Epidemiology of COVID-19 among Children and Adolescents in Sudan 2020–2021
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design Setting and Participants
2.2. Sample Size and Sampling Considerations
2.3. Study Variables and Data Collection
2.4. Data Management and Statistical Analysis
3. Results
3.1. Demographic and Clinical Characteristics of Study Participants
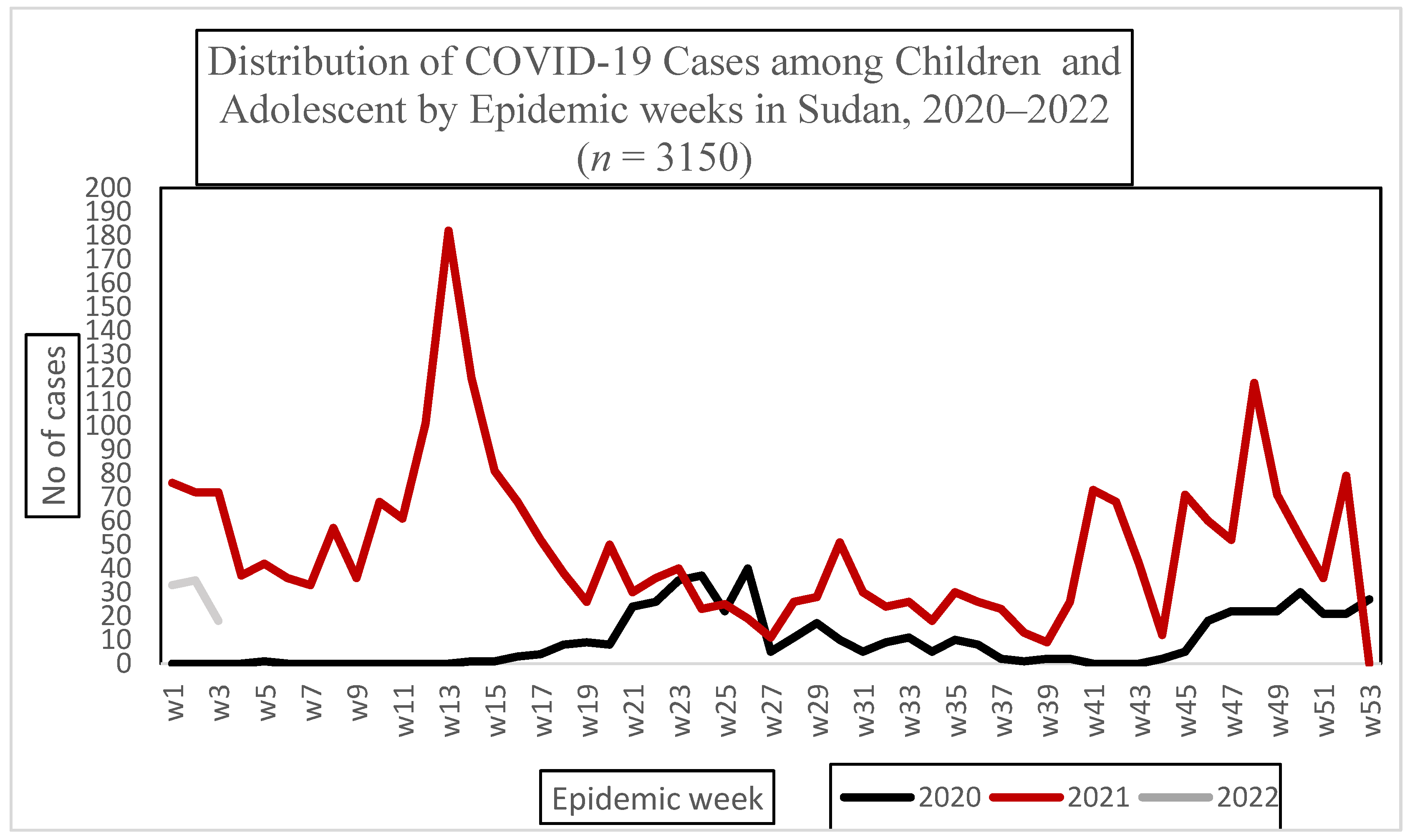
3.2. Descriptive Statistics
3.3. Factors Associated with COVID-19 Infection among Children and Adolescents in Sudan 2021
4. Discussion
Limitations of the Study
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhu, N.; Zhang, D.; Wang, W.; Li, X.; Yang, B.; Song, J. A Novel Coronavirus from Patients with Pneumonia in China, 2019. N. Engl. J. Med. 2020, 382, 727–733. [Google Scholar] [CrossRef] [PubMed]
- Cucinotta, D.; Vanelli, M. WHO Declares COVID-19 a Pandemic. Acta Bio Med. Atenei Parm. 2020, 91, 157. [Google Scholar]
- World Health Organization. COVID-19 Disease in Children and Adolescents. Sci. Brief 2021, 1–10. Available online: https://www.who.int/publications/i/item/WHO-2019-nCoV-Sci_Brief-Children_and_adolescents-2021.1 (accessed on 22 February 2023).
- Nikolopoulou, G.B.; Maltezou, H.C. COVID-19 in Children: Where do we Stand? Arch. Med. Res. 2022, 53, 1–8. [Google Scholar] [CrossRef]
- Khemiri, H.; Ayouni, K.; Triki, H.; Haddad-Boubaker, S. SARS-CoV-2 infection in pediatric population before and during the Delta (B.1.617.2) and Omicron (B.1.1.529) variants era. Virol. J. 2022, 19, 144. [Google Scholar] [CrossRef]
- Naja, M.; Wedderburn, L.; Ciurtin, C. COVID-19 infection in children and adolescents. Br. J. Hosp. Med. 2020, 81, 1–10. [Google Scholar] [CrossRef]
- Felsenstein, S.; Hedrich, C.M. SARS-CoV-2 infections in children and young people. Clin. Immunol. 2020, 220, 108588. [Google Scholar] [CrossRef]
- Ludvigsson, J.F. Systematic review of COVID-19 in children shows milder cases and a better prognosis than adults. Acta Paediatr. 2020, 109, 1088–1095. [Google Scholar] [CrossRef]
- Galindo, R.; Chow, H.; Rongkavilit, C. COVID-19 in Children: Clinical Manifestations and Pharmacologic Interventions Including Vaccine Trials. Pediatr. Clin. N. Am. 2021, 68, 961–976. [Google Scholar] [CrossRef]
- Bahl, A.; Mielke, N.; Johnson, S.; Desai, A.; Qu, L. Severe COVID-19 outcomes in pediatrics: An observational cohort analysis comparing Alpha, Delta, and Omicron variants. Lancet Reg. Health -Am. 2023, 18, 100405. [Google Scholar] [CrossRef]
- Aini, S.H.S.; Ingh, M.E.S. Multisystem Inflammatory Syndrome Associated With COVID-19 in Children (MIS-C): A Systematic Review of Studies From India. Indian Pediatr. 2022, 2, 563–569. [Google Scholar] [CrossRef]
- Patel, P.A.; Chandrakasan, S.; Mickells, G.E.; Yildirim, I.; Kao, C.M.; Bennett, C.M. Severe pediatric COVID-19 presenting with respiratory failure and severe thrombocytopenia. Pediatrics 2020, 146, e20201437. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. A Clinical Case Definition for Post COVID-19 Condition in Children and Adolescents. 2023. Available online: https://www.who.int/publications/i/item/WHO-2019-nCoV-Post-COVID-19-condition-CA-Clinical-case-definition-2023-1 (accessed on 26 April 2023).
- Liu, D.; Liu, W.; Rodriguez, M.; Zhang, J.; Zhang, F. The Mental Health Impacts of COVID-19 on Pediatric Patients Following Recovery. Front. Psychol. 2021, 12, 628707. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, G.K.; Elbeh, K.; Gomaa, H.M.; Soliman, S. Does COVID-19 infection have an impact on children’s psychological problems? Middle East Curr. Psychiatry 2021, 28, 77. [Google Scholar] [CrossRef]
- Fameen, R.; Pravin K., R.; S., P.; V., R.; Bhattarai, B.; B.P., A. Acquired childhood aphasia as a consequence of COVID-19 and its differential diagnosis from speech–language pathologist perspective: A case study. Clin. Case Rep. 2022, 10, e6587. [Google Scholar] [CrossRef]
- Gaythorpe, K.A.; Bhatia, S.; Mangal, T.; Unwin, H.J.T.; Imai, N.; Cuomo-Dannenburg, G. Children’s role in the COVID-19 pandemic: A systematic review of early surveillance data on susceptibility, severity, and transmissibility. Sci. Rep. 2021, 11, 1–14. [Google Scholar]
- Xu, W.; Li, X.; Dozier, M.; He, Y.; Kirolos, A.; Lang, Z.; Mathews, C.; Siegfried, N.; Theodoratou, E. What is the evidence for transmission of COVID-19 by children in schools? A living systematic review. J. Glob. Health 2020, 10, 021104. [Google Scholar] [CrossRef]
- Stein-Zamir, C.; Abramson, N.; Shoob, H.; Libal, E.; Bitan, M.; Cardash, T.; Cayam, R. A large COVID-19 outbreak in a high school 10 days after schools’ reopening, Israel, May 2020. Eurosurveillance 2020, 25, 2001352. [Google Scholar] [CrossRef]
- Aiano, F.; Mensah, A.A.; McOwat, K.; Obi, C.; Vusirikala, A.; Powell, A.A.; Flood, J.; Bosowski, J.; Letley, L.; Jones, S.; et al. COVID-19 outbreaks following full reopening of primary and secondary schools in England: Cross-sectional national surveillance, November 2020. Lancet Reg. Health-Eur. 2021, 6, 100120. [Google Scholar] [CrossRef]
- Kabbashi, M.; Khairy, A.; Mohamed, A.; Abuobaida, D.; Malik, E. The proportion and determinants of COVID-19 infection among medical doctors in Sudan, 2020: A cross-sectional survey. PLoS ONE 2022, 17, e0268037. [Google Scholar] [CrossRef]
- Khairy, A.; Mahgoub, E.A.A.; Nimir, M.; Ahmed, M.; Jubara, M.; Altayab, D.E. Acceptability of COVID-19 vaccination among health care workers in Sudan: A cross-sectional survey. East. Mediterr. Health J. 2023. Available online: https://www.emro.who.int/in-press/research/acceptability-of-covid-19-vaccination-among-health-care-workers-in-sudan-a-cross-sectional-survey.html (accessed on 26 April 2023).
- World Health Organization. Global COVID-19 Vaccination Strategy in a Changing World. July 2022. Available online: https://www.who.int/publications/m/item/global-covid-19-vaccination-strategy-in-a-changing-world--july-2022-update (accessed on 26 April 2023).
- World Health Organization. Age Group Codelist. World Health Organization. 2016. Available online: https://apps.who.int/gho/data/node.searo-metadata.AGEGROUP?lang=en (accessed on 26 April 2023).
- Wang, P.; Lu, J.; Jin, Y.; Zhu, M.; Wang, L.; Chen, S. Statistical and network analysis of 1212 COVID-19 patients in Henan, China. Int. J. Infect. Dis. 2020, 95, 391–398. [Google Scholar] [CrossRef] [PubMed]
- Adedeji, I.A.; Abdu, Y.M.; Bashir, M.F.; Adamu, A.S.; Gwarzo, G.D.; Yaro, B.S.; Musa, A.A.; Hassan, Z.I.; Maigoro, A.M. Profile of children with COVID-19 infection: A cross sectional study from north-east nigeria. Pan Afr. Med. J. 2020, 35 (Suppl. S2), 145. [Google Scholar] [CrossRef]
- Viner, R.M.; Mytton, O.T.; Bonell, C.; Melendez-Torres, G.J.; Ward, J.; Hudson, L.; Waddington, C.; Thomas, J.; Russell, S.; van der Klis, F.; et al. Susceptibility to SARS-CoV-2 Infection among Children and Adolescents Compared with Adults: A Systematic Review and Meta-Analysis. JAMA Pediatr. 2021, 175, 143–156. [Google Scholar] [CrossRef]
- Purwati, N.H.; Noprida, D.; Agustia, W.; Imroatun, T.; Sarini, S.; Sahariah, S.; Polapa, D. Impact of Age and Gender on the Incidence of COVID-19 in Children at Pasar Rebo Hospital, Jakarta. KnE Life Sci. 2022, 7, 460–466. [Google Scholar] [CrossRef]
- Mustafa, N.M.; Selim, L.A. Characterisation of COVID-19 Pandemic in Paediatric Age Group: A Systematic Review and Meta-Analysis. J. Clin. Virol. 2020, 128, 104395. [Google Scholar] [CrossRef]
- Ya’qoub, L.; Elgendy, I.Y.; Pepine, C.J. Sex and gender differences in COVID-19: More to be learned! Am. Heart J. Plus: Cardiol. Res. Pract. 2021, 3, 100011. [Google Scholar] [CrossRef]
- Kumar, L.; Kahlon, N.; Jain, A.; Kaur, J.; Singh, M.; Pandey, A.K. Loss of smell and taste in COVID-19 infection in adolescents. Int. J. Pediatr. Otorhinolaryngol. 2021, 142, 110626. [Google Scholar] [CrossRef]
- Tan, B.K.J.; Han, R.; Zhao, J.J.; Tan, N.K.W.; Quah, E.S.H.; Tan, C.J.W. Prognosis and persistence of smell and taste dysfunction in patients with COVID-19: Meta-analysis with parametric cure modelling of recovery curves. BMJ 2022, 378, e069503. [Google Scholar] [CrossRef]
| Background Characteristics | n | (%) | |
|---|---|---|---|
| Age | <1 year | 79 | 2.5 |
| 1–4 years | 88 | 2.8 | |
| 5–9 years | 278 | 8.8 | |
| 10–14 years | 593 | 18.8 | |
| 15 and over | 2112 | 67.1 | |
| Gender | Male | 1635 | 51.9 |
| Female | 1515 | 48.1 | |
| Result of PCR | Negative | 1809 | 57.4 |
| Positive | 1341 | 42.6 | |
| Present of symptoms | Symptomatic | 1385 | 44.0 |
| Asymptomatic | 1765 | 56.0 | |
| * Clinical Presentation | |||
| Fever | 866 | 27.5 | |
| Cough | 735 | 23.3 | |
| Shortness of breath | 393 | 12.5 | |
| Sore throat | 581 | 18.4 | |
| Headache | 658 | 20.9 | |
| Loss of smell and taste | 159 | 5.0 | |
| joint pain | 100 | 3.2 | |
| Muscle pain | 106 | 3.4 | |
| Back pain | 16 | 0.5 | |
| Fatigue | 130 | 4.1 | |
| Vomiting | 14 | 0.4 | |
| Diarrheal | 66 | 2.1 | |
| Nausea | 08 | 0.3 | |
| Unconsciousness | 04 | 0.1 | |
| Runny Nose | 104 | 3.3 | |
| loss of appetite | 05 | 0.2 |
| Factors Associated with Positive COVID-19 Test | Result | Chi-Squared | p-Value | ||
|---|---|---|---|---|---|
| Negative | Positive | ||||
| Age | <1 year | 66 (83.5%) | 13 (16.5%) | 32.688 | 0.001 |
| 1–4 years | 56 (63.6%) | 32 (36.4% | |||
| 5–9 years | 177 (63.6%) | 101 (36.4%) | |||
| 10–14 years | 346 (58.3%) | 247 (41.7%) | |||
| 15 and more | 1164 (55.1%) | 948 (44.9%) | |||
| Gender | Male | 951 (58.4%) | 677 (41.6%) | 1.886 | 0.170 |
| Female | 844 (56%) | 664 (44.0%) | |||
| Symptoms | Asymptomatic | 905 (51.2%) | 860 (48.8%) | 62.179 | 0.001 |
| Fever (yes) | 548 (63.2%) | 318 (36.8% | 16.724 | 0.001 | |
| Cough (yes) | 495 (67.3%) | 240 (32.7%) | 38.576 | 0.001 | |
| Shortness of breath (yes) | 261 (66.4%) | 132 (33.6%) | 14.823 | 0.001 | |
| Sore throat (yes) | 371 (63.8%) | 210 (36.2%) | 12.036 | 0.01 | |
| Headache (yes) | 441 (67%) | 217 (33%) | 31.306 | 0.001 | |
| Loss smell and taste (yes) | 72 (45.3%) | 87 (54.7%) | 10.104 | 0.001 | |
| Joint pain (yes) | 69 (69%) | 31 (31%) | 5.656 | 0.017 | |
| Muscle pain (yes) | 78 (73.5%) | 28 (26.5%) | 11.711 | 0.001 | |
| Back pain (yes) | 12 (75%) | 04 (25.0%) | 2.031 | 0.15 | |
| Fatigue (yes) | 79 (60.8%) | 51 (39.2%) | 0.619 | 0.431 | |
| Vomiting (yes) | 11 (78.6%) | 03 (21.4%) | 2.571 | 0.109 | |
| Diarrhea (yes) | 44 (66.7%) | 22 (33.3%) | 2.353 | 0.125 | |
| Nausea (yes) | 05 (62.5%) | 03 (37.5%) | 0.084 | 0.771 | |
| Unconsciousness (yes) | 02 (50%) | 02 (50%) | 0.090 | 0.764 | |
| Runny Nose (yes) | 80 (76.9%) | 24 (23.1%) | 16.718 | 0.001 | |
| Loss of appetite (yes) | 03 (60%) | 02 (40%) | 0.014 | 0.907 | |
| Outcome | Death | 06 (100%) | 0 (0%) | 8.109 | 0.004 |
| Factors Associated with Positive COVID-19 Test | OR | 95% C.I | P | |
| Age | <1 year | |||
| 1–4 years | 3.0 | (1.4–6.3) | 0.001 | |
| 5–9 years | 3.1 | (1.6–5.9) | 0.001 | |
| 10–14 years | 3.7 | (2.0–6.9) | 0.001 | |
| 15 and over | 3.4 | (1.9–6.4) | 0.001 | |
| Gender | Female | 1.3 | (1.1–1.5) | 0.01 |
| Male | ||||
| Symptoms | Fever | 1.0 | (0.7–1.2) | 0.72 |
| Cough | 0.8 | (0.6–1.0) | 0.04 | |
| Shortness of breath | 1.0 | (0.7–1.3) | 0.78 | |
| Sore throat | 1.0 | (0.8–1.3) | 0.91 | |
| Headache | 0.7 | (0.5–0.9) | 0.001 | |
| Loss of smell and taste | 2.1 | (1.4–3.3) | 0.001 | |
| Joint pain | 1.4 | (0.7–2.9) | 0.31 | |
| Muscle pain | 0.7 | (0.3–1.3) | 0.25 | |
| Back pain | 0.5 | (0.1–1.7) | 0.28 | |
| Fatigue | 1.3 | (0.8–2.0) | 0.32 | |
| Vomiting | 0.5 | (0.1–1.9) | 0.31 | |
| Diarrhea | 0.9 | (0.5–1.7) | 0.79 | |
| Nausea | 1.9 | (0.4–9.6) | 0.42 | |
| Unconsciousness | 1.3 | (0.2–9.6) | 0.77 | |
| Runny Nose | 0.6 | (0.3–0.9) | 0.02 | |
| Loss of appetite | 1.3 | (0.2–8.6) | 0.82 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khairy, A.; Elhussein, N.; Elbadri, O.; Mohamed, S.; Malik, E.M. Epidemiology of COVID-19 among Children and Adolescents in Sudan 2020–2021. Epidemiologia 2023, 4, 247-254. https://doi.org/10.3390/epidemiologia4030025
Khairy A, Elhussein N, Elbadri O, Mohamed S, Malik EM. Epidemiology of COVID-19 among Children and Adolescents in Sudan 2020–2021. Epidemiologia. 2023; 4(3):247-254. https://doi.org/10.3390/epidemiologia4030025
Chicago/Turabian StyleKhairy, Amna, Narmin Elhussein, Omer Elbadri, Sanad Mohamed, and Elfatih M. Malik. 2023. "Epidemiology of COVID-19 among Children and Adolescents in Sudan 2020–2021" Epidemiologia 4, no. 3: 247-254. https://doi.org/10.3390/epidemiologia4030025
APA StyleKhairy, A., Elhussein, N., Elbadri, O., Mohamed, S., & Malik, E. M. (2023). Epidemiology of COVID-19 among Children and Adolescents in Sudan 2020–2021. Epidemiologia, 4(3), 247-254. https://doi.org/10.3390/epidemiologia4030025






