Recent Research Process of Carbon Engineering on Na3V2(PO4)3 for Sodium-Ion Battery Cathodes: A Mini Review
Abstract
1. Introduction
2. The Structure and Properties of NVP
3. The Modification Methods to Improve the Conductivity of NVP
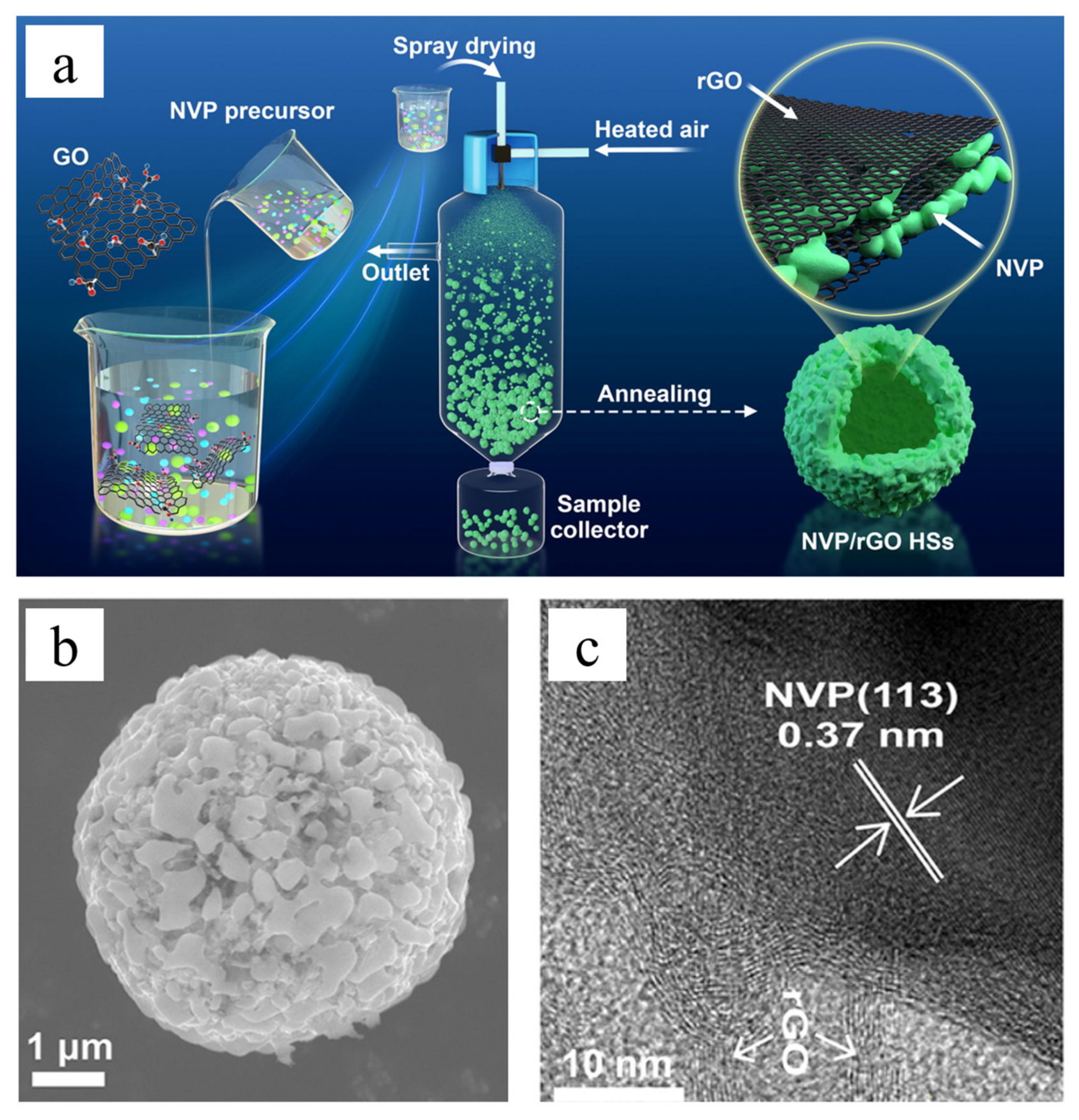
3.1. Graphene
3.2. Carbon Nanotubes
3.3. Other Carbon Coatings
3.4. Heteroatom Doping
4. Conclusions and Outlook
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- He, Z.; Jiang, Y.; Meng, W.; Zhu, J.; Liu, Y.; Dai, Y.; Wang, L.L. Advanced LiTi2(PO4)3@N-doped carbon anode for aqueous lithium ion batteries. Electrochim. Acta 2016, 222, 1491–1500. [Google Scholar] [CrossRef]
- Marom, R.; Amalraj, S.F.; Leifer, N.; Jacob, D.; Aurbach, D. A review of advanced and practical lithium battery materials. J. Mater. Chem. 2011, 21, 9938–9954. [Google Scholar] [CrossRef]
- Goodenough, J.B.; Park, K.S. The Li-ion rechargeable battery: A perspective. J. Am. Chem. Soc. 2013, 135, 1167–1176. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Chen, Y.; Luo, L.; Ming, X.; Li, J.; Lei, T.; Deng, S.; Guo, J.; Zhu, J.; Chang, S. Surface in-situ reconstruction of LiNi0.8Co0.1Mn0.1O2 cathode materials interacting with antimony compounds and the electrochemical performances. J. Electroanal. Chem. 2019, 854, 113582. [Google Scholar] [CrossRef]
- Cao, X.; Sun, Q.; Zhu, L.; Xie, L. Na3V2(PO4)3 nanoparticles confined in functional carbon framework towards high-rate and ultralong-life sodium storage. J. Alloys Compd. 2019, 791, 296–306. [Google Scholar] [CrossRef]
- Yi, H.; Lin, L.; Ling, M.; Lv, Z.; Li, R.; Fu, Q.; Zhang, H.; Zheng, Q.; Li, X. Scalable and economic synthesis of high-performance Na3V2(PO4)2F3 by a solvothermal-ball-milling method. ACS Energy Lett. 2019, 4, 1565–1571. [Google Scholar] [CrossRef]
- Liu, T.; Zhang, Y.; Jiang, Z.; Zeng, X.; Ji, J.; Li, Z.; Gao, X.; Sun, M.; Lin, Z.; Ling, M.; et al. Exploring competitive features of stationary sodium ion batteries for electrochemical energy storage. Energy Environ. Sci. 2019, 12, 1512–1533. [Google Scholar] [CrossRef]
- Zhang, B.W.; Ma, K.X.; Lv, X.; Shi, K.; Wang, Y.; Nian, Z.Y.; Li, Y.H.; Wang, L.; Dai, L.; He, Z.X. Recent advances of NASICON-Na3V2(PO4)3 as cathode for sodium-ion batteries: Synthesis, modifications, and perspectives. J. Alloys Compd. 2021, 867, 159060. [Google Scholar] [CrossRef]
- Chagas, L.G.; Jeong, S.; Hasa, I.; Passerini, S. Ionic liquid-based electrolytes for sodium-ion batteries: Tuning properties to enhance the electrochemical performance of manganese-based layered oxide cathode. ACS Appl. Mater. Inter. 2019, 11, 22278–22289. [Google Scholar] [CrossRef]
- Gu, Y.; Xiao, F.; Xu, K.; Pan, X.; Li, J.; Xu, C.; Zhou, X. Outstanding electrochemical performance of sodium vanadium phosphate cathode co-modified by carbon-coating and titanium-doping for Na-ion batteries. Ceram. Int. 2019, 45, 12570–12574. [Google Scholar] [CrossRef]
- Zhao, J.; Gao, Y.; Liu, Q.; Meng, X.; Chen, N.; Wang, C.; Du, F.; Chen, G. High rate capability and enhanced cyclability of Na3V2(PO4)2F3 cathode by in situ coating of carbon nanofibers for sodium-ion battery applications. Chem. Eur. J. 2018, 24, 2913–2919. [Google Scholar] [CrossRef] [PubMed]
- Slater, M.D.; Kim, D.; Lee, E.; Johnson, C.S. Sodium-ion batteries. Adv. Funct. Mater. 2013, 23, 947–958. [Google Scholar] [CrossRef]
- Kim, S.W.; Seo, D.H.; Ma, X.; Ceder, G.; Kang, K. Electrode materials for rechargeable sodium-ion batteries: Potential alternatives to current lithium-ion batteries. Adv. Energy Mater. 2012, 2, 710. [Google Scholar] [CrossRef]
- Zhou, Y.X.; Zhang, L.L.; Yang, X.L.; Huang, Y.H.; Ding, X.K.; Ma, D.; Wang, J.Q. Synthesis of nanosheet-structured Na3V2(PO4)3/C as high-performance cathode material for sodium ion batteries using anthracite as carbon source. Ceram. Int. 2017, 43, 2333. [Google Scholar] [CrossRef]
- Khan, Z.; Vagin, M.; Crispin, X. Can hybrid Na–air batteries outperform nonaqueous Na–O2 batteries? Adv. Sci. 2020, 7, 1902866. [Google Scholar] [CrossRef]
- Zhao, Y.; Yang, D.; He, T.; Li, J.; Wei, L.; Wang, D.; Wang, Y.; Wang, X.; Chen, G.; Wei, Y. Vacancy engineering in VS2 nanosheets for ultrafast pseudocapacitive sodium ionstorage. Chem. Eng. J. 2021, 421, 129715. [Google Scholar] [CrossRef]
- Lv, Z.Q.; Ling, M.X.; Yue, M.; Li, X.F.; Song, M.M.; Zheng, Q.; Zhang, H.M. Vanadium-based polyanionic compounds as cathode materials for sodium-ion batteries: Toward high-energy and high-power applications. J. Energy Chem. 2021, 55, 361–390. [Google Scholar] [CrossRef]
- Chen, S.; Wu, C.; Shen, L.; Zhu, C.; Huang, Y.; Xi, K.; Maier, J.; Yu, Y. Challenges and Perspectives for NASICON-Type Electrode Materials for Advanced Sodium-Ion Batteries. Adv. Mater. 2017, 29, 1700431. [Google Scholar] [CrossRef]
- Takeda, Y.; Nakahara, K.; Nishijima, M.; Imanishi, N.; Yamamoto, O.; Takano, M.; Kanno, R. Crystal chemistry and physical properties of La2−xSrxNiO4 (0 ≤ x ≤ 1.6). Mater. Res. Bull. 1990, 25, 293–306. [Google Scholar] [CrossRef]
- Ma, X.; Chen, H.; Ceder, G. Electrochemical properties of monoclinic NaMnO2. J. Electrochem. Soc. 2011, 158, A1307. [Google Scholar] [CrossRef]
- Komaba, S.; Takei, C.; Nakayama Ogata, T.A.; Yabuuchi, N. Electrochemical intercalation activity of layered NaCrO2 vs. LiCrO2. Electrochem. Commun. 2010, 12, 355. [Google Scholar] [CrossRef]
- Hamani, D.; Ati, M.; Tarascon, J.M.; Rozier, P. NaxVO2 as possible electrode for Na-ion batteries. Electrochem. Commun. 2011, 13, 938. [Google Scholar] [CrossRef]
- Recham, N.; Chotard, J.N.; Dupont, L.; Djell, K.; Armand, M.; Tarasconz, J.M. Ionothermal synthesis of sodium-based fluorophosphate cathode materials. J. Electrochem. Soc. 2009, 156, A993. [Google Scholar] [CrossRef]
- Pu, X.J.; Wang, H.M.; Yuan, T.C.; Cao, S.N.; Liu, S.Y.; Xu, L.; Yang, H.X.; Ai, X.P.; Chen, Z.X.; Cao, Y.L. Na4Fe3(PO4)2P2O7/C nanospheres as low-cost, high-performance cathode material for sodium-ion batteries. Energy Stor. Mater. 2019, 22, 330–336. [Google Scholar] [CrossRef]
- Rajagopalan, R.; Chen, B.; Zhang, Z.; Wu, X.L.; Du, Y.; Huang, Y.; Li, B.; Zong, Y.; Wang, J.; Nam, G.H.; et al. Improved Reversibility of Fe3+/Fe4+ Redox Couple in Sodium Super Ion Conductor Type Na3Fe2(PO4)3 for Sodium-Ion Batteries. Adv. Mater. 2017, 29, 1605694. [Google Scholar] [CrossRef]
- Xia, X.; Cao, Y.; Yao, L.; Yang, H.; Zhang, J. MCNT-Reinforced Na3Fe2(PO4)3 as Cathode Material for Sodium-Ion Batteries. Arab J. Sci. Eng. 2020, 45, 143–151. [Google Scholar] [CrossRef]
- Piernas Muñoz, M.J.; Martínez, E.C. Prussian Blue Based Batteries; Springer: Cham, Switzerland, 2018; pp. 9–22. [Google Scholar]
- Qian, J.; Wu, C.; Cao, Y.; Ma, Z.; Huang, Y.; Ai, X.; Yang, H. Prussian blue cathode materials for sodium-ion batteries and other ion batteries. Adv. Energy Mater. 2018, 8, 1702619. [Google Scholar] [CrossRef]
- You, Y.; Wu, X.L.; Yin, Y.X.; Guo, Y.-G. High-quality Prussian blue crystals as superior cathode materials for room-temperature sodium-ion batteries. Energy Environ. Sci. 2014, 7, 1643. [Google Scholar] [CrossRef]
- Wang, B.; Han, Y.; Wang, X.; Bahlawane, N.; Pan, H.; Yan, M.; Jiang, Y. Prussian blue analogs for rechargeable batteries. Iscience 2018, 3, 110. [Google Scholar] [CrossRef]
- Fernández, N.; Sánchez-Fontecoba, P.; Castillo-Martínez, E.; Carretero-González, J.; Rojo, T.; Armand, M. Polymeric redox-active electrodes for sodium-ion batteries. Chem. Sus. Chem. 2018, 11, 311–319. [Google Scholar] [CrossRef]
- Mendiboure, A.; Delmas, C.; Hagenmuller, P. Electrochemical intercalation and deintercalation of NaxMnO2 bronzes. J. Solid State Chem. 1985, 57, 323–331. [Google Scholar] [CrossRef]
- Manikandan, P.; Heo, S.; Kim, H.W.; Jeong, H.Y.; Lee, E.; Kim, Y. Structural characterization of layered Na0.5Co0.5Mn0.5O2 material as a promising cathode for sodium-ion batteries. J. Power Source 2017, 80, 442–449. [Google Scholar] [CrossRef]
- Komaba, S.; Yabuuchi, N.; Nakayama, T.; Ogata, A.; Ishikawa, T.; Nakai, I. Study on the reversible electrode reaction of Na1-xNi0.5Mn0.5O2 for a rechargeable sodium-ion battery. Inorg. Chem. 2012, 51, 6211–6220. [Google Scholar] [CrossRef] [PubMed]
- Han, D.W.; Ku, J.H.; Kim, R.H.; Yun, D.J.; Lee, S.S.; Doo, S.G. Aluminum manganese oxides with mixed crystal structure: High-energy-density cathodes for rechargeable sodium batteries. Chem. Sus. Chem. 2014, 7, 1870–1875. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Shen, J.; Wang, T. Electrochemical study of Na0.66Ni0.33Mn0.67−xMoxO2 as cathode material for sodium-ion battery. J. Alloys Compd. 2017, 709, 481–486. [Google Scholar] [CrossRef]
- Ding, F.X.; Zhao, C.L.; Zhou, D.; Meng, Q.S.; Xiao, D.D.; Zhang, Q.Q.; Niu, Y.S.; Li, Y.Q.; Rong, X.H.; Lu, Y.X.; et al. A Novel Ni-rich O3-Na[Ni0.60Fe0.25Mn0.15]O2 Cathode for Na-ion Batteries. Energy Storage Mater. 2020, 30, 420–430. [Google Scholar] [CrossRef]
- Fang, Y.J.; Yu, X.Y.; Xiong, W.L. A practical high-energy cathode for sodium-ion batteries based on uniform P2-Na0. 7CoO2 microspheres. Angew. Chem. Int. Ed. 2017, 21, 5801–5805. [Google Scholar] [CrossRef]
- Wang, L.; Song, J.; Qiao, R.M.; Wray, L.A.; Hossain, M.A.; Chuang, Y.D.; Yang, W.L.; Lu, Y.H.; Evans, D. Rhombohedral Prussian White as Cathode for Rechargeable Sodium-Ion Batteries. J. Am. Chem. Soc. 2015, 137, 2548–2554. [Google Scholar] [CrossRef]
- Hwang, J.; Kim, J. A supercritical methanol route for the synthesis of sodium iron oxide submicron plates for use as a cathode material for sodium-ion batteries. Mater. Lett. 2017, 206, 100–104. [Google Scholar] [CrossRef]
- Zhou, D.; Huang, W.; Lv, X.; Zhao, F. A novel P2/O3 bi phase Na0.67Fe0.425Mn0.425Mg0.15O2 as cathode for high-performance sodium-ion batteries. J. Power Source 2019, 421, 147–155. [Google Scholar] [CrossRef]
- Li, A.H.; Feng, Z.Y.; Sun, Y.; Shang, L.M.; Xu, L.Q. Porous organic polymer/RGO composite as high performance cathode for half and full sodium ion batteries. J. Power Source 2017, 343, 424–430. [Google Scholar] [CrossRef]
- Luo, C.; Fan, X.L.; Ma, Z.H.; Gao, T.; Wang, C.S. Self-Healing Chemistry between Organic Material and Binder for Stable Sodium-Ion Batteries. Chem 2017, 3, 1050–1062. [Google Scholar] [CrossRef]
- Li, J.; Chen, Y.; Zhou, T.; Shi, H.; Zheng, Z.; Wang, Y.; Guo, L. Dual-carbon coated Na3V2(PO4)3 derived from reduced graphene oxide and nanocellulose with porous structure for high performance sodium-ion batteries. Appl. Surf. Sci. 2023, 610, 155553. [Google Scholar] [CrossRef]
- Li, H.; Chen, X.; Jin, T.; Bao, W.; Zhang, Z.; Jiao, L. Robust graphene layer modified Na2MnP2O7 as a durable high-rate and high energy cathode for Na-ion batteries. Energy Storage Mater. 2019, 16, 383–390. [Google Scholar] [CrossRef]
- Zaghib, K.; Julien, C.M. Structure and electrochemistry of FePO4·2H2O hydrate. J. Power Source 2005, 142, 279–284. [Google Scholar] [CrossRef]
- Karami, H.; Taala, F. Synthesis, characterization and application of Li3Fe2(PO4)3 nanoparticles as cathode of lithium-ion rechargeable batteries. J. Power Source 2011, 196, 6400–6411. [Google Scholar] [CrossRef]
- Yin, Y.; Hu, Y.; Wu, P.; Zhang, H.; Cai, C. A graphene-amorphous FePO4 hollow nanosphere hybrid as a cathode material for lithium ion batteries. Chem. Commun. 2012, 48, 2137–2139. [Google Scholar] [CrossRef]
- Guo, B.; Ruan, H.; Zheng, C.; Fei, H.; Wei, M. Hierarchical LiFePO4 with a controllable growth of the (010) facet for lithiumion batteries. Sci. Rep. 2013, 3, 2788–2793. [Google Scholar] [CrossRef]
- Zhang, S.M.; Zhang, J.X.; Xu, S.J.; Yuan, X.J.; Tan, T. Synthesis, morphological analysis and electrochemical performance of iron hydroxyl phosphate as a cathode material for lithium ion batteries. J. Power Source 2013, 243, 274–279. [Google Scholar] [CrossRef]
- Pu, X.; Wang, H.; Zhao, D.; Yang, H.; Ai, X.; Cao, S.; Chen, Z.; Cao, Y. Recent progress in rechargeable sodium-ion batteries: Toward high-power applications. Small 2019, 15, 1805427. [Google Scholar] [CrossRef]
- Niu, Y.; Zhang, Y.; Xu, M. A review on pyrophosphate framework cathode materials for sodium-ion batteries. J. Mater. Chem. A 2019, 7, 15006–15025. [Google Scholar] [CrossRef]
- Guo, S.P.; Li, J.C.; Xu, Q.T.; Ma, Z.; Xue, H.G. Recent achievements on polyanion-type compounds for sodium-ion batteries: Syntheses, crystal chemistry and electrochemical performance. J. Power Source 2017, 361, 285–299. [Google Scholar] [CrossRef]
- Wang, X.; Wang, W.; Zhu, B.; Qian, F.; Fang, Z. Mo-doped Na3V2(PO4)3@C composites for high stable sodium ion battery cathode. Front. Mater. Sci. 2018, 12, 53–63. [Google Scholar] [CrossRef]
- Li, X.; Huang, Y.; Wang, J.; Miao, L.; Li, Y.; Liu, Y.; Qiu, Y.; Fang, C.; Han, J.; Huang, Y. High valence Mo-doped Na3V2(PO4)3/C as a high rate and stable cycle-life cathode for sodium battery. J. Mater. Chem. A 2018, 6, 1390–1396. [Google Scholar] [CrossRef]
- Zhu, Q.; Wang, M.; Nan, B.; Shi, H.; Zhang, X.; Deng, Y.; Wang, L.; Chen, Q.; Lu, Z. Core/shell nanostructured Na3V2(PO4)3/C/TiO2 composite nanofibers as a stable anode for sodium-ion batteries. J. Power Source 2017, 362, 147–159. [Google Scholar] [CrossRef]
- Zheng, W.; Huang, X.; Ren, Y.; Wang, H.; Zhou, S.; Chen, Y.; Ding, X.; Zhou, T. Porous spherical Na3V2(PO4)3/C composites synthesized via a spray drying-assisted process with high-rate performance as cathode materials for sodium-ion batteries. Solid State Ion. 2017, 308, 161–166. [Google Scholar] [CrossRef]
- Li, G.; Jiang, D.; Wang, H.; Lan, X.; Zhong, H.; Jiang, Y. Glucose-assisted synthesis of Na3V2(PO4)3 composite as an electrode material for high-performance sodium-ion batteries. J. Power Source 2014, 265, 325–334. [Google Scholar] [CrossRef]
- Tao, S.; Cui, P.X.; Huang, W.F.; Yu, Z.; Wang, X.B.; Wei, S.H.; Liu, D.B.; Song, L.; Chu, W.S. Sol-gel design strategy for embedded Na3V2(PO4)3 particles into carbon matrices for high-performance sodium-ion batteries. Carbon 2016, 96, 1028–1033. [Google Scholar] [CrossRef]
- Zhu, C.B.; Song, K.P.; Aken, P.A.; Maier, V.J.; Yu, Y. Carbon-coated Na3V2(PO4)3 embedded in porous carbon matrix: An ultrafast Na-storage cathode with the potential of outperforming Li cathodes. Nano Lett. 2014, 14, 2175–2180. [Google Scholar] [CrossRef]
- Li, S.; Dong, Y.F.; Xu, L.; Xu, X.; He, L.; Mai, L.Q. Effect of carbon matrix dimensions on the electrochemical properties of Na3V2(PO4)3 nanograins for high-performance symmetric sodium-ion batteries. Adv. Mater. 2014, 26, 3358–3363. [Google Scholar] [CrossRef]
- Zhu, X.M.; Fang, Y.J.; Ai, X.P.; Yang, H.X.; Cao, Y.L. Na3V2(PO4)3/C nanocomposite synthesize via pre-reduction process as high-performance cathode material for sodium-ion batteries. J. Alloys Compd. 2015, 646, 170–174. [Google Scholar] [CrossRef]
- Jian, Z.L.; Zhao, L.; Pan, H.L.; Hu, Y.S.; Li, H.; Chen, W.; Chen, L.Q. Carbon coated Na3V2(PO4)3 as novel electrode material for sodium ion batteries. Electrochem. Commun. 2012, 14, 86–89. [Google Scholar] [CrossRef]
- Cheng, D.; Li, Y.; Zhang, J.; Tian, M.; Wang, B.; He, Z.; Dai, L.; Wang, L. Recent advances in electrospun carbon fiber electrode for vanadium redox flow battery: Properties, structures, and perspectives. Carbon 2020, 170, 527–542. [Google Scholar] [CrossRef]
- Jiang, Y.; Zeng, L.; Wang, J.; Li, W.; Pana, F.; Yu, Y. A carbon coated NASICON structure material embedded in porous carbon enabling superior sodium storage performance: NaTi2(PO4)3 as an example. Nanoscale 2015, 7, 14723. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Wang, W.; Wang, Y.; Feng, P.; Wang, K.; Cheng, S.; Jiang, K. Controllable construction of 3D-skeleton-carbon coated Na3V2(PO4)3 for high-performance sodium ion battery cathode. Nano Energy 2016, 20, 11. [Google Scholar] [CrossRef]
- Fang, J.; Wang, S.; Li, Z.; Chen, H.; Xia, L.; Ding, L.; Wang, H. Porous Na3V2 (PO4)3@C nanoparticles enwrapped in three-dimensional graphene for high performance sodium-ion batteries. J. Mater. Chem. A 2016, 4, 1180. [Google Scholar] [CrossRef]
- Li, H.; Yu, X.; Bai, Y.; Wu, F.; Wu, C.; Liu, L.Y.; Yang, X.Q. Effects of Mg doping on the remarkably enhanced electrochemical performance of Na3V2(PO4)3 cathode materials for sodium ion batteries. J. Mater. Chem. A 2015, 3, 9578. [Google Scholar] [CrossRef]
- Aragón, M.J.; Lavela, P.; Alcántara, R.; Tirado, J.L. Effect of aluminum doping on carbon loaded Na3V2(PO4)3 as cathode material for sodium-ion batteries. Electrochim. Acta 2015, 180, 824. [Google Scholar] [CrossRef]
- Zhu, C.; Kopold, P.; van Aken, P.A.; Maier, J.; Yu, Y. High power-high energy sodium battery based on threefold interpenetrating network. Adv. Mater. 2016, 28, 2409. [Google Scholar] [CrossRef]
- Song, W.; Ji, X.; Wu, Z.; Zhu, Y.; Yang, Y.; Chen, J.; Jing, M.; Li, F.; Banks, C.E. First exploration of Na-ion migration pathways in the NASICON structure Na3V2(PO4)3. J. Mater. Chem. A 2014, 2, 5358. [Google Scholar] [CrossRef]
- Xiao, H.; Huang, X.; Ren, Y.; Wang, H.; Ding, J.; Zhou, S.; Ding, X.; Chen, Y. Enhanced sodium ion storage performance of Na3V2(PO4)3 with N-doped carbon by folic acid as carbon-nitrogen source. J. Alloys Compd. 2018, 732, 454–459. [Google Scholar] [CrossRef]
- Klee, R.; Aragon, M.J.; Lavela, P.; Alcantara, R.; Tirado, J.L. Na3V2(PO4)3/C nanorods with improved electrode-electrolyte interface as cathode material for sodium-ion batteries. ACS Appl. Mater. Interfaces 2016, 8, 23151–23159. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.Y.; Kim, H.; Shakoor, R.A.; Jung, Y.; Choi, J.W. Electrochemical and Thermal Properties of NASICON Structured Na3V2(PO4)3 as a Sodium Rechargeable Battery Cathode: A Combined Experimental and Theoretical Study. J. Electrochem. Soc. 2012, 159, A1393–A1397. [Google Scholar] [CrossRef]
- Bianchini, M.; Fauth, F.; Brisset, N.; Weill, F.; Masquelier, E.; Croguennec, L. Comprehensive investigation of the Na3V2(PO4)2F3–NaV2(PO4)2F3 system by operando high resolution synchrotron X-ray diffraction. Chem. Mater. 2015, 27, 3009–3020. [Google Scholar] [CrossRef]
- Liu, J.; Tang, K.; Song, K.; van Aken, P.A.; Yu, Y.; Maier, J. Electrospun Na3V2(PO4)3/C nanofibers as stable cathode materials for sodium-ion batteries. Nanoscale 2014, 6, 5081–5086. [Google Scholar] [CrossRef]
- Xiao, H.; Huang, X.; Ren, Y.; Ding, X.; Chen, Y.; Zhou, S. Improved electrochemical performance of Na3V2(PO4)3 modified by N-doped carbon coating and Zr doping. Solid State Ion. 2019, 333, 93–100. [Google Scholar] [CrossRef]
- Rui, X.; Sun, W.; Wu, C.; Yu, Y.; Yan, Q. An Advanced Sodium-Ion Battery Composed of Carbon Coated Na3V2(PO4)3 in a Porous Graphene Network. Adv. Mater. 2015, 27, 6670–6676. [Google Scholar] [CrossRef]
- Chang, X.Q.; Zhu, Q.Z.; Sun, N.; Guan, Y.B.; Wang, R.; Zhao, J.H.; Feng, M.Y.; Xu, B. Graphene-bound Na3V2(PO4)3 film electrode with excellent cycle and rate performance for Na-ion batteries. Electrochim. Acta 2018, 296, 282–290. [Google Scholar] [CrossRef]
- Xu, J.Y.; Gu, E.L.; Zhang, Z.Z.; Xu, Z.H.; Xu, Y.F.; Du, Y.C.; Zhu, X.S.; Zhou, X.S. Fabrication of porous Na3V2(PO4)3/reduced graphene oxide hollow spheres with enhanced sodium storage performance. J. Colloid Interface Sci. 2020, 567, 84–91. [Google Scholar] [CrossRef]
- Xu, Y.N.; Wei, Q.L.; Xu, C.; Li, Q.D.; An, Q.Y.; Zhang, P.F.; Sheng, J.Z.; Zhou, L.; Mai, L.Q. Layer-by-Layer Na3V2(PO4)3 Embedded in Reduced Graphene Oxide as Superior Rate and Ultralong-Life Sodium-Ion Battery Cathode. Adv. Energy Mater. 2016, 6, 1600389. [Google Scholar] [CrossRef]
- Shen, W.; Li, H.; Guo, Z.; Wang, C.; Li, Z.; Xu, Q.; Liu, H.; Wang, Y.; Xia, Y. Double-Nanocarbon Synergistically Modified Na3V2(PO4)3: An Advanced Cathode for High-Rate and Long-Life Sodium-Ion Batteries. ACS Appl. Mater. Interfaces 2016, 8, 15341–15351. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Zhang, B.; Wang, X.; Dong, P.; Tong, H.; Zheng, J.C.; Yu, W.; Zhang, J. CNT-Decorated Na3V2(PO4)3 Microspheres as a High-Rate and Cycle-Stable Cathode Material for Sodium Ion Batteries. ACS Appl. Mater. Interfaces 2018, 10, 3590–3595. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.Q.; Chen, Y.J.; Cheng, J.; Tian, Z.Y.; Wang, C.; Wu, G.P.; Liu, C.C.; Wang, Y.Z.; Li, G. Constructing dimensional gradient structure of Na3V2(PO4)3/C@CNTs-WC by wolfram substitution for superior sodium storage. Chem. Eng. J. 2021, 420, 130453. [Google Scholar] [CrossRef]
- Duan, W.C.; Zhu, Z.Q.; Li, H.; Hu, Z.; Zhang, K.; Cheng, F.Y.; Chen, J. Na3V2(PO4)3@C core–shell nanocomposites for rechargeable sodium-ion batteries. J. Mater. Chem. A 2014, 2, 8668. [Google Scholar] [CrossRef]
- Li, S.J.; Ge, P.; Zhang, C.T.; Sun, W.; Hou, H.S.; Ji, X.B. The electrochemical exploration of double carbon-wrapped Na3V2(PO4)3: Towards long-time cycling and superior rate sodium-ion battery cathode. J. Power Source 2017, 366, 249–258. [Google Scholar] [CrossRef]
- Jiang, Y.; Yang, Z.; Li, W.; Zeng, L.; Pan, F.; Wang, M.; Wei, X.; Hu, G.; Gu, L.; Yu, Y. Nanoconfined Carbon-Coated Na3V2(PO4)3 Particles in Mesoporous Carbon Enabling Ultralong Cycle Life for Sodium-Ion Batteries. Adv. Energy Mater. 2015, 5, 1402104. [Google Scholar] [CrossRef]
- Wang, G.; Liu, H.; Liu, J.; Qiao, S.; Lu, G.M.; Munroe, P.; Ahn, H. Mesoporous LiFePO4/C Nanocomposite Cathode Materials for High Power Lithium Ion Batteries with Superior Performance. Adv. Mater. 2010, 22, 4944. [Google Scholar] [CrossRef]
- Zhang, H.; Tao, H.; Jiang, Y.; Jiao, Z.; Wu, M.; Zhao, B. Ordered CoO/CMK-3 nanocomposites as the anode materials for lithium-ion batteries. J. Power Source 2010, 195, 2950–2955. [Google Scholar] [CrossRef]
- Hong, X.D.; Huang, X.B.; Ren, Y.R.; Wang, H.Y.; Ding, X.; Jin, J.L. Superior Na-storage performance of Na3V2(PO4)3/C-Ag composites as cathode material for Na-ion battery. J. Alloys Compd. 2020, 822, 153587. [Google Scholar] [CrossRef]
- Wang, Y.P.; Zhao, K.J.; Wang, k.; Li, H.H.; Jiang, H.B.; Chen, L. N-doped carbon confined Na3V2(PO4)3 derived from an organophosphonic acid as a high-performance cathode for sodium-ion batteries. J. Alloys Compd. 2020, 844, 156118. [Google Scholar] [CrossRef]
- Jiang, Y.; Wu, Y.; Chen, Y.; Qi, Z.; Shi, J.; Gu, L.; Yu, Y. Design Nitrogen (N) and Sulfur (S) co-Doped 3D Graphene Network Architectures for High-Performance Sodium Storage. Small 2018, 14, 1703471. [Google Scholar] [CrossRef] [PubMed]
- Ren, W.H.; Yao, X.H.; Niu, C.J.; Zheng, Z.P.; Zhao, K.N.; An, Q.Y.; Wei, Q.L.; Yan, M.Y.; Zhang, L.; Mai, L.Q. Cathodic polarization suppressed sodium-ion full cell with a 3.3 V high-voltage. Nano Energy 2016, 28, 216–223. [Google Scholar] [CrossRef]
- Kajiyama, S.; Kikkawa, J.; Hoshino, J.; Okubo, M.; Hosono, E. Assembly of Na3V2(PO4)3 Nanoparticles Confined in a One-Dimensional Carbon Sheath for Enhanced Sodium-Ion Cathode Properties. Chem. Eur. J. 2014, 20, 12636–12640. [Google Scholar] [CrossRef] [PubMed]
- Shen, W.; Wang, C.; Xu, Q.J.; Liu, H.M.; Wang, Y.G. Nitrogen-Doping-Induced Defects of a Carbon Coating Layer Facilitate Na-Storage in Electrode Materials. Adv. Energy Mater. 2015, 5, 1400982. [Google Scholar] [CrossRef]
- Yi, H.M.; Li, D.; Lv, Z.Q.; Li, R.; Ling, M.; Zhang, H.; Zheng, Q.; Li, X. Constructing high-performance 3D porous self-standing electrodes with various morphologies and shapes by a flexible phase separation-derived method. J. Mater. Chem. A 2019, 7, 22550–22558. [Google Scholar] [CrossRef]
- Shen, W.; Li, H.; Wang, C.; Li, Z.H.; Xu, Q.J.; Liu, H.M.; Wang, Y.G. Improved electrochemical performance of the Na3V2(PO4)3 cathode by B-doping of the carbon coating layer for sodium-ion batteries. J. Mater. Chem. A 2015, 3, 15190–15201. [Google Scholar] [CrossRef]
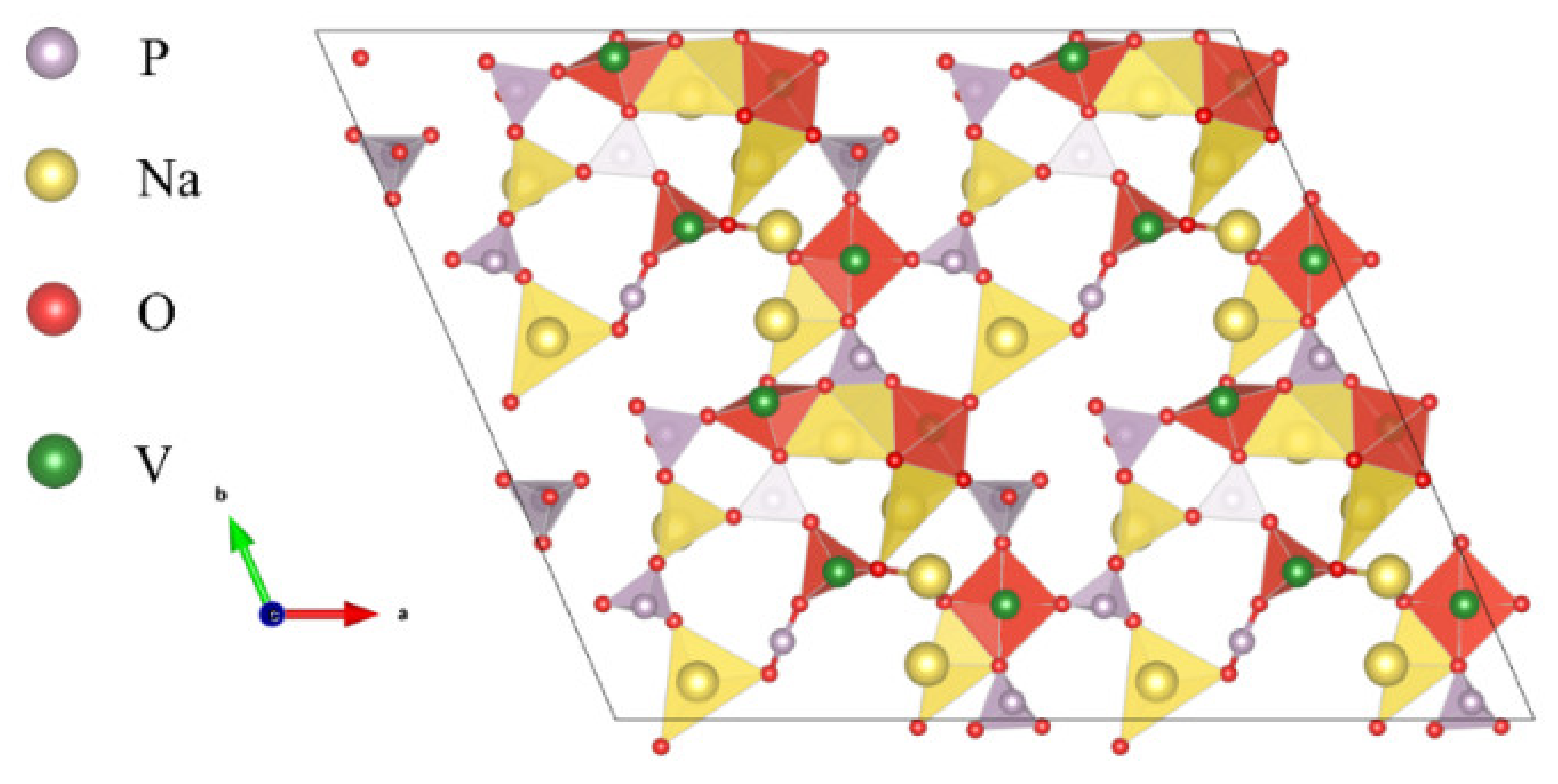







| Metal | Na | Li |
|---|---|---|
| Atoms radius/Å | 1.86 | 1.52 |
| Ionic radius/Å | 1.02 | 0.76 |
| Standard electrode potential/V | −2.71 | −3.04 |
| Earth crust abundance | 2.8% | 0.0065% |
| Theoretical specific capacity/(mah/g) | 1166 | 3862 |
| Materials | Voltage | Reversible Capacity | Rate Performance | Ref. |
|---|---|---|---|---|
| NaMnO2 | 2.59 V | C/30, 149 mAh/g | C/10, 144 mAh/g | [32] |
| Na0.5Co0.5Mn0.5O2 | 2.8 V | C/2, 141 mAh/g | 5 C, 80 mAh/g | [33] |
| NaNi0.5Mn0.5O2 | >2.5 V | C/50, 125 mAh/g | C/30, 100 mAh/g | [34] |
| NaAl0.1Mn0.9O2 | 2.56 V | - | C/10, 145 mAh/g | [35] |
| Na0.66Ni0.33Mn0.62Mo0.05O2 | 3.25 V | 34 mA/g, 125 mAh/g | 1 C, 112 mAh/g | [36] |
| NaNi0.60Fe0.25Mn0.15O2 | 3.1 V | C/2, 190 mAh/g | 2 C, 134 mAh/g | [37] |
| Na0.7CoO2 | 2.8 V | 0.04 C, 125 mAh/g | 16 C, 64 mAh/g | [38] |
| Na1.92Fe[Fe(CN)6] | 3.2 V | C/10, 157 mAh/g | 10 C, 145 mAh/g | [39] |
| NaFeO2 | 3.4 V | 10 mA/g, 94 mAh/g | - | [40] |
| Na0.67Fe0.425Mn0.425Mg0.15O2 | 3.6 V | C/10, 146.6 mAh/g | 2 C, 58.4 mAh/g | [41] |
| Na2PDHBQS/RGO | 1.4 V | C/10, 228 mAh/g | 4 C, 147 mAh/g | [42] |
| Na2C6O6 | 2.1 V | C/10, 190 mAh/g | 10 C, 95 mAh/g | [43] |
| CNF-GC-NVP | 3.4 V | 1 C, 123.77 mAh/g | 60 C, 95.59 mAh/g | [44] |
| Na2MnP2O7 | 3.6 V | C/10, 94 mAh/g | 10 C, 55 mAh/g | [45] |
| Composition | Morphology | Rate Performance | Cycle Number | Ref. |
|---|---|---|---|---|
| Na3V2(PO4)3@AC | Nanoparticle | 5 C, 100.6 mAh/g | 200 | [44] |
| C@Na3V2(PO4)3@pC | Double-shell nanospheres | 10 C, 103 mAh/g | 1000 | [60] |
| Na3V2(PO4)3-F/C | Nanofibers | C/10, 103 mAh/g | 50 | [76] |
| NVP@C@rGO | 3D porous composites | 1 C, 115 mAh/g | 10,000 | [78] |
| Graphene-bound NVP | 3D continuous network | 30 C, 71.9 mAh/g | 1000 | [79] |
| NVP/rGO HSs | Hollow spheres | 1 C, 109 mAh/g | 400 | [80] |
| NVP@C+N@CNTs | Nanoparticle with CNTs | C/5, 86.5 mAh/g | 400 | [82] |
| NVP/CNT | Microsphere | 10 C, 88.5 mAh/g | 150 | [83] |
| NVP/C@CNTs-WC | Nanoparticle with CNTs | 50 C, 84.5 mAh/g | 400 | [84] |
| NVP@C | Double carbon-wrapped | 1 C, 100 mAh/g | 40 | [85] |
| Na3V2(PO4)3@C@CMK-3 | 3D CMK-3 | 1 C, 115 mAh/g | 2000 | [87] |
| NVP/C-Ag | Particle | 10 C, 95 mAh/g | 500 | [90] |
| NVP-NC | Particle | 10 C, 94 mAh/g | 600 | [91] |
| Na3V2(PO4)3@C@3DPGFs-NS | 3D porous composites | 20 C, 86 mAh/g | 12,000 | [92] |
| Na3V2(PO4)3@C | 3D nanofibers | 10 C, 110 mAh/g | 1000 | [93] |
| Na3V2(PO4)3/CS | Core-sheath nanowires | 1 C, 94 mAh/g | 50 | [94] |
| Na3V2(PO4)3@CNT-G | 3D continuous free-standing foam | 30 C, 109 mAh/g | 2000 | [95] |
| Na3V2(PO4)3@C | 3D porous self-standing | C/2,110 mAh/g | 2000 | [96] |
| Na3V2(PO4)3@C-B0.38 | Nanoparticles | C/5, 87.1 mAh/g | 50 | [97] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, Y.; Li, H. Recent Research Process of Carbon Engineering on Na3V2(PO4)3 for Sodium-Ion Battery Cathodes: A Mini Review. Electron. Mater. 2023, 4, 17-32. https://doi.org/10.3390/electronicmat4010003
He Y, Li H. Recent Research Process of Carbon Engineering on Na3V2(PO4)3 for Sodium-Ion Battery Cathodes: A Mini Review. Electronic Materials. 2023; 4(1):17-32. https://doi.org/10.3390/electronicmat4010003
Chicago/Turabian StyleHe, Yaxuan, and Haibo Li. 2023. "Recent Research Process of Carbon Engineering on Na3V2(PO4)3 for Sodium-Ion Battery Cathodes: A Mini Review" Electronic Materials 4, no. 1: 17-32. https://doi.org/10.3390/electronicmat4010003
APA StyleHe, Y., & Li, H. (2023). Recent Research Process of Carbon Engineering on Na3V2(PO4)3 for Sodium-Ion Battery Cathodes: A Mini Review. Electronic Materials, 4(1), 17-32. https://doi.org/10.3390/electronicmat4010003







