozic Mijovski, M.; Tasic, J.; Jug, B. HFpEF and Atrial Fibrillation: The Enigmatic Interplay of Dysmetabolism, Biomarkers, and Vascular Endothelial Dysfunction. Dis. Markers 2022, 2022, 9539676. [Google Scholar] [CrossRef] [PubMed]
- Tu, W.J.; Qiu, H.C.; Cao, J.L.; Liu, Q.; Zeng, X.W.; Zhao, J.Z. Decreased Concentration of Irisin Is Associated with Poor Functional Outcome in Ischemic Stroke. Neurotherapeutics 2018, 15, 1158–1167. [Google Scholar] [CrossRef]
- Zhu, H.; Liu, M.; Zhang, N.; Pan, H.; Lin, G.; Li, N.; Wang, L.; Yang, H.; Yan, K.; Gong, F. Serum and Adipose Tissue mRNA Levels of ATF3 and FNDC5/Irisin in Colorectal Cancer Patients With or Without Obesity. Front. Physiol. 2018, 9, 1125. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Kim, D.Y. Aquarobic exercises improve the serum blood irisin and brain-derived neurotrophic factor levels in elderly women. Exp. Gerontol. 2018, 104, 60–65. [Google Scholar] [CrossRef]
- Staiger, H.; Bohm, A.; Scheler, M.; Berti, L.; Machann, J.; Schick, F.; Machicao, F.; Fritsche, A.; Stefan, N.; Weigert, C.; et al. Common genetic variation in the human FNDC5 locus, encoding the novel muscle-derived ‘browning’ factor irisin, determines insulin sensitivity. PLoS ONE 2013, 8, e61903. [Google Scholar] [CrossRef]
- Miranda, M.; Morici, J.F.; Zanoni, M.B.; Bekinschtein, P. Brain-Derived Neurotrophic Factor: A Key Molecule for Memory in the Healthy and the Pathological Brain. Front. Cell Neurosci. 2019, 13, 363. [Google Scholar] [CrossRef] [PubMed]
- Papadopoulou, S.K. Sarcopenia: A Contemporary Health Problem among Older Adult Populations. Nutrients 2020, 12, 1293. [Google Scholar] [CrossRef]
- Coelho-Junior, H.J.; Picca, A.; Calvani, R.; Marzetti, E. Prescription of resistance training for sarcopenic older adults: Does it require specific attention? Ageing Res. Rev. 2022, 81, 101720. [Google Scholar] [CrossRef]
- Clark, D.; Nakamura, M.; Miclau, T.; Marcucio, R. Effects of Aging on Fracture Healing. Curr. Osteoporos. Rep. 2017, 15, 601–608. [Google Scholar] [CrossRef] [PubMed]
- Feske, S.K. Ischemic Stroke. Am. J. Med. 2021, 134, 1457–1464. [Google Scholar] [CrossRef]
- Zhao, R. Irisin at the crossroads of inter-organ communications: Challenge and implications. Front. Endocrinol. 2022, 13, 989135. [Google Scholar] [CrossRef]
- Guo, M.; Yao, J.; Li, J.; Zhang, J.; Wang, D.; Zuo, H.; Zhang, Y.; Xu, B.; Zhong, Y.; Shen, F.; et al. Irisin ameliorates age-associated sarcopenia and metabolic dysfunction. J. Cachexia Sarcopenia Muscle 2023, 14, 391–405. [Google Scholar] [CrossRef]
- Jia, J.; Yu, F.; Wei, W.P.; Yang, P.; Zhang, R.; Sheng, Y.; Shi, Y.Q. Relationship between circulating irisin levels and overweight/obesity: A meta-analysis. World J. Clin. Cases 2019, 7, 1444–1455. [Google Scholar] [CrossRef] [PubMed]
- Rana, K.S.; Arif, M.; Hill, E.J.; Aldred, S.; Nagel, D.A.; Nevill, A.; Randeva, H.S.; Bailey, C.J.; Bellary, S.; Brown, J.E. Plasma irisin levels predict telomere length in healthy adults. Age 2014, 36, 995–1001. [Google Scholar] [CrossRef] [PubMed]
- Khavinson, V.; Kuznik, B.I.; Tarnovskaya, S.I.; Lin’kova, N.S. Short Peptides and Telomere Length Regulator Hormone Irisin. Bull Exp. Biol. Med. 2016, 160, 347–349. [Google Scholar] [CrossRef] [PubMed]
- Mohammad Rahimi, G.R.; Hejazi, K.; Hofmeister, M. The effect of exercise interventions on Irisin level: A systematic review and meta-analysis of randomized controlled trials. EXCLI J. 2022, 21, 524–539. [Google Scholar] [PubMed]
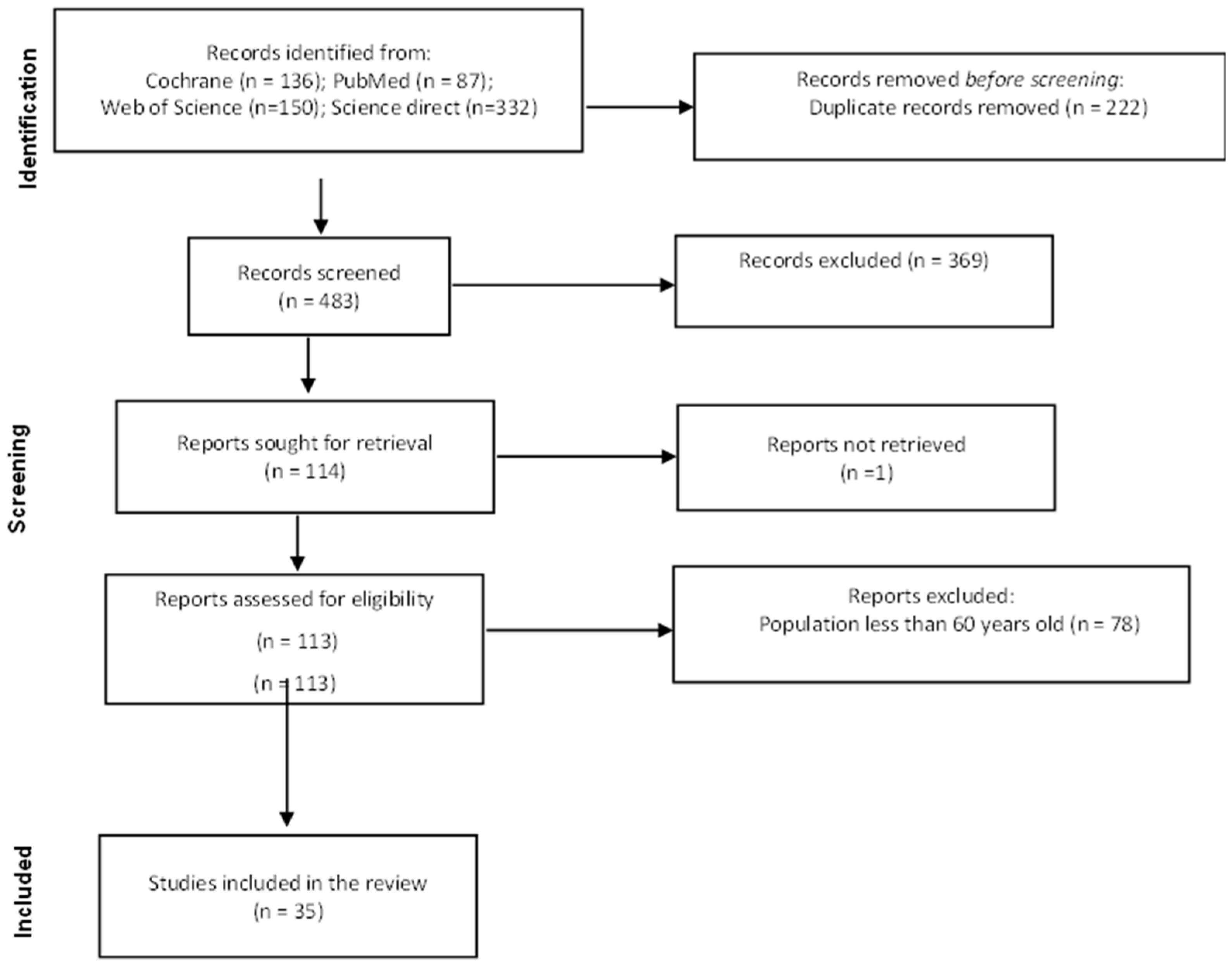

| Author, Year, Country | Study Design | Sample Size | Mean Age | Patients-Associated Condition | Irisin | Outcomes | ||
|---|---|---|---|---|---|---|---|---|
| Healthy older adults | ||||||||
| Rodziewicz-Flis et al., 2023 Poland [17] | Randomized control trial | 41 Balance training, n = 15 Dance training group, n = 14 Control group, n = 12 | 71.2 ± 5.5 | Disease-free | Balance training group: from 14.5 ± 3.5 to 16.4 ± 4.4 ng/mL; p = 0.029 Folk dance training group: from 15.6 ± 4.3 to 17.6 ± 4.5 ng/mL; p = 0.022 Control group: unchanged | Folk dance and balance training improved physical performance and blood pressure, accompanied by an increase in irisin levels. The folk dance training group had increased insulin sensitivity. | ||
| Gmiat et al., 2018 Poland [18] | Randomized control trial | 45 women Beginner group, n = 20 Advanced group, n = 25 | 68 ± 5.12 | Disease-free | Beginners: baseline 12 ± 5 observed change (−22 ± 72%) Advance: baseline 13 ± 8 observed change 4 ± 49% | No direct correlation was noted between vitamin D and cognitive function. The amelioration of cognitive functions may be explained by an increase in irisin and an elevated uptake of tryptophan. | ||
| Solianik, et al., 2022 Lithuania [19] | Randomized control study | 30 (women) Tai chi, n = 15 Control; n = 15 | 60–79 years | Disease-free | - | Tai Chi increased irisin levels (p < 0.001). | ||
| Kujawski et al., 2022 Poland [20] | Two-arm single-blind randomized control trial | 69 Sitting callisthenic, n = 31 Resistance training, n = 38 | 64.6 ± 4 (Resistance training) 67.7 ± 6 (Sitting callisthenic balance) | Disease-free | Resistance group (irisin μg/mL) Before: 19.54 ± 3.3, after 20.46 ± 4 Sitting callisitic group Before: 18.66 ± 4.3; after: 17.63 ± 5.2 | Changes in irisin were related to set-shifting and short-term memory. | ||
| Pazokian, et al., 2022 Iran [21] | Randomized trial | 30 (men) Functional training with blood flow restriction, n = 10 Functional training, n = 10 Control group, n = 10 | 67.7 ± 5.8 | Disease-free | No difference in the level of irisin between the groups ((F = 0.6, p = 0.561, η2 = 0.04) | No changes in irisin serum levels. | ||
| Rioux et al., 2021 Canada [22] | Quasi-experimental randomized trial | 26 Older adults, n =13 Younger adults, n = 13 | 68.00 (64.50–69.50) (Older adults) 24.00 (22.00–30.50) (Younger adults) | Disease-free | No changes | Circuit training did not increase irisin levels. | ||
| Bizjak et al., 2021 Germany Iran [23] | Clinical trial (pilot study) | 28 Low physical fitness, n =14 High physical fitness, n = 14 | 75.25 ± 5.44 | Disease-free | High physical fitness participants had a higher basal level of irisin than low physical fitness participants (p = 0.0195) | Higher basal irisin serum levels in the high physical fitness group revealed slightly beneficial molecular serum and muscle adaptations. | ||
| Planella-Farrugia et al., 2019 [24] | Prospective and controlled clinical trial | 34 Control group, n = 20 Resistance exercise group, n = 14 Resistance exercise + nutritional support group, n = 9 | 66.4 ± 4.6 (Control) 64.9 ± 5.5 (Resistance exercise) 71.2 ± 3.3 (Resistance exercise + nutritional support group) | Disease-free | Resistance exercise + nutritional support Baseline: 3 ± 1.1 Follow-up: 2.6 ± 1.3, p = 0.030) Resistance training Baseline: 3.1 ± 0.8 Follow-up 2.4 ± 0.3, p = 0.011 Control Baseline: 3.1 ± 0.9 Follow-up: 3.5 ± 1.1 | Circulating irisin constitutes a marker for improved muscular performance in older adults. | ||
| Sanchis-Gomar et al., 2014 Spain [25] | Cohort | 2158 | 100–104 (Italian) 100–116 (Japanese) 100–111 (Spanish) | Disease-free | Genotype frequencies between centenarians and controls Spanish cohort | No differences between genotype/allele frequencies of the two SNPs associated with in vivo insulin sensitivity in centenarians versus controls. | ||
| rs726344 χ2 = 2.821, p = 0.244; rs16835198 χ2 = 1.540, p = 0.463 | Italian Cohort rs726344 χ2 = 0.122, p = 0.941; rs16835198 χ2 = 1.128, p = 0.569 | Japanese cohort rs726344- is not present in the cohort rs16835198 χ2 = 5.337, p < 0.069) | ||||||
| Kim and Kim, 2018 South Korea [24] | - | 26 women Control group, n = 12 Aquarobic exercise group, n = 14 | 71.43 ± 4.45 (Control) 71.77 ± 3.07 (Aquarobic exercise) | Disease-free | Control group Pre: −165.76 ± 12.53; Post: 157.14 ± 13.97 Aquarobic exercise group: pre: −174.85 ± 11.6; post: 203.62 ± 16.44 | Aquarobic exercises increase the serum irisin and BDNF levels. | ||
| Gmiat et al., 2017 Poland [26] | - | 27 | 67 ± 8 | Disease-free Nordic walking training | Patients with less than 20 ng/mL of vitamin D: Baseline: 11 ± 3 observed changed −23 ± 60% Patients with more than 20 ng/mL of vitamin D Baseline: 10 ± 3 observed changed 5 ± 55 | Nordic walking training irisin and improves the uptake of leucine among women with higher baseline vitamin D. | ||
| Miyamoto-Mikami et al., 2015 Japan [27] | - | 53 Healthy young adults, n = 25 Middle-aged/aged older adults, n = 28 | 69 ± 6 (Control) 65 ± 8 (Training group) | Disease-free | Control Pre: 142.8 ± 8.7 Post: 144.4 ± 9.2 Training Pre: 140.6 ± 26.7 Post: 140.6 ± 26.7 | Secreted irisin may have a role in the exercise-induced alteration of abdominal visceral fat in middle-aged and older adults. | ||
| Prestes et al., 2015 Brazil [28] | - | 72 (women) | 66.90 ± 7.56 (Control) 66.20 ± 6.05 (Linear Periodization group) 65.52 ± 4.72 (Undulating periodization group) | Disease-free | Control: 169.62 ± 36.55 Linear periodization group 230.00 ± 55.88 Undulating periodization group: 202.10 ± 52.30 | Although resistance training did not induce a significant effect on body composition and cytokines, the authors identified a group of people that have an increment > 80th percentile (>14.12%) of irisin, suggesting that not all people respond in the same way to physical activity. | ||
| Emanuele et al., 2014 [29] | - | Centenarians, n = 79 Patients with precocious acute myocardial infarction, n = 178 Young controls, n = 180 | 100–104 (Centenarians) 28–39 (Patients with precocious acute myocardial infarction) 27–39 (Young controls) | Disease-free | Centenarians: 35.3 ± 5.5 Patients with precocious acute myocardial infarction: 15.1 ± 5.4 Young controls: 20.7 ± 6.3 | Serum irisin is highest in disease-free centenarians compared with young, healthy controls and young patients with myocardial infarction. | ||
| Brain diseases | ||||||||
| Lima-Filho et al., 2023 Brazil [30] | Cross-sectional | 725 Cognitive unimpaired, n =240 Cognitive impairment, n = 485 | 73.8 ± 7.37 | Cognitive impairment | - | Patients carrying the FNDC5 rs1746661(T) allele presented hypometabolism in Memory-linked brain regions and increased brain amyloid-β PET load. | ||
| Mucher et al., 2021 Austria [31] | Cross-sectional study | 112 Athletes, n = 56 Controls, n =58 | 66 (62–68) (Athletes) 66 (63–69) (Controls) | Depression | Irisin [z-score] Athletes: −0.13 [−0.86–0.33] Control: −0.02 [−0.63–0.65] Differences: U = 1359.0; p = 0.225 | Circulating irisin and the multifunctional cytokine/myokine IL-6 are associated with depressive symptoms among older adults. | ||
| Conti et al., 2019 Italy [32] | Cross-sectional study | 60 Alzheimer’s disease, n = 40 Control, n = 20 | 77.6 ± 5.6 (Alzheimer’s disease) 78.7 ± 5.7 (Control) | Alzheimer’s disease | Irisin serum levels were elevated in A/A+ patients (+10.0%; p < 0.05) | Irisin is not useful as a surrogate marker for agitation in AD but might represent secondary outcomes when testing drugs for behavioral dysfunction, implying elevated motor activity. | ||
| Damirchi, Hosseini, and Babaei, 2018 Iran [33] | Randomized control trial (small scale study) | 54 (women) Control group, n = 9 Mental training, n = 15 Physical training, n = 15 Mix training, n = 15 | 60–85 69.11 ± 4.69 (Control) 67.9 ± 3.75 (Mental training) 68.81± 3.68 (Physical training) 67.76 ± 4.69 (Mix training) | Mild cognitive impairment | Irisin concentration (ng/mL) Control: baseline 13.67 ± 5.23; post-intervention: 12.87 ± 4.95 Physical training: baseline 11.23 ± 2.77 post-intervention: 11.47 ± 3.08 Mental exercise: baseline 10.57 ± 1.99 post-intervention: 9.92 ± 1.66 Physical training + mental exercise; baseline 10.38 ± 1.03 | The authors did not observe changes in irisin levels, contradicting a previous study performed by them. | ||
| Küster et al., 2017 Germany [34] | Clinical trial | 47 | 71.2 (60–88) | Dementia | Irisin (M) Cognitive training: Baseline 55.2 ± 9.9 Physical training group: Baseline 57.9 ± 10.6 Waitlist group Baseline 56.4 ± 14.1 | Irisin and BDNF correlated positively with cognitive function. | ||
| COPD | ||||||||
| Lage et al., 2022 Brazil [35] | Cross-sectional study | 86 COPD, n = 43 No COPD, n = 43 | 73.9 (COPD) 72.7 (No COPD) | COPD and sarcopenia | No COPD 1062.8 pg/mL (909.6–1216.2) COPD 904.6 pg/mL (794.3–1014.8) | Plasma irisin levels and inflammation are decreased in older adults with COPD and sarcopenia. | ||
| Sugiyama et al., 2017 Japan [36] | - | 40 | 73 ± 9.3 | COPD | - | Decreased serum irisin levels are related to emphysema in patients with COPD and are involved in epithelial apoptosis, resulting in emphysema. | ||
| Kureya et al., 2016 [37] | - | 53 Smokers with COPD, n = 24 Smokers without COPD, n = 13 Non-smokers, n = 16 | 71 (62–78) (Non-smokers) 66 (62–71) (Smokers without COPD) 70 (65–74) (Smokers with COPD) | COPD | smokers with COPD patients: 26.3 (22.6–32.4) ng/mL; smokers without COPD: 53.7 (46.7–62.8) ng/mL; non-smokers: 58.5 (42.8–78.9) ng/mL | Soluble a-klotho is one possible factor involved in reduced irisin release from skeletal muscle. The disruption of irisin leads to abnormal energy homeostasis in COPD. | ||
| Ijiri et al., 2015 Japan [38] | - | 99 Control, n = 27 COPD, n = 72 | 70 (62–75) (Control) 70 (66–74) (COPD) | COPD | COPD patients: 31.6 (22.7–40.4) ng/mL; control subjects: 50.7 (39.3–65.8) ng/mL; p < 0.001 | Serum irisin level may prove to be a valuable biomarker in clinical follow-up of COPD. | ||
| Fractures | ||||||||
| Yan et al., 2018 China [39] | Cross-sectional, case-control study | 160 women | 70–90 | Hip fracture | Cases (361.5 ± 140.0 ng/mL vs. control 478.5 ± 159.6 ng/mL, p < 0.001) | Low concentrations of irisin are associated with an increased risk of hip fractures. | ||
| Ruan et al., 2018 [40] | - | 6 | Over 80 | Osteoporotic fracture or oblique inguinal hernia | 0.20–186 ng/mL (cSf) | Glycosylated form of irisin is present in human cerebrospinal fluid. Irisin was not detected in plasma samples by using mass spectrometry. | ||
| Obesity | ||||||||
| Weber-Rajek et al., 2019 Poland [41] | Randomized control trial | 49 (women) | 67.00 ±6 (Control) 62.50 ± 2.0 (Experimental group) | Obesity | experimental group: 9.02 ± 2.67 control group: 5.91 ± 1.77 | The authors observed a weak positive correlation between irisin and body mass index, however without statistical significance. | ||
| Sahin-Efe et al., 2018 USA [42] | Cross-sectional and a prospective case-control study | 216 | 69.5 ± 9.2 (Non-obese normal fasting glucose) 66.9 ± 7.9 (Non-obese with impaired fasting glucose) 69.4 ± 8.6 (Obese with normal fasting glucose) 67.7 ± 7.4 (Obese with impaired fasting glucose) | Obesity | Non-obese normal fasting glucose: 123.6 ± 12.1 Non-obese with impaired fasting glucose: 124.8 ± 16.8 Obese with normal fasting glucose: 147.0 ± 16.2 Obese with impaired fasting glucose: 172.5 ± 13.0 | Obese individuals with impaired fasting glucose have higher circulating irisin concentrations than non-obese subjects with normal glucose tolerance. Irisin concentrations do not predict the risk of developing diabetes prospectively. | ||
| Tibana et al., 2017 Brazil [43] | - | 49 (women) Non-obese, n =23 Obese, n = 26 | 68.0 ± 6.2 (Non-obese) 66.5 ± 5.0 (Obese) | Obesity | Baseline Irisin concentration was 214.7 ± 53.2 ng Post-intervention mL for the non-obese and 225.0 ± 54.6 ng/mL for the obese group (184.1 ± 72.5 ng/mL; p = 0.011; 1 –ß = 0.95) with no change for the obese group (228.2 ± 59.5 ng/mL; p = 0.79) | No changes were observed in circulating irisin levels. | ||
| Sarcopenia | ||||||||
| Alsaawi et al., 2022 Saudi Arabia [44] | Cross-sectional study | 131 (Women) Sarcopenia, n =26 No sarcopenia n = 131 | 65.9 ± 5.5 | Sarcopenia | No sarcopenia: 180.8 ± 44.3 ng/L Sarcopenia: 145.8 ± 11.6 ng/L; p = 0.001 | Irisin was significantly lower in the sarcopenia group. No associations were found with physical activity or dietary and lifestyle habits. | ||
| Baek et al., 2022 Republic of Korea [45] | Cross-sectional study | 143 Sarcopenia, n =23 | 69.5 ± 6.16 | Sarcopenia | Mean concentration 6.02 ± 1.46 ng/mL | Low irisin was associated with sarcopenia (OR = 0.97; 95% CI, 0.95–0.99; p = 0.002). No association was found between serum irisin levels and clinical muscle parameters. | ||
| Tsai et al., 2022 Turkey [46] | Not reported | 72 No sarcopenia in older adults, n = 24 Sarcopenia in older adults, n = 24 Younger adults, n = 24 | 79.0 ± 5.9 (Older without sarcopenia) 79.4 ± 6.2 (Older adults with sarcopenia) | Sarcopenia | No sarcopenia 2.8 ng/mL (2.5, 3.2) Sarcopenia 3.1 ng/mL (2.2, 3.3) | No changes in irisin serum levels. | ||
| Park et al., 2019 Republic of Korea [47] | 153 (women) | 72.20 ± 5.96 | Sarcopenia | Irisin was associated with sarcopenia (odds-ratio = 1.95, 95% confidence interval 1.33–2.87, p-value = 0.001) | In postmenopausal women, serum irisin may be used as a biomarker for sarcopenia. | |||
| Vascular disorders | ||||||||
| Bosanac et al., 2022 Slovenia [48] | Cross-sectional study | 52 | 80.6 ± 6.6 | Cardiovascular diseases | All (7.7) (3.5–19.5) | HFpEF with AF 4.8 (2.6–12.7) | HFpEF with AF 13.5 (7.1–31.5) | HFpEF and AF groups have significantly lower irisin levels compared to patients with HFpEF but without AF. |
| Tu et al., 2018 China [49] | Cross-sectional study | 1530 | 66 (57–77) | Ischemic stroke | quartile 1 (<67.1 ng/mL), quartile 2 (67.1–87.8 ng/mL), quartile 3 (87.9–136.4 ng/mL), and quartile 4 (>136.4 ng/mL) | Irisin can be useful in predicting poor functional outcomes in ischemic patients. | ||
| Others | ||||||||
| Zhu et al., 2018 [50] | - | Control, n = 40 Cancer, n = 76 | 61.0 (59.0–66.0) (Control) 68.0 (62.0–76.0) (Cancer) | Colorectal cancer | Patient with colorectal cancer and normal weight: 0.17 ± 0.01 control 0.22 ± 0.01 μg/mL, p < 0.05 | Individuals with high activating transcription factor 3 (ATF3) and low irisin levels were more likely to have colorectal cancer. | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Plácido, A.I.; Azevedo, D.; Herdeiro, M.T.; Morgado, M.; Roque, F. Understanding the Role of Irisin in Longevity and Aging: A Narrative Review. Epidemiologia 2025, 6, 1. https://doi.org/10.3390/epidemiologia6010001
Plácido AI, Azevedo D, Herdeiro MT, Morgado M, Roque F. Understanding the Role of Irisin in Longevity and Aging: A Narrative Review. Epidemiologia. 2025; 6(1):1. https://doi.org/10.3390/epidemiologia6010001
Chicago/Turabian StylePlácido, Ana I., Daniela Azevedo, Maria Teresa Herdeiro, Manuel Morgado, and Fátima Roque. 2025. "Understanding the Role of Irisin in Longevity and Aging: A Narrative Review" Epidemiologia 6, no. 1: 1. https://doi.org/10.3390/epidemiologia6010001
APA StylePlácido, A. I., Azevedo, D., Herdeiro, M. T., Morgado, M., & Roque, F. (2025). Understanding the Role of Irisin in Longevity and Aging: A Narrative Review. Epidemiologia, 6(1), 1. https://doi.org/10.3390/epidemiologia6010001









