Far-Red to Near Infrared Emissive Aqueous Nanoparticles Based on a New Organic Material with Three BODIPY Dyes at the Periphery of the Core: A Combined Experimental and Theoretical Study
Abstract
1. Introduction
2. Experimental Section
2.1. Materials
2.2. Instrumentation
2.3. Dynamic Light Scattering (DLS)
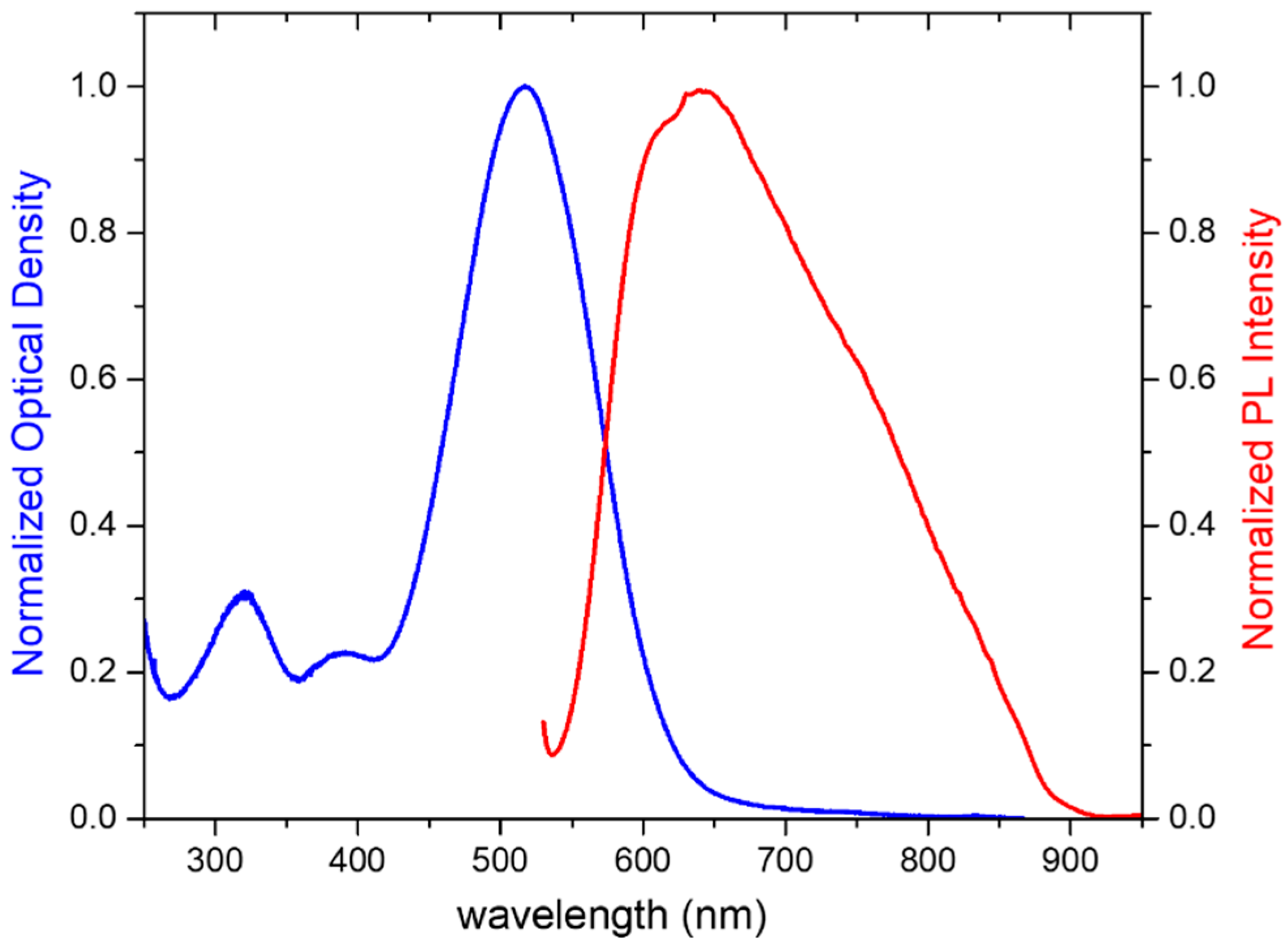
2.4. Absorption and Photoluminescence
- ΦX, is the photoluminescence quantum yield of the unknown sample,
- F, is the area of the integration of the emission intensities,
- n, is the refractive index of the sample and the reference,
- A, is the solution optical density at the excitation wavelength.
2.5. Theoretical Calculations
3. Results and Discussion
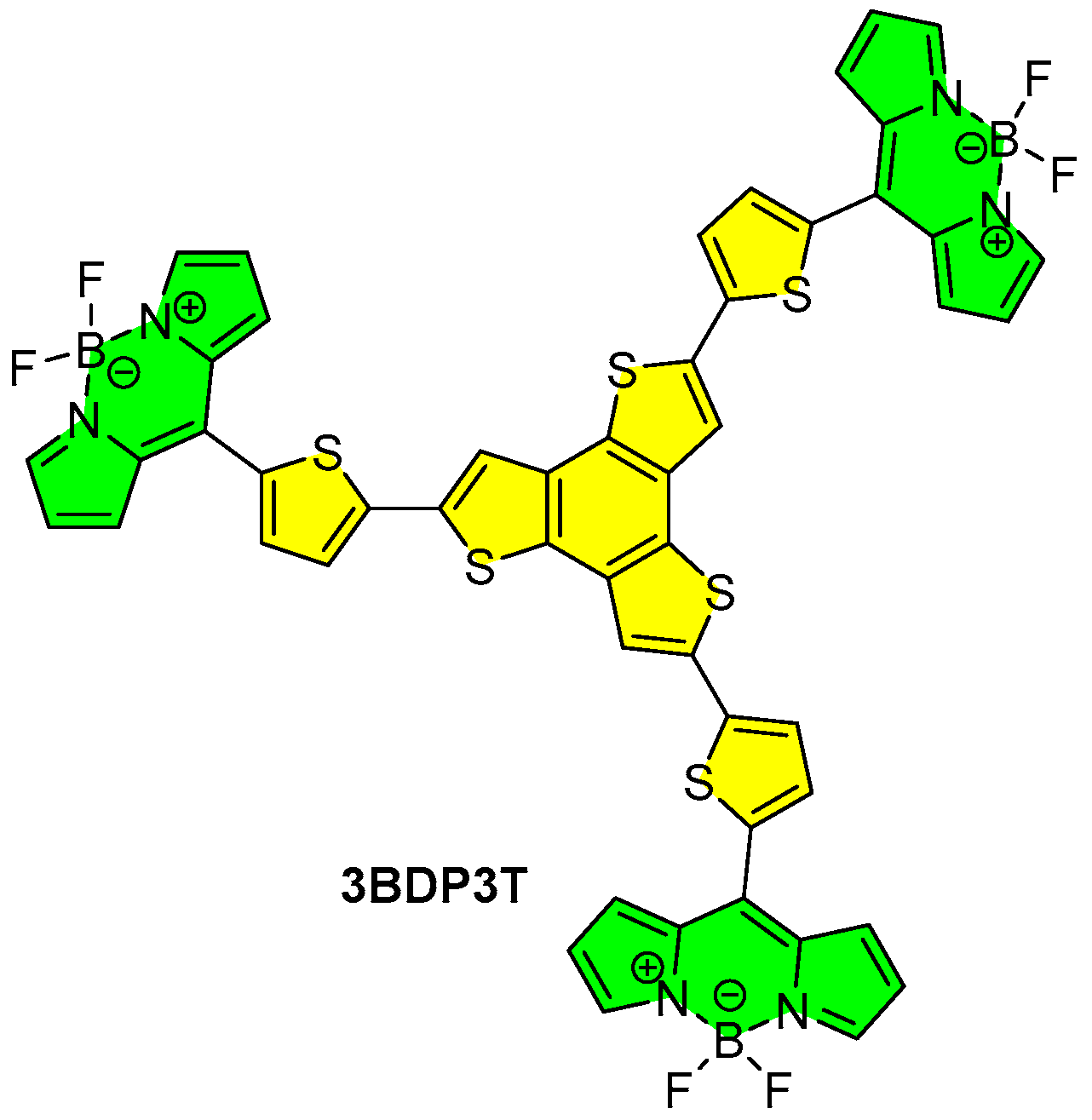
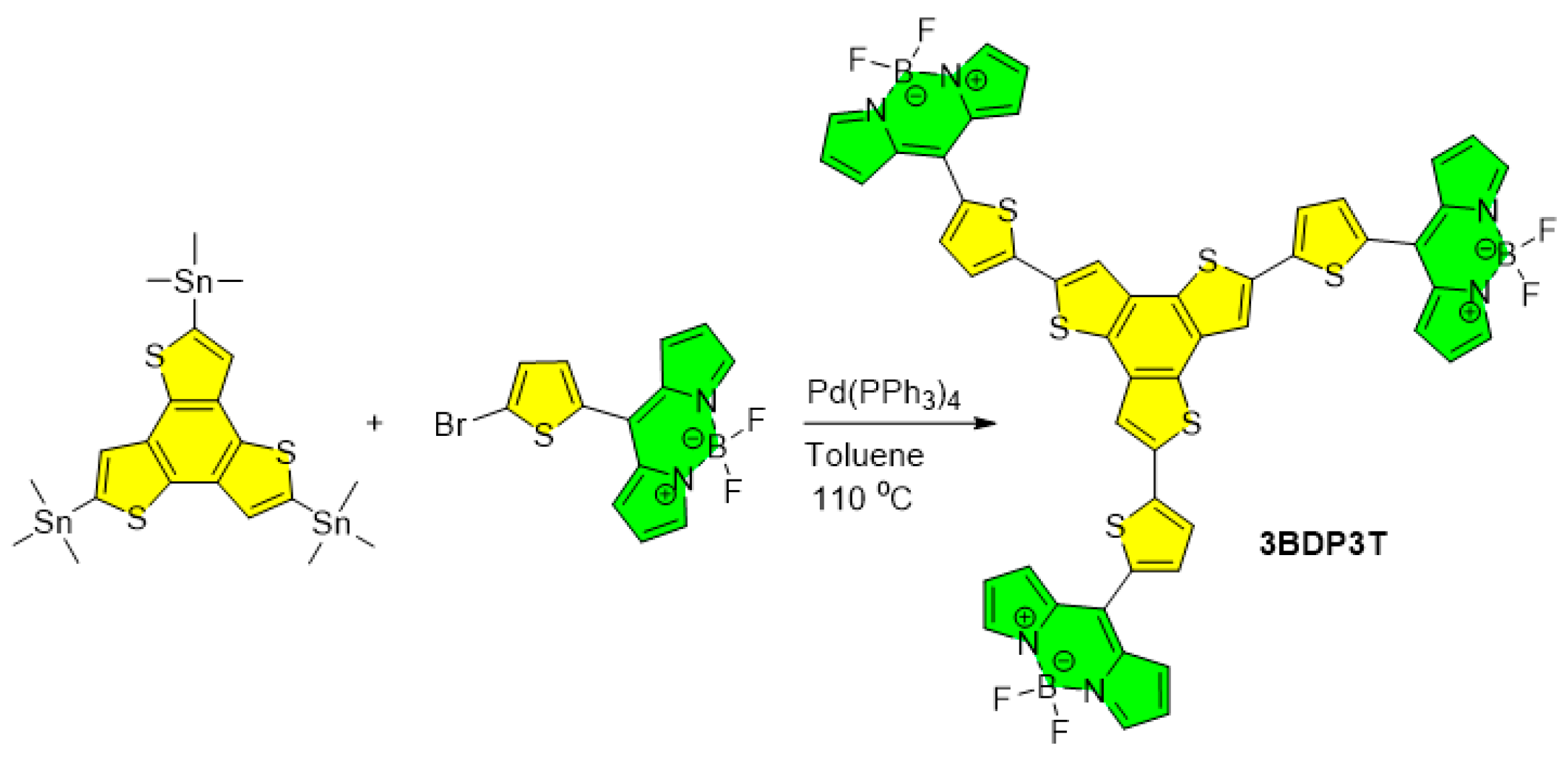
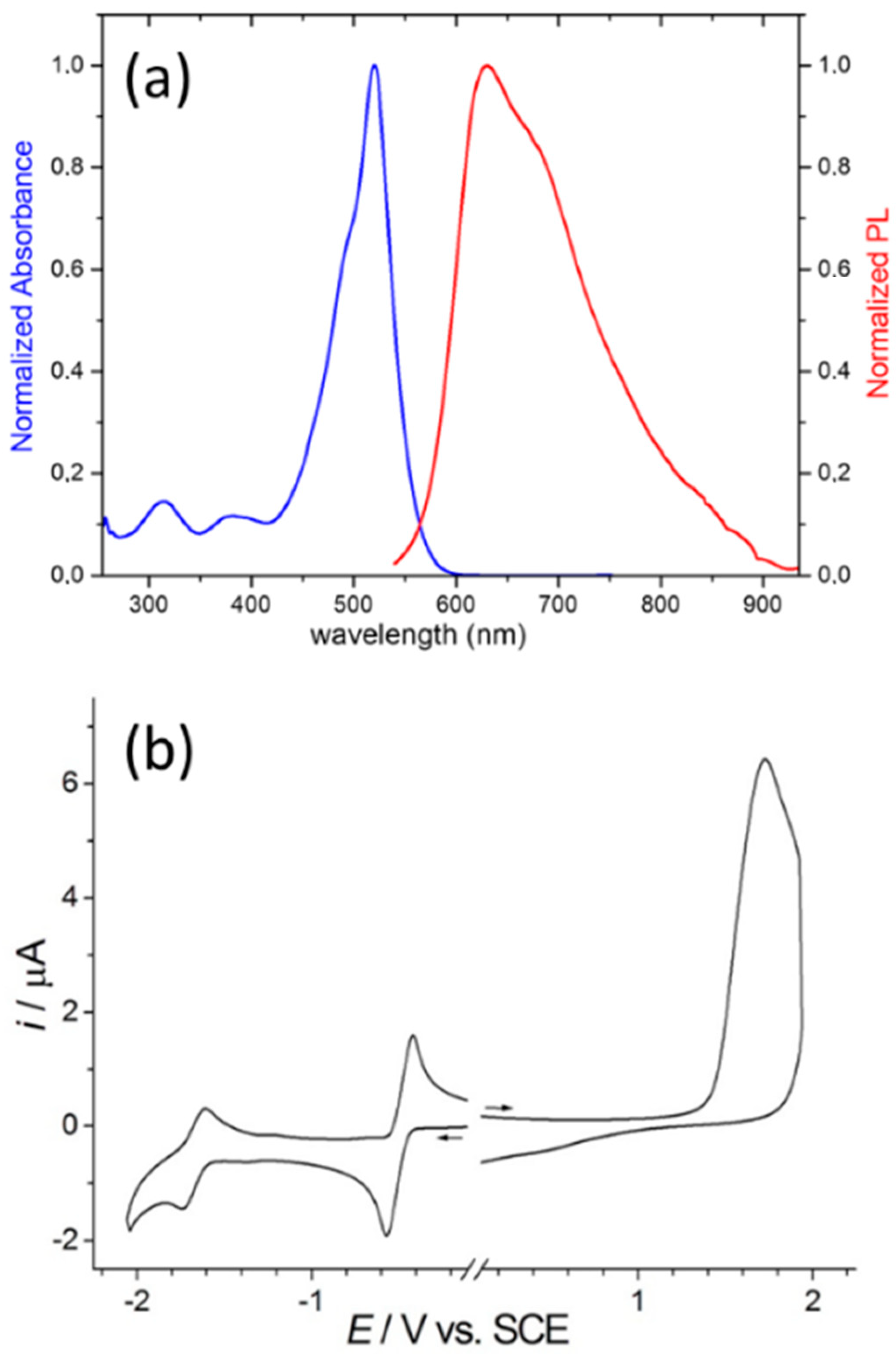
3.1. Synthesis and Properties Characterization
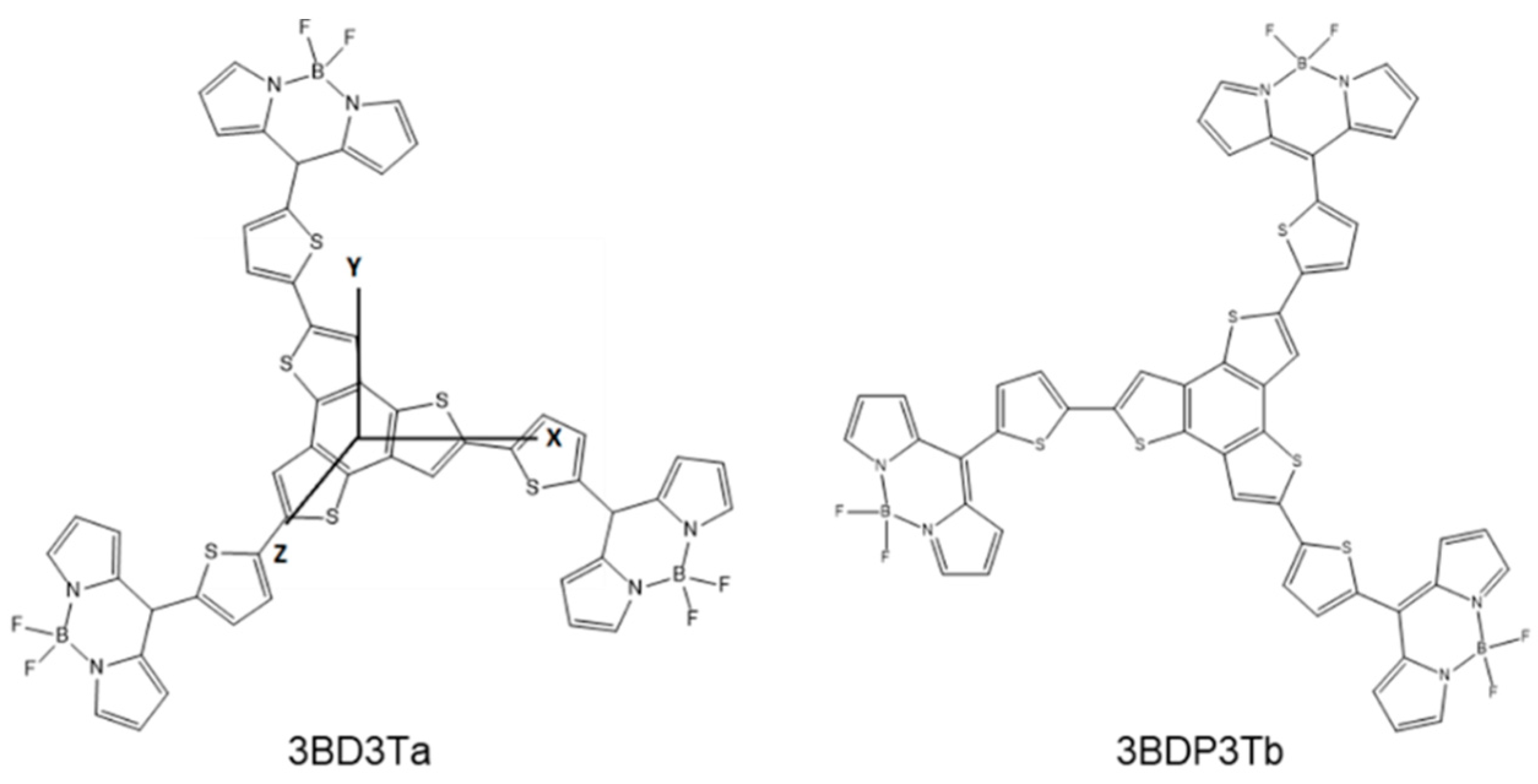
3.2. Theoretical Calculations
3.3. Preparation of Nanoparticles
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Yan, C.; Barlow, S.; Wang, Z.; Yan, H.; Jen, A.K.-Y.; Marder, S.R.; Zhan, X. Non-fullerene acceptors for organic solar cells. Nat. Rev. Mater. 2018, 3, 18003. [Google Scholar] [CrossRef]
- Zhang, J.; Tan, H.S.; Guo, X.; Facchetti, A.; Yan, H. Material insights and challenges for non-fullerene organic solar cells based on small molecular acceptors. Nat. Energy 2018, 3, 720–731. [Google Scholar] [CrossRef]
- Hou, J.; Inganäs, O.; Friend, R.H.; Gao, F. Organic solar cells based on non-fullerene acceptors. Nat. Mater. 2018, 17, 119–128. [Google Scholar] [CrossRef]
- Cheng, P.; Li, G.; Zhan, X.; Yang, Y. Next-generation organic photovoltaics based on non-fullerene acceptors. Nat. Photon. 2018, 12, 131–142. [Google Scholar] [CrossRef]
- Nowak-Król, A.; Wagener, R.; Kraus, F.; Mishra, A.; Bäuerle, P.; Würthner, F. Modulation of Band Gap and P-: Versus n-Semiconductor Character of ADA Dyes by Core and Acceptor Group Variation. Org. Chem. Front. 2016, 3, 545–555. [Google Scholar] [CrossRef]
- Wadsworth, A.; Moser, M.; Marks, A.; Little, M.S.; Gasparini, N.; Brabec, C.J.; Baran, D.; McCulloch, I. Critical review of the molecular design progress in non-fullerene electron acceptors towards commercially viable organic solar cells. Chem. Soc. Rev. 2019, 48, 1596–1625. [Google Scholar] [CrossRef]
- Squeo, B.M.; Gregoriou, V.G.; Avgeropoulos, A.; Baysec, S.; Allard, S.; Scherf, U.; Chochos, C.L. BODIPY-based Polymeric Dyes as Emerging Horizon Materials for Biological Sensing and Organic Electronic Applications. Prog. Polym. Sci. 2017, 71, 26–52. [Google Scholar] [CrossRef]
- Loudet, A.; Burgess, K. BODIPY Dyes and Their Derivatives: Syntheses and Spectroscopic Properties. Chem. Rev. 2007, 107, 4891–4932. [Google Scholar] [CrossRef]
- Boens, N.; Leen, V.; Dehaen, W. Fluorescent Indicators Based on BODIPY. Chem. Soc. Rev. 2012, 41, 1130–1172. [Google Scholar] [CrossRef]
- Zampetti, A.; Minotto, A.; Squeo, B.M.; Gregoriou, V.G.; Allard, S.; Scherf, U.; Chochos, C.L.; Cacialli, F. Highly Efficient Solid-State Near-Infrared Organic Light-Emitting Diodes Incorporating A-D-A Dyes Based on α,β-Unsubstituted “BODIPY” Moieties. Sci. Rep. 2017, 7, 1–7. [Google Scholar] [CrossRef]
- Treibs, A.; Kreuzer, F.-H. Difluorboryl-Komplexe von Di-Und Tripyrrylmethenen. Justus Liebigs Ann. Chem. 1968, 718, 208–223. [Google Scholar] [CrossRef]
- Sathyamoorthi, G.; Boyer, J.H.; Allik, T.H.; Chandra, S. Laser Active Cyanopyromethene- BF2, Corn Plexes. Heteroat. Chem. 1994, 5, 403–407. [Google Scholar] [CrossRef]
- Pavlopoulos, T.G.; Shah, M.; Boyer, J.H. Laser Action from a Tetramethylpyrromethene-BF2 Complex. Appl. Opt. 1988, 27, 4998. [Google Scholar] [CrossRef]
- Wang, D.; Miyamoto, R.; Shiraishi, Y.; Hirai, T. BODIPY-Conjugated Thermoresponsive Copolymer as a Fluorescent Thermometer Based on Polymer Microviscosity. Langmuir 2009, 25, 13176–13182. [Google Scholar] [CrossRef]
- Rosenthal, J.; Lippard, S.J. Direct Detection of Nitroxyl in Aqueous Solution Using a Tripodal Copper(II) BODIPY Complex. J. Am. Chem. Soc. 2010, 132, 5536–5537. [Google Scholar] [CrossRef]
- Lee, C.Y.; Hupp, J.T. Dye Sensitized Solar Cells: TiO2 Sensitization with a Bodipy-Porphyrin Antenna System. Langmuir 2010, 26, 3760–3765. [Google Scholar] [CrossRef]
- Godoy, J.; Vives, G.; Tour, J.M. Synthesis of Highly Fluorescent BODIPY-Based Nanocars. Org. Lett. 2010, 12, 1464–1467. [Google Scholar] [CrossRef]
- Terai, T.; Nagano, T. Fluorescent probes for bioimaging applications. Curr. Opin. Chem. Biol. 2008, 12, 515–521. [Google Scholar] [CrossRef]
- Ulrich, G.; Ziessel, R.; Harriman, A. The Chemistry of Fluorescent Bodipy Dyes: Versatility Unsurpassed. Angew. Chem. Int. Ed. 2008, 47, 1184–1201. [Google Scholar] [CrossRef]
- Carvalho, P.M.; Felício, M.R.; Santos, N.C.; Gonçalves, S.; Domingues, M.M. Application of Light Scattering Techniques to Nanoparticle Characterization and Development. Front. Chem. 2018, 6, 1–17. [Google Scholar] [CrossRef]
- Demas, J.N.; Crosby, G.A. The measurement of Photoluminescence Quantum Yields. J. Chem. Phys. 1971, 75, 991. [Google Scholar]
- Biczók, L.; Bérces, T.; Márta, F. Substituent, Solvent, and Temperature Effect son Radiative and Nonradiative Processes of Singlet Excited Fluorenone Derivatives. J. Phys. Chem. 1993, 97, 8895–8899. [Google Scholar] [CrossRef]
- Fries, F.; Reineke, S. Statistical Treatment of Photoluminescence Quantum Yield Measurements. Sci. Rep. 2019, 9, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Cohen, H.D.; Roothaan, C.C.J. Electric Dipole Polarizability of Atoms by the Hartree-Fock Method. I. Theory for Closed-Shell Systems. J. Chem. Phys. 1965, 43, S34–S39. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09. Revision A.02; Gaussian, Inc.: Wallingford, UK, 2009. [Google Scholar]
- Tomasi, J.; Mennucci, B.; Cammi, R. Quantum Mechanical Continuum Solvation Models. Chem. Rev. 2005, 105, 2999–3093. [Google Scholar] [CrossRef]
- Cossi, M.; Barone, V.; Cammi, R.; Tomasi, J. Ab Initio Study of Solvated Molecules: A New Implementation of the Polarizable Continuum Model. Chem. Phys. Lett. 1996, 255, 327–335. [Google Scholar] [CrossRef]
- Elmowafy, E.M.; Tiboni, M.; Soliman, M.E. Biocompatibility, Biodegradation and Biomedical Applications of Poly(Lactic Acid)/Poly(Lactic-Co-Glycolic Acid) Micro and Nanoparticles; Springer: Singapore, 2019; Volume 49. [Google Scholar]
- Li, K.; Liu, B. Polymer Encapsulated Conjugated Polymer Nanoparticles for Fluorescence Bioimaging. J. Mater. Chem. 2012, 22, 1257–1264. [Google Scholar] [CrossRef]
- Ozdemir, M.; Choi, D.; Kwon, G.; Zorlu, Y.; Cosut, B.; Kim, H.; Facchetti, A.; Kim, C.; Usta, H. Solution-Processable BODIPY-Based Small Molecules for Semiconducting Microfibers in Organic Thin-Film Transistors. ACS Appl. Mater. Interfaces 2016, 8, 14077–14087. [Google Scholar] [CrossRef]
- He, S.; Song, J.; Qu, J.; Cheng, Z. Crucial breakthrough of second near-infrared biological window fluorophores: Design and synthesis toward multimodal imaging and theranostics. Chem. Soc. Rev. 2018, 47, 4258. [Google Scholar] [CrossRef]
- Martin, R.L. Natural Transition Orbitals. J. Chem. Phys. 2003, 118, 4775–4777. [Google Scholar] [CrossRef]
- Muller, P. Glossary of terms used in physical organic chemistry. Pure Appl. Chem. 1994, 66, 1077–1184. [Google Scholar] [CrossRef]
- Huang, S.; Zhang, Q.; Shiota, Y.; Nakagawa, T.; Kuwabara, K.; Yoshizawa, K.; Adachi, C. Computational Prediction for Singlet- and Triplet-Transition Energies of Charge-Transfer Compounds. J. Chem. Theory Comput. 2013, 9, 3872–3877. [Google Scholar] [CrossRef] [PubMed]
- Avramopoulos, A.; Otero, N.; Karamanis, P.; Pouchan, C.; Papadopoulos, M.G. A Computational Study of the Interaction and Polarization Effects of Complexes Involving Molecular Graphene and C60 or a Nucleobases. J. Phys. Chem. A 2016, 120, 284–298. [Google Scholar] [CrossRef] [PubMed]
- Miletić, T.; Fermi, A.; Orfanos, I.; Avramopoulos, A.; De Leo, F.; Demitri, N.; Bergamini, G.; Ceroni, P.; Papadopoulos, M.G.; Couris, S.; et al. Tailoring Colors by O Annulation of Polycyclic Aromatic Hydrocarbons. Chem.-A Eur. J. 2017, 23, 2363–2378. [Google Scholar] [CrossRef]
- Sweeney, S.J.; Mukherjee, J. Optoelectronic Devices and Materials. In Springer Handbook of Electronic and Photonic Materials; Springer: Berlin/Heidelberg, Germany, 2017. [Google Scholar]
- Khalid, M.; Ali, A.; Jawaria, R.; Asghar, M.A.; Asim, S.; Khan, M.U.; Hussain, R.; ur Rehman, M.F.; Ennis, C.J.; Akram, M.S. First principles study of electronic and nonlinearoptical properties of A–D–p–A and D–A–D–p–A configured compounds containing novel quinoline–carbazole derivatives. RSC Adv. 2020, 10, 22273–22283. [Google Scholar] [CrossRef]
- Gooch, J.W. Encyclopedic Dictionary of Polymers; Springer: Berlin/Heidelberg, Germany, 2011. [Google Scholar]
- Clafton, S.N.; Beattie, D.A.; Mierczynska-Vasilev, A.; Acres, R.G.; Morgan, A.C.; Kee, T.W. Chemical Defects in the Highly Fluorescent Conjugated Polymer Dots. Langmuir 2010, 26, 17785–17789. [Google Scholar] [CrossRef]
- Lu, T.; Chen, F. Multiwfn: A Multifunctional Wavefunction Analyzer. J. Comput. Chem. 2012, 33, 580–592. [Google Scholar] [CrossRef]
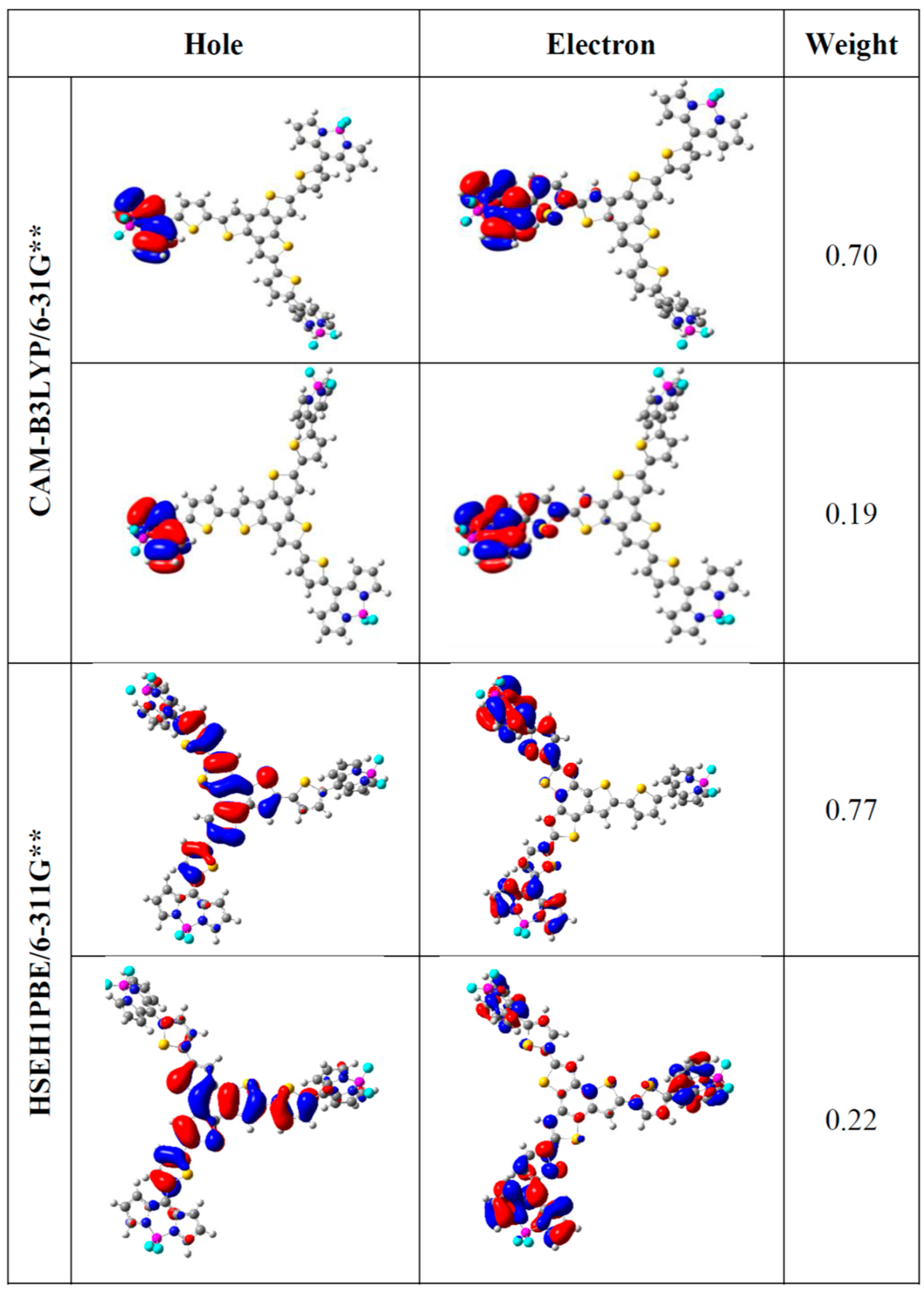
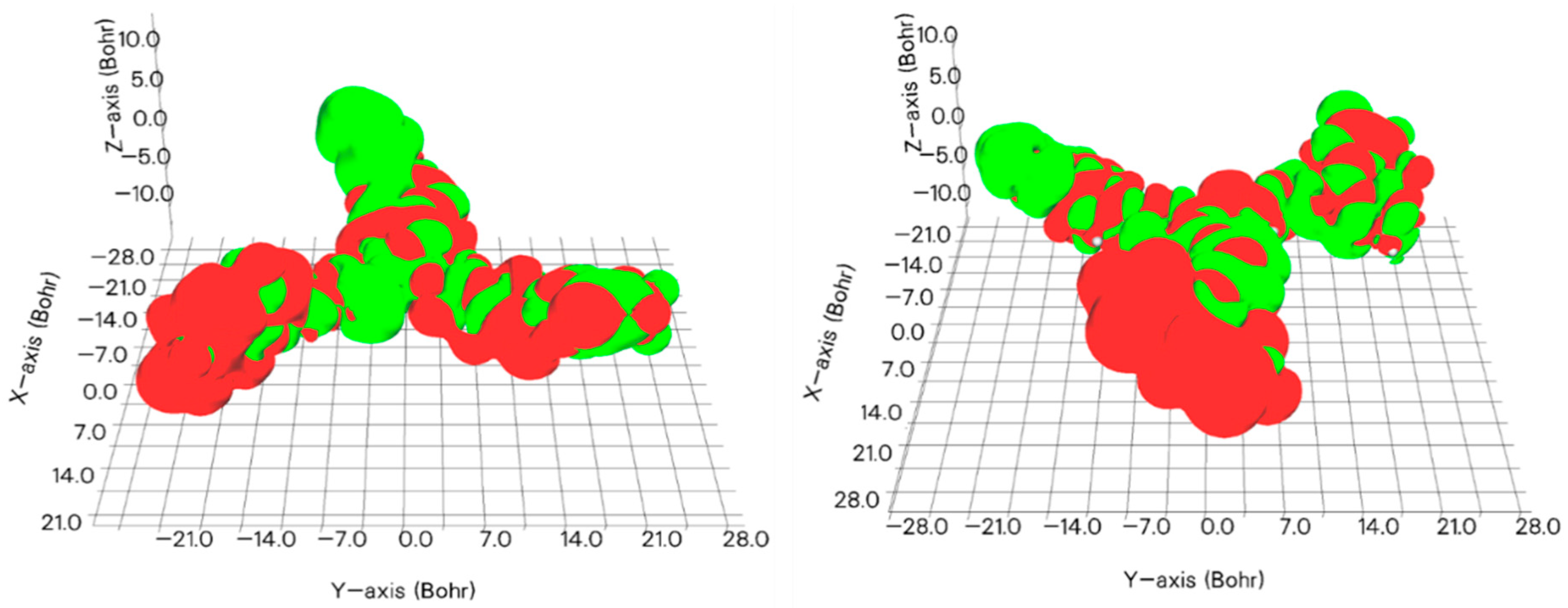


| Molecule 1 | EHOMO | ELUMO | |EHOMO − ELUMO| |
|---|---|---|---|
| CAM-B3LYP/6-31G** | −0.256 | −0.079 | 0.177 |
| CAM-B3LYP/6-311G** | −0.263 | −0.088 | 0.175 |
| PBE1PBE/6-31G** | −0.223 | −0.113 | 0.11 |
| HSEH1PBE/6-311G** | −0.215 | −0.132 | 0.083 |
| B3PW91/6-311G** | −0.223 | −0.125 | 0.098 |
| BLYP/6-31G** | −0.180 | −0.120 | 0.06 |
| B3LYP/6-31G** | −0.220 | −0.116 | 0.104 |
| MP2/6-311G** | −0.281 | −0.010 | 0.271 |
| Exp.2 | −0.225 | −0.144 | 0.081 |
| Molecule | μ | α | β | γ (×106) | Eexc/λ | Etot |
|---|---|---|---|---|---|---|
| 3BDP3Ta | ||||||
| G.P 1 | 1.1603 | 962.53 | 16,053 | 2.713 | −5350.7473 9 | |
| Sol. 2 | 1.4783 | 1193.43 | 28,903 | 5.253 | 2.78/445.5 3 | |
| 1.4734 | 1253.94 | 31,534 | 5.774 | |||
| (0.114) 4,7 | (1524.4) 4,7 | (17,300) 4,7 | (10.7) 4,7 | 2.18/569.4 5 | ||
| 1.4675 | 1371.15 | 72,505 | 19.25 | 2.23/556.8 6 | ||
| (0.108) 5,7 | (1699.6) 5,7 | (41,700) 5,7 | (34.9) 5,7 | 2.10/590.0 5,11 | ||
| (0.117) 7,8 | (1506.9) 7,8 | (15,400) 7,8 | (8.5) 7,8 | |||
| Exp. 10 | 1.633 4,11 | 1390.7 4,11 | 3685 4,11 | 520 | ||
| 3BDP3Tb | ||||||
| G.P 1 | −5350.7514 9 | |||||
| Sol 2,3 | 0.095 | 1201.5 | 400 | 5.88 | 2.78/446.0 3 | |
| 0.114 3,11 | 1326.2 4,11 | 577 4,11 | 2.15/576.0 5 | |||
| 2.08/597.0 5,11 | ||||||
| Exp. 10 | 520 |
| Molecule 1/A | μ 2 | α 2 | β 2 | γ (×106) 2 | EHOMO3 | ELUMO3 | |EHOMO − ELUMO| 3 | Eexc/λ 3 |
|---|---|---|---|---|---|---|---|---|
| Bodipy | 1.474 | 1193 | 2890 | 5.25 | −0.215 | −0.132 | 0.083 | 2.18/569.4 |
| NO2 | 1.561 | 656 | 1625 | 2.54 | −0.225 | −0.127 | 0.098 | 2.62/473 |
| CN | 1.629 | 632 | 636 | 1.57 | −0.219 | −0.104 | 0.115 | 3.09/400.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Squeo, B.M.; Avramopoulos, A.; Nega, A.D.; Pavlou, A.; Siskos, M.G.; Koralli, P.; Schiza, A.; Dimitrakopoulou-Strauss, A.; Gregoriou, V.G.; Chochos, C.L. Far-Red to Near Infrared Emissive Aqueous Nanoparticles Based on a New Organic Material with Three BODIPY Dyes at the Periphery of the Core: A Combined Experimental and Theoretical Study. Electron. Mater. 2021, 2, 24-38. https://doi.org/10.3390/electronicmat2010003
Squeo BM, Avramopoulos A, Nega AD, Pavlou A, Siskos MG, Koralli P, Schiza A, Dimitrakopoulou-Strauss A, Gregoriou VG, Chochos CL. Far-Red to Near Infrared Emissive Aqueous Nanoparticles Based on a New Organic Material with Three BODIPY Dyes at the Periphery of the Core: A Combined Experimental and Theoretical Study. Electronic Materials. 2021; 2(1):24-38. https://doi.org/10.3390/electronicmat2010003
Chicago/Turabian StyleSqueo, Benedetta M., Aggelos Avramopoulos, Alkmini D. Nega, Aristea Pavlou, Michael G. Siskos, Panagiota Koralli, Andriana Schiza, Antonia Dimitrakopoulou-Strauss, Vasilis G. Gregoriou, and Christos L. Chochos. 2021. "Far-Red to Near Infrared Emissive Aqueous Nanoparticles Based on a New Organic Material with Three BODIPY Dyes at the Periphery of the Core: A Combined Experimental and Theoretical Study" Electronic Materials 2, no. 1: 24-38. https://doi.org/10.3390/electronicmat2010003
APA StyleSqueo, B. M., Avramopoulos, A., Nega, A. D., Pavlou, A., Siskos, M. G., Koralli, P., Schiza, A., Dimitrakopoulou-Strauss, A., Gregoriou, V. G., & Chochos, C. L. (2021). Far-Red to Near Infrared Emissive Aqueous Nanoparticles Based on a New Organic Material with Three BODIPY Dyes at the Periphery of the Core: A Combined Experimental and Theoretical Study. Electronic Materials, 2(1), 24-38. https://doi.org/10.3390/electronicmat2010003








