Sleep Deprivation and Its Impact on Insulin Resistance
Abstract
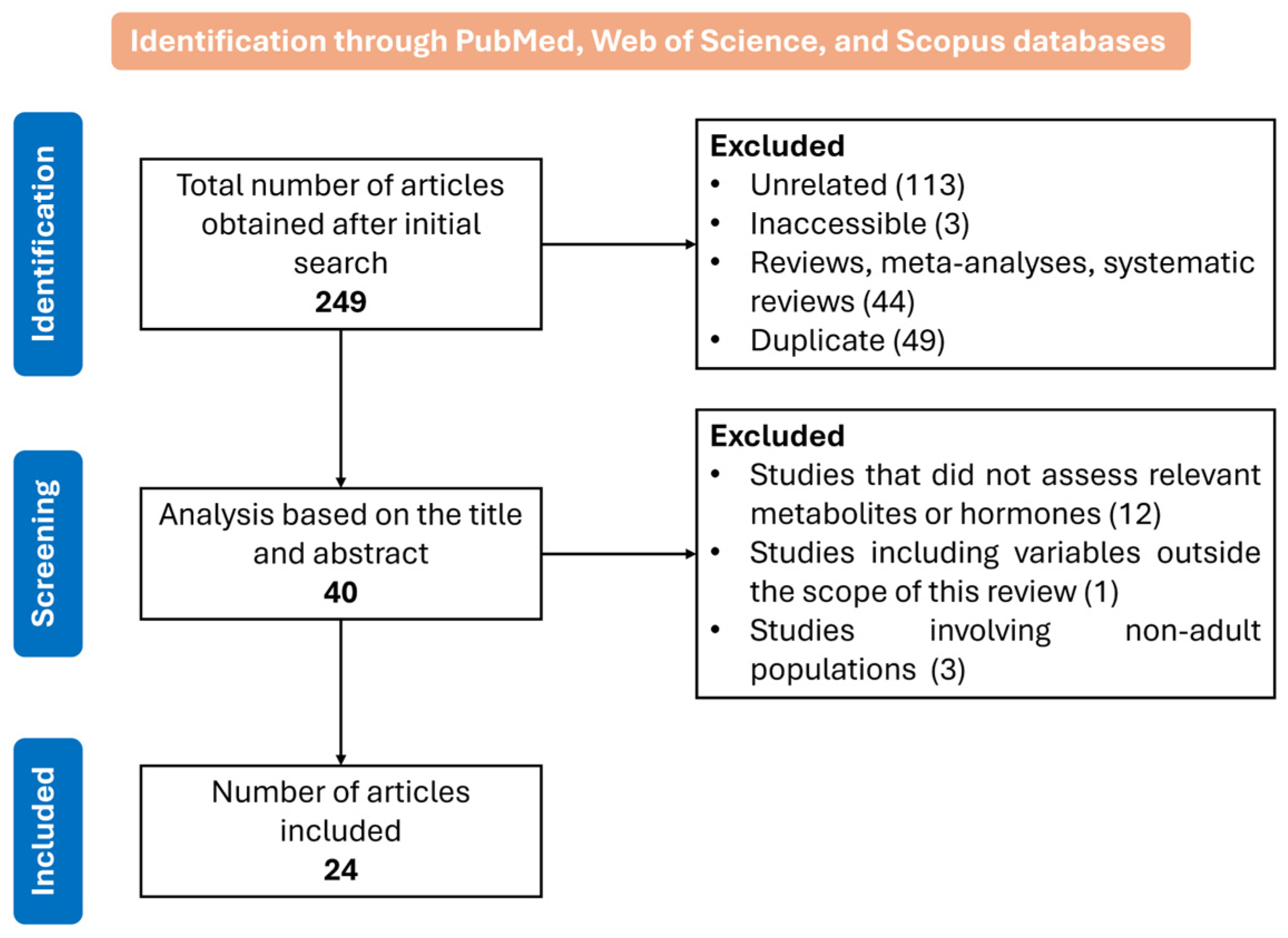
1. Introduction
2. Materials and Methods
3. Human Evidence on the Impact of Sleep Deprivation on Insulin Sensitivity and Metabolic Regulation
3.1. Effects of Sleep Deprivation on Insulin Sensitivity and Glucose Metabolism
3.2. Effects of Sleep Deprivation on Lipolysis, Non-Esterified Fatty Acids, and Insulin Sensitivity
3.3. Impact of Sleep Deprivation on Fatty Acid and Acylcarnitine Metabolism
3.4. Role of Cortisol, Catecholamines, and Growth Hormone in Sleep Deprivation
4. Animal Evidence on the Impact of Sleep Deprivation on Insulin Sensitivity and Metabolic Function
5. Discussion
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| ACTH | Adrenocorticotropic hormone |
| ALT | Alanine aminotransferase |
| AST | Aspartate transaminase |
| AUC | Area under the curve |
| BMI | Body mass index |
| CDC | Centers for Disease Control and Prevention |
| CRH | Corticotropin-releasing hormone |
| CRP | C-reactive protein |
| CSD | Chronically sleep deprived |
| DI | Disposition index |
| EGP | Endogenous glucose production |
| FFA | Free fatty acid |
| FGF-21 | Fibroblast growth factor 21 |
| GGT | Gamma-glutamyl transferase |
| GH | Growth hormone |
| GLP-1 | Glucagon-like peptide-1 |
| HDL | High-density lipoprotein |
| HEC | Hyperinsulinemic–euglycemic clamp |
| HOMA-IR | Homeostasis Model Assessment of Insulin Resistance |
| HPA | Hypothalamic–pituitary–adrenal |
| hsCRP | High-sensitivity C-reactive protein |
| IL | Interleukin |
| IPGTT | Intraperitoneal glucose tolerance test |
| ISI | Insulin sensitivity index |
| ITT | Insulin tolerance test |
| IVGTT | Intravenous glucose tolerance test |
| LDL | Low-density lipoprotein |
| MCR | Metabolic clearance rate |
| MetS | Metabolic syndrome |
| MMPM | Modified multiple platform method |
| NEFA | Non-esterified fatty acids |
| NHANES | National Health and Nutrition Examination Survey |
| NREM | Non-rapid eye movement |
| NSAIDs | Nonsteroidal anti-inflammatory drugs |
| OGIS | Oral glucose insulin sensitivity |
| OGTT | Oral glucose tolerance test |
| pIRS-1 | Phosphorylated insulin receptor substrate-1 |
| PKB | Protein kinase B |
| pNFkB | Phosphorylated nuclear factor kappa B |
| PYY | Peptide YY |
| QUICKI | Quantitative insulin sensitivity check index |
| RBP4 | Retinol-binding protein 4 |
| REM | Rapid eye movement |
| T1DM | Type 1 diabetes mellitus |
| T2DM | Type 2 diabetes mellitus |
| TG | Triglycerides |
| TLR4 | Toll-like receptor 4 |
| TNF-α | Tumor necrosis factor alpha |
| WAT | White adipose tissue |
References
- Baranwal, N.; Yu, P.K.; Siegel, N.S. Sleep physiology, pathophysiology, and sleep hygiene. Prog. Cardiovasc. Dis. 2023, 77, 59–69. [Google Scholar] [CrossRef]
- Liew, S.C.; Aung, T. Sleep deprivation and its association with diseases—A review. Sleep Med. 2021, 77, 192–204. [Google Scholar] [CrossRef]
- Chaput, J.P.; McHill, A.W.; Cox, R.C.; Broussard, J.L.; Dutil, C.; da Costa, B.G.G.; Sampasa-Kanyinga, H.; Wright, K.P., Jr. The role of insufficient sleep and circadian misalignment in obesity. Nat. Rev. Endocrinol. 2023, 19, 82–97. [Google Scholar] [CrossRef]
- Li, J.; Vitiello, M.V.; Gooneratne, N.S. Sleep in Normal Aging. Sleep Med. Clin. 2018, 13, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Bishir, M.; Bhat, A.; Essa, M.M.; Ekpo, O.; Ihunwo, A.O.; Veeraraghavan, V.P.; Mohan, S.K.; Mahalakshmi, A.M.; Ray, B.; Tuladhar, S.; et al. Sleep Deprivation and Neurological Disorders. BioMed Res. Int. 2020, 2020, 5764017. [Google Scholar] [CrossRef] [PubMed]
- Arnaud, C.; Bochaton, T.; Pepin, J.L.; Belaidi, E. Obstructive sleep apnoea and cardiovascular consequences: Pathophysiological mechanisms. Arch. Cardiovasc. Dis. 2020, 113, 350–358. [Google Scholar] [CrossRef]
- Tripathy, D.; Wessman, Y.; Gullstrom, M.; Tuomi, T.; Groop, L. Importance of obtaining independent measures of insulin secretion and insulin sensitivity during the same test: Results with the Botnia clamp. Diabetes Care 2003, 26, 1395–1401. [Google Scholar] [CrossRef]
- Placzkowska, S.; Pawlik-Sobecka, L.; Kokot, I.; Piwowar, A. Indirect insulin resistance detection: Current clinical trends and laboratory limitations. Biomed. Pap. Med. Fac. Univ. Palacky. Olomouc Czech Repub. 2019, 163, 187–199. [Google Scholar] [CrossRef] [PubMed]
- Petrelli, A.; Cugnata, F.; Carnovale, D.; Bosi, E.; Libman, I.M.; Piemonti, L.; Cuthbertson, D.; Sosenko, J.M. HOMA-IR and the Matsuda Index as predictors of progression to type 1 diabetes in autoantibody-positive relatives. Diabetologia 2024, 67, 290–300. [Google Scholar] [CrossRef]
- Leproult, R.; Holmback, U.; Van Cauter, E. Circadian misalignment augments markers of insulin resistance and inflammation, independently of sleep loss. Diabetes 2014, 63, 1860–1869. [Google Scholar] [CrossRef]
- Rao, M.N.; Neylan, T.C.; Grunfeld, C.; Mulligan, K.; Schambelan, M.; Schwarz, J.M. Subchronic sleep restriction causes tissue-specific insulin resistance. J. Clin. Endocrinol. Metab. 2015, 100, 1664–1671. [Google Scholar] [CrossRef]
- Wang, X.; Greer, J.; Porter, R.R.; Kaur, K.; Youngstedt, S.D. Short-Term Moderate Sleep Restriction Decreases Insulin Sensitivity in Young Healthy Adults. Sleep Health 2016, 2, 63–68. [Google Scholar] [CrossRef]
- Broussard, J.L.; Chapotot, F.; Abraham, V.; Day, A.; Delebecque, F.; Whitmore, H.R.; Tasali, E. Sleep restriction increases free fatty acids in healthy men. Diabetologia 2015, 58, 791–798. [Google Scholar] [CrossRef]
- Depner, C.M.; Melanson, E.L.; Eckel, R.H.; Snell-Bergeon, J.K.; Perreault, L.; Bergman, B.C.; Higgins, J.A.; Guerin, M.K.; Stothard, E.R.; Morton, S.J.; et al. Ad libitum Weekend Recovery Sleep Fails to Prevent Metabolic Dysregulation during a Repeating Pattern of Insufficient Sleep and Weekend Recovery Sleep. Curr. Biol. 2019, 29, 957–967.e954. [Google Scholar] [CrossRef] [PubMed]
- Radcliffe, P.N.; Whitney, C.C.; Fagnant, H.S.; Wilson, M.A.; Finlayson, G.; Smith, T.J.; Karl, J.P. Severe sleep restriction suppresses appetite independent of effects on appetite regulating hormones in healthy young men without obesity. Physiol. Behav. 2021, 237, 113438. [Google Scholar] [CrossRef] [PubMed]
- Kanagasabai, T.; Riddell, M.C.; Ardern, C.I. Inflammation, Oxidative Stress, and Antioxidant Micronutrients as Mediators of the Relationship Between Sleep, Insulin Sensitivity, and Glycosylated Hemoglobin. Front. Public Health 2022, 10, 888331. [Google Scholar] [CrossRef]
- Eckel, R.H.; Depner, C.M.; Perreault, L.; Markwald, R.R.; Smith, M.R.; McHill, A.W.; Higgins, J.; Melanson, E.L.; Wright, K.P., Jr. Morning Circadian Misalignment during Short Sleep Duration Impacts Insulin Sensitivity. Curr. Biol. 2015, 25, 3004–3010. [Google Scholar] [CrossRef] [PubMed]
- Deng, H.B.; Tam, T.; Zee, B.C.; Chung, R.Y.; Su, X.; Jin, L.; Chan, T.C.; Chang, L.Y.; Yeoh, E.K.; Lao, X.Q. Short Sleep Duration Increases Metabolic Impact in Healthy Adults: A Population-Based Cohort Study. Sleep 2017, 40, zsx130. [Google Scholar] [CrossRef]
- Li, X.; Lin, L.; Lv, L.; Pang, X.; Du, S.; Zhang, W.; Na, G.; Ma, H.; Zhang, Q.; Jiang, S.; et al. U-shaped relationships between sleep duration and metabolic syndrome and metabolic syndrome components in males: A prospective cohort study. Sleep Med. 2015, 16, 949–954. [Google Scholar] [CrossRef]
- So-Ngern, A.; Chirakalwasan, N.; Saetung, S.; Chanprasertyothin, S.; Thakkinstian, A.; Reutrakul, S. Effects of Two-Week Sleep Extension on Glucose Metabolism in Chronically Sleep-Deprived Individuals. J. Clin. Sleep Med. 2019, 15, 711–718. [Google Scholar] [CrossRef]
- Cedernaes, J.; Lampola, L.; Axelsson, E.K.; Liethof, L.; Hassanzadeh, S.; Yeganeh, A.; Broman, J.E.; Schioth, H.B.; Benedict, C. A single night of partial sleep loss impairs fasting insulin sensitivity but does not affect cephalic phase insulin release in young men. J. Sleep Res. 2016, 25, 5–10. [Google Scholar] [CrossRef]
- Mateus Brandao, L.E.; Espes, D.; Westholm, J.O.; Martikainen, T.; Westerlund, N.; Lampola, L.; Popa, A.; Vogel, H.; Schurmann, A.; Dickson, S.L.; et al. Acute sleep loss alters circulating fibroblast growth factor 21 levels in humans: A randomised crossover trial. J. Sleep Res. 2022, 31, e13472. [Google Scholar] [CrossRef] [PubMed]
- van den Berg, R.; Mook-Kanamori, D.O.; Donga, E.; van Dijk, M.; van Dijk, J.G.; Lammers, G.J.; van Kralingen, K.W.; Prehn, C.; Adamski, J.; Romijn, J.A.; et al. A single night of sleep curtailment increases plasma acylcarnitines: Novel insights in the relationship between sleep and insulin resistance. Arch. Biochem. Biophys. 2016, 589, 145–151. [Google Scholar] [CrossRef]
- van Dijk, D.; Balkau, B.; Segrestin, B.; Gottsater, M.; Gabriel, R.; Hatunic, M.; Mari, A.; Dekker, J.M.; Rutters, F.; Group, E.-R.S. Associations between sleep duration and sleep debt with insulin sensitivity and insulin secretion in the EGIR-RISC Study. Diabetes Metab. 2019, 45, 375–381. [Google Scholar] [CrossRef]
- Davies, S.K.; Ang, J.E.; Revell, V.L.; Holmes, B.; Mann, A.; Robertson, F.P.; Cui, N.; Middleton, B.; Ackermann, K.; Kayser, M.; et al. Effect of sleep deprivation on the human metabolome. Proc. Natl. Acad. Sci. USA 2014, 111, 10761–10766. [Google Scholar] [CrossRef]
- Sweeney, E.L.; Jeromson, S.; Hamilton, D.L.; Brooks, N.E.; Walshe, I.H. Skeletal muscle insulin signaling and whole-body glucose metabolism following acute sleep restriction in healthy males. Physiol. Rep. 2017, 5, e13498. [Google Scholar] [CrossRef]
- Wilms, B.; Leineweber, E.M.; Molle, M.; Chamorro, R.; Pommerenke, C.; Salinas-Riester, G.; Sina, C.; Lehnert, H.; Oster, H.; Schmid, S.M. Sleep Loss Disrupts Morning-to-Evening Differences in Human White Adipose Tissue Transcriptome. J. Clin. Endocrinol. Metab. 2019, 104, 1687–1696. [Google Scholar] [CrossRef] [PubMed]
- Wilms, B.; Chamorro, R.; Hallschmid, M.; Trost, D.; Forck, N.; Schultes, B.; Molle, M.; Sayk, F.; Lehnert, H.; Schmid, S.M. Timing Modulates the Effect of Sleep Loss on Glucose Homeostasis. J. Clin. Endocrinol. Metab. 2019, 104, 2801–2808. [Google Scholar] [CrossRef] [PubMed]
- Venancio, D.P.; Suchecki, D. Prolonged REM sleep restriction induces metabolic syndrome-related changes: Mediation by pro-inflammatory cytokines. Brain Behav. Immun. 2015, 47, 109–117. [Google Scholar] [CrossRef]
- de Oliveira, E.M.; Visniauskas, B.; Sandri, S.; Migliorini, S.; Andersen, M.L.; Tufik, S.; Fock, R.A.; Chagas, J.R.; Campa, A. Late effects of sleep restriction: Potentiating weight gain and insulin resistance arising from a high-fat diet in mice. Obesity 2015, 23, 391–398. [Google Scholar] [CrossRef]
- Xu, X.; Wang, L.; Zhang, Y.; Su, T.; Chen, L.; Zhang, Y.; Ma, W.; Xie, Y.; Wang, T.; Yang, F.; et al. Effects of chronic sleep deprivation on glucose homeostasis in rats. Sleep Biol. Rhythms 2016, 14, 321–328. [Google Scholar] [CrossRef][Green Version]
- Shigiyama, F.; Kumashiro, N.; Tsuneoka, Y.; Igarashi, H.; Yoshikawa, F.; Kakehi, S.; Funato, H.; Hirose, T. Mechanisms of sleep deprivation-induced hepatic steatosis and insulin resistance in mice. Am. J. Physiol. Endocrinol. Metab. 2018, 315, E848–E858. [Google Scholar] [CrossRef]
- Brouwer, A.; Asare Bediako, I.; Paszkiewicz, R.L.; Kolka, C.M.; Bergman, R.N.; Broussard, J.L. Impact of sleep deprivation and high-fat feeding on insulin sensitivity and beta cell function in dogs. Diabetologia 2020, 63, 875–884. [Google Scholar] [CrossRef]
- Kahn, S.E.; Cooper, M.E.; Del Prato, S. Pathophysiology and treatment of type 2 diabetes: Perspectives on the past, present, and future. Lancet 2014, 383, 1068–1083. [Google Scholar] [CrossRef]
- Antza, C.; Kostopoulos, G.; Mostafa, S.; Nirantharakumar, K.; Tahrani, A. The links between sleep duration, obesity and type 2 diabetes mellitus. J. Endocrinol. 2021, 252, 125–141. [Google Scholar] [CrossRef]
- Zhu, B.; Shi, C.; Park, C.G.; Zhao, X.; Reutrakul, S. Effects of sleep restriction on metabolism-related parameters in healthy adults: A comprehensive review and meta-analysis of randomized controlled trials. Sleep Med. Rev. 2019, 45, 18–30. [Google Scholar] [CrossRef] [PubMed]
- Spiegel, K.; Tasali, E.; Penev, P.; Van Cauter, E. Brief communication: Sleep curtailment in healthy young men is associated with decreased leptin levels, elevated ghrelin levels, and increased hunger and appetite. Ann. Intern. Med. 2004, 141, 846–850. [Google Scholar] [CrossRef] [PubMed]
- Cedernaes, J.; Fanelli, F.; Fazzini, A.; Pagotto, U.; Broman, J.E.; Vogel, H.; Dickson, S.L.; Schioth, H.B.; Benedict, C. Sleep restriction alters plasma endocannabinoids concentrations before but not after exercise in humans. Psychoneuroendocrinology 2016, 74, 258–268. [Google Scholar] [CrossRef] [PubMed]
- Benedict, C.; Barclay, J.L.; Ott, V.; Oster, H.; Hallschmid, M. Acute sleep deprivation delays the glucagon-like peptide 1 peak response to breakfast in healthy men. Nutr. Diabetes 2013, 3, e78. [Google Scholar] [CrossRef]
- Rao, R.; Somvanshi, P.; Klerman, E.B.; Marmar, C.; Doyle, F.J., 3rd. Modeling the Influence of Chronic Sleep Restriction on Cortisol Circadian Rhythms, with Implications for Metabolic Disorders. Metabolites 2021, 11, 483. [Google Scholar] [CrossRef]
- Irwin, M.R.; Olmstead, R.; Carroll, J.E. Sleep Disturbance, Sleep Duration, and Inflammation: A Systematic Review and Meta-Analysis of Cohort Studies and Experimental Sleep Deprivation. Biol. Psychiatry 2016, 80, 40–52. [Google Scholar] [CrossRef] [PubMed]
- Park, H.K.; Kwak, M.K.; Kim, H.J.; Ahima, R.S. Linking resistin, inflammation, and cardiometabolic diseases. Korean J. Intern. Med. 2017, 32, 239–247. [Google Scholar] [CrossRef] [PubMed]
- Farjo, K.M.; Farjo, R.A.; Halsey, S.; Moiseyev, G.; Ma, J.X. Retinol-binding protein 4 induces inflammation in human endothelial cells by an NADPH oxidase- and nuclear factor kappa B-dependent and retinol-independent mechanism. Mol. Cell Biol. 2012, 32, 5103–5115. [Google Scholar] [CrossRef] [PubMed]
- Oeltmann, T.; Carson, R.; Shannon, J.R.; Ketch, T.; Robertson, D. Assessment of O-methylated catecholamine levels in plasma and urine for diagnosis of autonomic disorders. Auton. Neurosci. 2004, 116, 1–10. [Google Scholar] [CrossRef]
- Donga, E.; van Dijk, M.; van Dijk, J.G.; Biermasz, N.R.; Lammers, G.J.; van Kralingen, K.W.; Corssmit, E.P.; Romijn, J.A. A single night of partial sleep deprivation induces insulin resistance in multiple metabolic pathways in healthy subjects. J. Clin. Endocrinol. Metab. 2010, 95, 2963–2968. [Google Scholar] [CrossRef]
- DeFronzo, R.A. Dysfunctional fat cells, lipotoxicity and type 2 diabetes. Int. J. Clin. Pract. Suppl. 2004, 58, 9–21. [Google Scholar] [CrossRef]
- Spiegel, K.; Leproult, R.; Colecchia, E.F.; L’Hermite-Baleriaux, M.; Nie, Z.; Copinschi, G.; Van Cauter, E. Adaptation of the 24-h growth hormone profile to a state of sleep debt. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2000, 279, R874–R883. [Google Scholar] [CrossRef]
| System | Main Effects |
|---|---|
| Metabolic |
|
| Cardiovascular |
|
| Immune |
|
| Neurological |
|
| Psychological |
|
| Author and Year | Sample | Analyzed Markers | Results |
|---|---|---|---|
| Leproult et al., 2014 [10] | 26 healthy adults | Glucose, hsCRP | SR reduced insulin sensitivity without compensatory insulin increase, and inflammation increased. Mean hsCRP levels did not vary significantly in the SR group. |
| Davies et al., 2014 [25] | 12 healthy men | 171 metabolites (acylcarnitines) | Most metabolites remained unchanged; only 16% were significantly increased, including 8 acylcarnitines, serotonin, taurine, tryptophan, 13 glycerophospholipids, and 3 sphingolipids. |
| Deng et al., 2014 [18] | 162,121 individuals | Fasting glucose, HDL, LDL, Cholesterol | <6 h sleep/day significantly increased risk of hypertension, prediabetes, diabetes, low HDL-C, hypertriglyceridemia, and MetS. >8 h sleep/day lowered risk of hypertriglyceridemia and MetS. |
| Li et al., 2015 [19] | 4774 participants without MetS | Fasting glucose, BMI, waist circumference | <6 h sleep led to increased fasting glucose; BMI and waist circumference did not vary. Long sleep led to increased risk of elevated fasting glucose. |
| Eckel et al., 2015 [17] | 8 women and 8 men | OGTT, IVGTT, Melatonin | 21% decrease in insulin sensitivity. Longer elevated melatonin levels associated with worse sensitivity. Compensatory insulin secretion increased. |
| Rao et al., 2015 [11] | 14 healthy men and women | OGTT, NEFA, β-hydroxybutyrate | No changes in fasting glucose/insulin. Glucose AUC and insulin levels unchanged, but insulin AUC increased 20% after SR. 25% decrease in whole-body insulin sensitivity and 29% decrease in peripheral sensitivity. SR increased NEFA use by 62%, β-hydroxybutyrate by 55%, urinary cortisol by 21%, urinary metanephrines by 8%, and normetanephrines by 18%. |
| Wang et al., 2015 [12] | 15 non-obese men | OGTT, Cortisol, Glucagon | Insulin levels significantly increased pre- and post-glucose. Insulin sensitivity indices (Matsuda, HOMA-IR, QUICKI) indicated reduced sensitivity. Glucagon increased after SR; cortisol unchanged. |
| Cedernaes et al., 2015 [21] | 16 men | OGTT, Glucose, Insulin | Elevated insulin in the morning after SR; HOMA-IR increased, suggesting insulin resistance. Glucose and cephalic insulin responses unchanged. One night of SR sufficient to impair insulin sensitivity. |
| Broussard et al., 2015 [13] | 19 healthy men | NEFA, GH, Noradrenaline, Cortisol, Glucose, Insulin, IVGTT | SR raised nocturnal/morning NEFA (15–30%), contributing to insulin resistance. Insulin sensitivity via IVGTT dropped 23%. NEFA plateaued in SR vs. normal decline. Cortisol peaked 2 h earlier, increased 23%. Noradrenaline and GH increased. |
| Van den Berg et al., 2015 [23] | 18 individuals (9 healthy, 7 T1DM; 2 excluded) | Glucose, FFA, Acylcarnitines | One night of SR increased peripheral insulin resistance and hepatic endogenous glucose production. Increased acylcarnitines. |
| Sweeney et al., 2017 [26] | 10 healthy male participants | OGTT, Muscle tissue | 2 nights of SR altered glucose regulation via whole-body insulin sensitivity reduction. PKB activity results were inconclusive. |
| Dijk et al., 2018 [24] | 1002 participants | OGTT, Glucose, Insulin | SR duration linked to lower insulin sensitivity. Insulin secretion remained unchanged. Sleep influences insulin resistance. |
| Wilms et al., 2018 [27] | 15 men | Glucose, Insulin, C-peptide, TG, FFA, Resistin, IL-8, RBP4 | SR reduced β-cell secretory capacity without increasing insulin resistance. No changes in TG and FFA. RBP4 increased; IL-8 and resistin unchanged. |
| Depner et al., 2019 [14] | 36 participants (18 men, 18 women) | Melatonin, Glucose, Insulin | SR decreased total insulin sensitivity (possibly brain/kidney-mediated). Weekend recovery reduced total, hepatic, and muscular insulin sensitivity. |
| Wilms et al., 2019 [28] | 15 men | IVGTT (insulin & glucose), Cortisol, Glucagon | 16% reduction in insulin sensitivity in both early/late SR. No differences in peripheral sensitivity. β-cell response reduced in both SR types. Glucagon levels unchanged overall but decreased with late-night SR. Cortisol levels varied by sleep phase. |
| So-ngern et al., 2019 [20] | 21 healthy participants (19 women, 2 men) | OGTT (glucose & insulin) | Sleep extension improved glycemic parameters: reduced fasting insulin resistance, increased insulin secretion, and improved β-cell function. |
| Radcliffe et al., 2021 [15] | 18 healthy men | Ghrelin, PYY, GLP-1, Insulin, Glucose | Lower postprandial PYY and GLP-1, higher glucose in SR vs. control. Fasting glucose stable; insulin unchanged. Postprandial ghrelin and insulin unaffected. |
| Mateus Brandão et al., 2021 [22] | 15 participants | FGF-21, Adipose & skeletal muscle tissue, OGTT, Cortisol | SR increased glucose AUC. Circulating FGF-21 increased post-SR; no tissue-specific production/response changes. Attenuated cortisol shift from night to morning. |
| Kanagasabai et al., 2022 [16] | 10,348 participants | CRP, GGT, Bilirubin, Carotenoids, Uric Acid, Vitamins A, C, D, E, Insulin, HbA1c | Poor sleep quality/duration affected oxidative stress and inflammation markers, potentially promoting systemic insulin resistance. |
| Author and Year | Sample | Analyzed Markers | Results |
|---|---|---|---|
| Venancio et al., 2014 [29] | 34 male Wistar rats | Leptin; TLR4 and pNFκB; pIRS-1 and hypothalamic leptin receptor; IL-6, IL-10 and TNF-α; Endotoxin; IPGTT | Expression of pro-inflammatory cytokines increased in the SR21 group. Elevated pNFκB in adipose tissue suggests it is the source of cytokine production. Higher glycemia at 30 min and delayed return to baseline glucose levels in the SR21 group. Elevated endotoxin levels in the SR21 group. |
| de Oliveira et al., 2015 [30] | Male C57BL mice | Glucose and insulin tolerance tests; Glycemia; Fasting leptin, insulin and adiponectin; Resistin and free glycerol | Metabolic alterations during sleep restriction appear to primarily affect adipose tissue, exacerbating the detrimental effects of diet-induced obesity. |
| Xu et al., 2016 [31] | 24 female Sprague-Dawley rats | IPGTT; ITT; Blood: glucose, AST, ALT, creatinine, triglycerides, total cholesterol, LDL and HDL; Body weight | Glucose intolerance developed in CSD rats after 3 months of sleep deprivation (via IPGTT). CSD rats showed marked increases in HOMA-IR and ITT, indicating reduced insulin sensitivity. |
| Shigiyama et al., 2018 [32] | Male C57BL/6J mice | Blood glucose; Plasma insulin; Glucagon; Corticosterone; ACTH; Hepatic triglycerides; Hepatic metabolites | Sleep restriction-induced hepatic steatosis and hepatic insulin resistance appear to be mediated by upregulation of lipogenic hepatic enzymes. |
| Brouwer et al., 2020 [33] | 24 adult mixed-breed dogs (>1 year old, normal weight) | Glucose tolerance test; NEFA; Cortisol; Epinephrine and norepinephrine; Insulin and glucose | A single night of experimental sleep deprivation impairs insulin sensitivity without compensatory β-cell response. Sleep deprivation impaired insulin sensitivity similar to that observed after 9 months of chronic high-fat diet. No evidence of adipose tissue insulin resistance observed in response to sleep deprivation. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pinheiro, M.C.; Costa, H.E.; Mariana, M.; Cairrao, E. Sleep Deprivation and Its Impact on Insulin Resistance. Endocrines 2025, 6, 49. https://doi.org/10.3390/endocrines6040049
Pinheiro MC, Costa HE, Mariana M, Cairrao E. Sleep Deprivation and Its Impact on Insulin Resistance. Endocrines. 2025; 6(4):49. https://doi.org/10.3390/endocrines6040049
Chicago/Turabian StylePinheiro, Margarida C., Henrique E. Costa, Melissa Mariana, and Elisa Cairrao. 2025. "Sleep Deprivation and Its Impact on Insulin Resistance" Endocrines 6, no. 4: 49. https://doi.org/10.3390/endocrines6040049
APA StylePinheiro, M. C., Costa, H. E., Mariana, M., & Cairrao, E. (2025). Sleep Deprivation and Its Impact on Insulin Resistance. Endocrines, 6(4), 49. https://doi.org/10.3390/endocrines6040049









