Precocious Puberty and Benign Variants in Female Children: Etiology, Diagnostic Challenges, and Clinical Management
Abstract
1. Introduction
2. Physiology of Puberty
2.1. Fetal Activation of the HPG Axis
2.2. Postnatal Reactivation: Mini-Puberty
2.3. Onset of True Puberty
2.4. Endocrine and Physiological Changes
2.5. Clinical Progression and Pubertal Staging
3. Disorders of Pubertal Development
3.1. Gonadotropin-Dependent Precocious Puberty
3.2. Gonadotropin-Independent Precocious Puberty
3.3. Benign Variants
3.3.1. Premature Thelarche
3.3.2. Premature Adrenarche
3.3.3. Premature Menarche
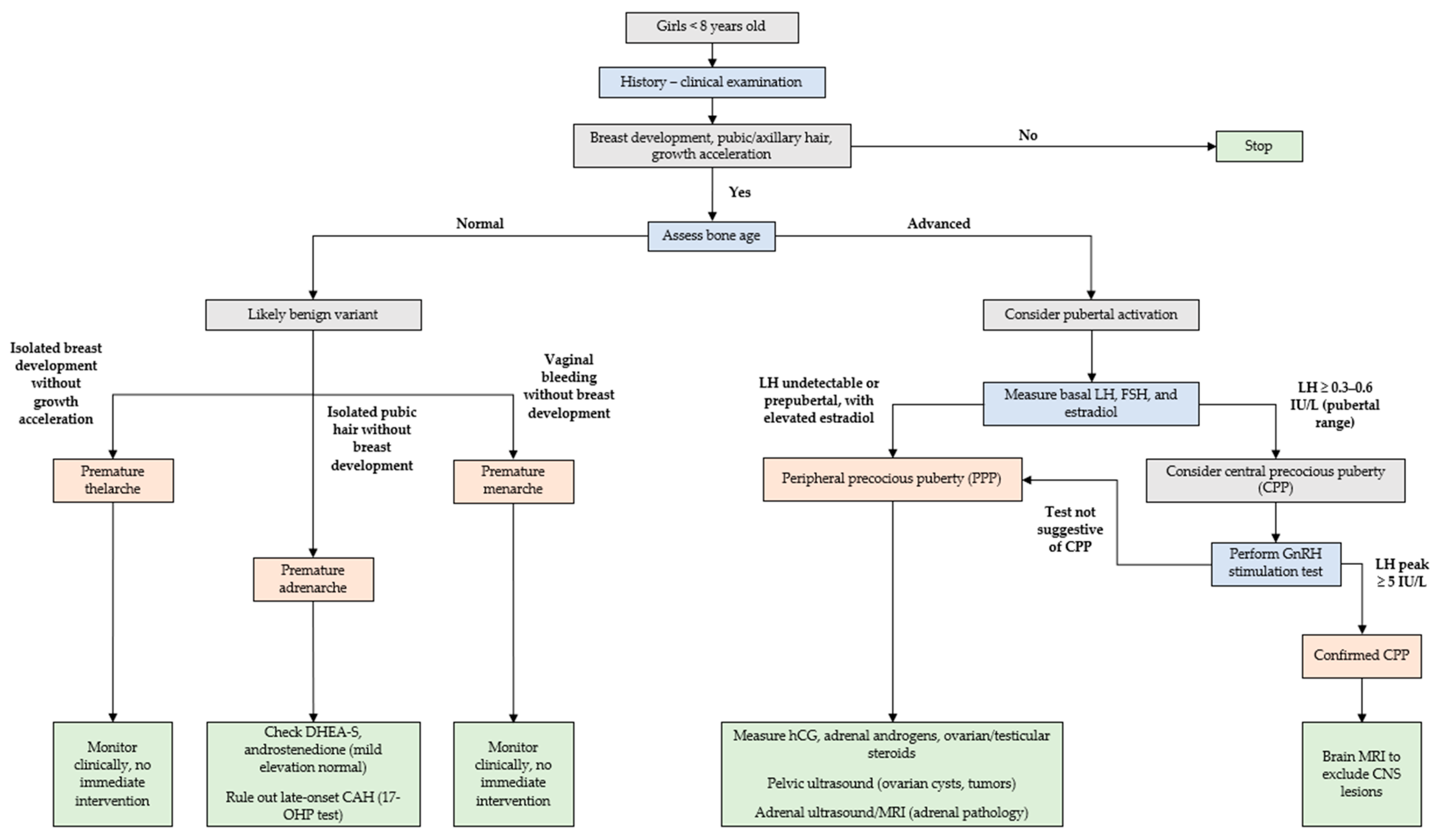
4. Management
4.1. History and Clinical Examination
4.2. Laboratory Tests
- Adrenal androgens: Elevated DHEA-S, androstenedione, and 17-OHP (and testosterone) levels may indicate premature adrenarche or non-classic CAH [59].
- IGF-1 and inhibin B: These markers provide insights into growth patterns and gonadal function, respectively [100].
- Thyroid function: Evaluation of TSH and free thyroxine is essential to exclude hypothyroidism, which can rarely present with pubertal changes [39].
4.3. Imaging
4.4. Treatment
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| 17-OHP | 17-hydroxyprogesterone |
| BMI | Body mass index |
| CAH | Congenital adrenal hyperplasia |
| CNS | Central nervous system |
| CPP | Central precocious puberty |
| DHEA | Dehydroepiandrosterone |
| DHEA-S | Dehydroepiandrosterone sulfate |
| EDCs | Endocrine-disrupting chemicals |
| FSH | Follicle-stimulating hormone |
| GnRH | Gonadotropin-releasing hormone |
| GnRHa | Gonadotropin-releasing hormone agonists |
| GPR54 | G-protein-coupled receptor 54 |
| HPG | Hypothalamic–pituitary–gonadal |
| IGF-1 | Insulin-like growth factor 1 |
| LH | Luteinizing hormone |
| MAS | McCune–Albright syndrome |
| MRI | Magnetic resonance imaging |
| PPP | Peripheral precocious puberty |
| SHBG | Sex hormone-binding globulin |
| TSH | Thyroid-stimulating hormone |
References
- Guarneri, A.M.; Kamboj, M.K. Physiology of Pubertal Development in Females. Pediatr. Med. 2019, 2, 42. [Google Scholar] [CrossRef]
- Spaziani, M.; Tarantino, C.; Tahani, N.; Gianfrilli, D.; Sbardella, E.; Lenzi, A.; Radicioni, A.F. Hypothalamo-Pituitary Axis and Puberty. Mol. Cell. Endocrinol. 2021, 520, 111094. [Google Scholar] [CrossRef] [PubMed]
- Janes, L. Hypothalamic-Pituitary-Gonadal (HPG) Axis. In Encyclopedia of Personality and Individual Differences; Zeigler-Hill, V., Shackelford, T.K., Eds.; Springer International Publishing: Cham, Switzerland, 2016; pp. 1–2. ISBN 978-3-319-28099-8. [Google Scholar]
- Micangeli, G.; Paparella, R.; Tarani, F.; Menghi, M.; Ferraguti, G.; Carlomagno, F.; Spaziani, M.; Pucarelli, I.; Greco, A.; Fiore, M.; et al. Clinical Management and Therapy of Precocious Puberty in the Sapienza University Pediatrics Hospital of Rome, Italy. Children 2023, 10, 1672. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Mukhopadhyay, S.; Dutta, D. Challenges and Controversies in Diagnosis and Management of Gonadotropin Dependent Precocious Puberty: An Indian Perspective. Indian J. Endocrinol. Metab. 2015, 19, 228. [Google Scholar] [CrossRef]
- Marshall, W.A.; Tanner, J.M. Variations in Pattern of Pubertal Changes in Girls. Arch. Dis. Child. 1969, 44, 291–303. [Google Scholar] [CrossRef]
- Berberoğlu, M. Precocious Puberty and Normal Variant Puberty: Definition, Etiology, Diagnosis and Current Management—Review. J. Clin. Res. Pediatr. Endocrinol. 2009, 1, 164–174. [Google Scholar] [CrossRef]
- Varimo, T.; Huttunen, H.; Miettinen, P.J.; Kariola, L.; Hietamäki, J.; Tarkkanen, A.; Hero, M.; Raivio, T. Precocious Puberty or Premature Thelarche: Analysis of a Large Patient Series in a Single Tertiary Center with Special Emphasis on 6- to 8-Year-Old Girls. Front. Endocrinol. 2017, 8, 213. [Google Scholar] [CrossRef]
- Algedik, P.; Kirmizibekmez, H.; Dursun, F. Psychological Effects of Precocious Puberty on Young Girls and Their Mothers. J. Pediatr. Endocrinol. Diabetes 2025, 4, 129–134. [Google Scholar] [CrossRef]
- Alotaibi, M.F. Physiology of Puberty in Boys and Girls and Pathological Disorders Affecting Its Onset. J. Adolesc. 2019, 71, 63–71. [Google Scholar] [CrossRef]
- Kuiri-Hänninen, T.; Sankilampi, U.; Dunkel, L. Activation of the Hypothalamic-Pituitary-Gonadal Axis in Infancy: Minipuberty. Horm. Res. Paediatr. 2014, 82, 73–80. [Google Scholar] [CrossRef]
- Clements, J.A.; Reyes, F.I.; Winter, J.S.D.; Faiman, C. Studies on Human Sexual Development. III. Fetal Pituitary and Serum, and Amniotic Fluid Concentrations of LH, CG, and FSH. J. Clin. Endocrinol. Metab. 1976, 42, 9–19. [Google Scholar] [CrossRef]
- Pilavdzic, D.; Kovacs, K.; Asa, S.L. Pituitary Morphology in Anencephalic Human Fetuses. Neuroendocrinology 1997, 65, 164–172. [Google Scholar] [CrossRef]
- Debieve, F.; Beerlandt, S.; Hubinont, C.; Thomas, K. Gonadotropins, Prolactin, Inhibin A, Inhibin B, and Activin A in Human Fetal Serum from Midpregnancy and Term Pregnancy. J. Clin. Endocrinol. Metab. 2000, 85, 270–274. [Google Scholar] [CrossRef]
- Troisi, R.; Potischman, N.; Roberts, J.M.; Harger, G.; Markovic, N.; Cole, B.; Lykins, D.; Siiteri, P.; Hoover, R.N. Correlation of Serum Hormone Concentrations in Maternal and Umbilical Cord Samples. Cancer Epidemiol. Biomark. Prev. Publ. Am. Assoc. Cancer Res. Cosponsored Am. Soc. Prev. Oncol. 2003, 12, 452–456. [Google Scholar]
- Winter, J.S.D.; Faiman, C.; Hobson, W.C.; Prasad, A.V.; Reyes, F.I. Pituitary-Gonadal Relations in Infancy. I. Patterns of Serum Gonadotropin Concentrations from Birth to Four Years of Age in Man and Chimpanzee. J. Clin. Endocrinol. Metab. 1975, 40, 545–551. [Google Scholar] [CrossRef]
- Kuiri-Hänninen, T.; Kallio, S.; Seuri, R.; Tyrväinen, E.; Liakka, A.; Tapanainen, J.; Sankilampi, U.; Dunkel, L. Postnatal Developmental Changes in the Pituitary-Ovarian Axis in Preterm and Term Infant Girls. J. Clin. Endocrinol. Metab. 2011, 96, 3432–3439. [Google Scholar] [CrossRef]
- Reyes, F.I.; Boroditsky, R.S.; Winter, J.S.D.; Faiman, C. Studies on Human Sexual Development. II. Fetal and Maternal Serum Gonadotropin and Sex Steroid Concentrations1. J. Clin. Endocrinol. Metab. 1974, 38, 612–617. [Google Scholar] [CrossRef]
- Kaplowitz, P.B.; Lee, P.A. Females with Breast Development before Three Years of Age. Endocrinol. Metab. Clin. N. Am. 2024, 53, 195–201. [Google Scholar] [CrossRef]
- Messager, S.; Chatzidaki, E.E.; Ma, D.; Hendrick, A.G.; Zahn, D.; Dixon, J.; Thresher, R.R.; Malinge, I.; Lomet, D.; Carlton, M.B.L.; et al. Kisspeptin Directly Stimulates Gonadotropin-Releasing Hormone Release via G Protein-Coupled Receptor 54. Proc. Natl. Acad. Sci. USA 2005, 102, 1761–1766. [Google Scholar] [CrossRef]
- De Roux, N.; Genin, E.; Carel, J.-C.; Matsuda, F.; Chaussain, J.-L.; Milgrom, E. Hypogonadotropic Hypogonadism Due to Loss of Function of the KiSS1-Derived Peptide Receptor GPR54. Proc. Natl. Acad. Sci. USA 2003, 100, 10972–10976. [Google Scholar] [CrossRef]
- d’Anglemont De Tassigny, X.; Fagg, L.A.; Dixon, J.P.C.; Day, K.; Leitch, H.G.; Hendrick, A.G.; Zahn, D.; Franceschini, I.; Caraty, A.; Carlton, M.B.L.; et al. Hypogonadotropic Hypogonadism in Mice Lacking a Functional Kiss1 Gene. Proc. Natl. Acad. Sci. USA 2007, 104, 10714–10719. [Google Scholar] [CrossRef]
- Iwasa, T.; Matsuzaki, T.; Kinouchi, R.; Gereltsetseg, G.; Murakami, M.; Nakazawa, H.; Yasui, T.; Irahara, M. Changes in the Responsiveness of Serum Leptin and Hypothalamic Neuropeptide Y mRNA Levels to Food Deprivation in Developing Rats. Int. J. Dev. Neurosci. 2011, 29, 377–380. [Google Scholar] [CrossRef]
- Roemmich, J.N.; Rogol, A.D. Role of Leptin during Childhood Growth and Development. Endocrinol. Metab. Clin. N. Am. 1999, 28, 749–764. [Google Scholar] [CrossRef]
- Kaplowitz, P.B.; Slora, E.J.; Wasserman, R.C.; Pedlow, S.E.; Herman-Giddens, M.E. Earlier Onset of Puberty in Girls: Relation to Increased Body Mass Index and Race. Pediatrics 2001, 108, 347–353. [Google Scholar] [CrossRef]
- Rege, J.; Turcu, A.F.; Kasa-Vubu, J.Z.; Lerario, A.M.; Auchus, G.C.; Auchus, R.J.; Smith, J.M.; White, P.C.; Rainey, W.E. 11-Ketotestosterone Is the Dominant Circulating Bioactive Androgen During Normal and Premature Adrenarche. J. Clin. Endocrinol. Metab. 2018, 103, 4589–4598. [Google Scholar] [CrossRef]
- Ibáñez, L.; Dimartino-Nardi, J.; Potau, N.; Saenger, P. Premature Adrenarche--Normal Variant or Forerunner of Adult Disease? Endocr. Rev. 2000, 21, 671–696. [Google Scholar] [CrossRef]
- Cole, T.J.; Ahmed, M.L.; Preece, M.A.; Hindmarsh, P.; Dunger, D.B. The Relationship between Insulin-like Growth Factor 1, Sex Steroids and Timing of the Pubertal Growth Spurt. Clin. Endocrinol. 2015, 82, 862–869. [Google Scholar] [CrossRef]
- Blumenthal, H.; Leen-Feldner, E.W.; Badour, C.L.; Trainor, C.D.; Babson, K.A. Pubertal Maturation and Cortisol Level in Response to a Novel Social Environment among Female Adolescents. J. Adolesc. 2014, 37, 893–900. [Google Scholar] [CrossRef]
- Wang, Y.; He, D.; Fu, C.; Dong, X.; Jiang, F.; Su, M.; Xu, Q.; Huang, P.; Wang, N.; Chen, Y.; et al. Thyroid Function Changes and Pubertal Progress in Females: A Longitudinal Study in Iodine-Sufficient Areas of East China. Front. Endocrinol. 2021, 12, 653680. [Google Scholar] [CrossRef]
- Marshall, W.A.; Tanner, J.M. Variations in the Pattern of Pubertal Changes in Boys. Arch. Dis. Child. 1970, 45, 13–23. [Google Scholar] [CrossRef]
- Alaaraj, N.; Soliman, A.T.; De Sanctis, V.; Hamed, N.; Alyafai, F.; Ahmed, S.; Khalil, A.; Bedair, E.; Elawwa, A. Growth, Bone Maturation and Ovarian Size in Girls with Early and Fast Puberty (EFP) and Effects of Three Years Treatment with GnRH Analogue (GnRHa): Early and Fast Puberty (EFP) and GnRH Analogue (GnRHa) Treatment. Acta Biomed. Atenei Parm. 2022, 92, e2021333. [Google Scholar] [CrossRef]
- Cheng, T.S.; Ong, K.K.; Biro, F.M. Trends Toward Earlier Puberty Timing in Girls and Its Likely Mechanisms. J. Pediatr. Adolesc. Gynecol. 2022, 35, 527–531. [Google Scholar] [CrossRef]
- Eckert-Lind, C.; Busch, A.S.; Petersen, J.H.; Biro, F.M.; Butler, G.; Bräuner, E.V.; Juul, A. Worldwide Secular Trends in Age at Pubertal Onset Assessed by Breast Development Among Girls: A Systematic Review and Meta-Analysis. JAMA Pediatr. 2020, 174, e195881. [Google Scholar] [CrossRef]
- Euling, S.Y.; Herman-Giddens, M.E.; Lee, P.A.; Selevan, S.G.; Juul, A.; SØrensen, T.I.A.; Dunkel, L.; Himes, J.H.; Teilmann, G.; Swan, S.H. Examination of US Puberty-Timing Data from 1940 to 1994 for Secular Trends: Panel Findings. Pediatrics 2008, 121, S172–S191. [Google Scholar] [CrossRef]
- Biro, F.M.; Greenspan, L.C.; Galvez, M.P.; Pinney, S.M.; Teitelbaum, S.; Windham, G.C.; Deardorff, J.; Herrick, R.L.; Succop, P.A.; Hiatt, R.A.; et al. Onset of Breast Development in a Longitudinal Cohort. Pediatrics 2013, 132, 1019–1027. [Google Scholar] [CrossRef] [PubMed]
- Biro, F.M.; Pajak, A.; Wolff, M.S.; Pinney, S.M.; Windham, G.C.; Galvez, M.P.; Greenspan, L.C.; Kushi, L.H.; Teitelbaum, S.L. Age of Menarche in a Longitudinal US Cohort. J. Pediatr. Adolesc. Gynecol. 2018, 31, 339–345. [Google Scholar] [CrossRef]
- Herman-Giddens, M.E.; Slora, E.J.; Wasserman, R.C.; Bourdony, C.J.; Bhapkar, M.V.; Koch, G.G.; Hasemeier, C.M. Secondary Sexual Characteristics and Menses in Young Girls Seen in Office Practice: A Study from the Pediatric Research in Office Settings Network. Pediatrics 1997, 99, 505–512. [Google Scholar] [CrossRef]
- Kaplowitz, P.; Bloch, C.; the Section on Endocrinology; Sills, I.N.; Bloch, C.A.; Casella, S.J.; Gonzalez, J.L.; Lynch, J.L.; Wintergerst, K.A. Evaluation and Referral of Children with Signs of Early Puberty. Pediatrics 2016, 137, e20153732. [Google Scholar] [CrossRef]
- Latronico, A.C.; Brito, V.N.; Carel, J.-C. Causes, Diagnosis, and Treatment of Central Precocious Puberty. Lancet Diabetes Endocrinol. 2016, 4, 265–274. [Google Scholar] [CrossRef]
- Alghamdi, A. Precocious Puberty: Types, Pathogenesis and Updated Management. Cureus 2023, 15, e47485. [Google Scholar] [CrossRef]
- De Sanctis, V.; Corrias, A.; Rizzo, V.; Bertelloni, S.; Urso, L.; Galluzzi, F.; Pasquino, A.M.; Pozzan, G.; Guarneri, M.P.; Cisternino, M.; et al. Etiology of Central Precocious Puberty in Males: The Results of the Italian Study Group for Physiopathology of Puberty. J. Pediatr. Endocrinol. Metab. 2000, 13, 687–694. [Google Scholar] [CrossRef]
- Mitrica, M.; Manole, A.M.; Toma, M.; Sirbu, O.M.; Sirbu, A.M.; Munteanu, A.E. Hypothalamic Hamartomas: A Narrative Review. Biomedicines 2025, 13, 371. [Google Scholar] [CrossRef]
- Scholly, J.; Bartolomei, F. Gelastic Seizures and the Hypothalamic Hamartoma Syndrome: Epileptogenesis beyond the Lesion? Handb. Clin. Neurol. 2021, 182, 143–154. [Google Scholar] [CrossRef]
- Huynh, Q.T.V.; Ho, B.T.; Le, N.Q.K.; Trinh, T.H.; Lam, L.H.T.; Nguyen, N.T.K.; Huang, S.-Y. Pathological Brain Lesions in Girls with Central Precocious Puberty at Initial Diagnosis in Southern Vietnam. Ann. Pediatr. Endocrinol. Metab. 2022, 27, 105–112. [Google Scholar] [CrossRef]
- Ogilvy-Stuart, A.L.; Clayton, P.E.; Shalet, S.M. Cranial Irradiation and Early Puberty. J. Clin. Endocrinol. Metab. 1994, 78, 1282–1286. [Google Scholar] [CrossRef]
- Dassa, Y.; Crosnier, H.; Chevignard, M.; Viaud, M.; Personnier, C.; Flechtner, I.; Meyer, P.; Puget, S.; Boddaert, N.; Breton, S.; et al. Pituitary Deficiency and Precocious Puberty after Childhood Severe Traumatic Brain Injury: A Long-Term Follow-up Prospective Study. Eur. J. Endocrinol. 2019, 180, 281–290. [Google Scholar] [CrossRef]
- Chatterjee, R.; Mukherjee, S.; Anuradha, S.; Naskar, P.; Basu, D. Pituitary Hormone Involvement in Tuberculous Meningitis. Afro-Egypt. J. Infect. Endem. Dis. 2023, 13, 218–224. [Google Scholar] [CrossRef]
- Pagani, S.; Calcaterra, V.; Acquafredda, G.; Montalbano, C.; Bozzola, E.; Ferrara, P.; Gasparri, M.; Villani, A.; Bozzola, M. MKRN3 and KISS1R Mutations in Precocious and Early Puberty. Ital. J. Pediatr. 2020, 46, 39. [Google Scholar] [CrossRef]
- Canton, A.P.M.; Tinano, F.R.; Guasti, L.; Montenegro, L.R.; Ryan, F.; Shears, D.; de Melo, M.E.; Gomes, L.G.; Piana, M.P.; Brauner, R.; et al. Rare Variants in the MECP2 Gene in Girls with Central Precocious Puberty: A Translational Cohort Study. Lancet Diabetes Endocrinol. 2023, 11, 545–554. [Google Scholar] [CrossRef]
- Kagami, M.; Nagasaki, K.; Kosaki, R.; Horikawa, R.; Naiki, Y.; Saitoh, S.; Tajima, T.; Yorifuji, T.; Numakura, C.; Mizuno, S.; et al. Temple Syndrome: Comprehensive Molecular and Clinical Findings in 32 Japanese Patients. Genet. Med. 2017, 19, 1356–1366. [Google Scholar] [CrossRef]
- Katz, J.; Ratnam, S.; Listernick, R.H.; Habiby, R.L.; Gutmann, D.H. Precocious Puberty in Children with Neurofibromatosis Type 1. J. Pediatr. 2025, 278, 114440. [Google Scholar] [CrossRef] [PubMed]
- Bercaw-Pratt, J. Peripheral Precocious Puberty. In Female Puberty; Dietrich, J.E., Ed.; Springer: New York, NY, USA, 2014; pp. 79–88. ISBN 978-1-4939-0911-7. [Google Scholar]
- Schmidt, H.; Kiess, W. Central Precocious Puberty in a Girl with McCune-Albright Syndrome Responds to Treatment with GnRH Analogue. J. Pediatr. Endocrinol. Metab. 1998, 11, 77–82. [Google Scholar] [CrossRef] [PubMed]
- Eugster, E.A. Peripheral Precocious Puberty: Causes and Current Management. Horm. Res. Paediatr. 2009, 71, 64–67. [Google Scholar] [CrossRef]
- Knific, T.; Lazarevič, M.; Žibert, J.; Obolnar, N.; Aleksovska, N.; Šuput Omladič, J.; Battelino, T.; Avbelj Stefanija, M. Final Adult Height in Children with Central Precocious Puberty—A Retrospective Study. Front. Endocrinol. 2022, 13, 1008474. [Google Scholar] [CrossRef]
- Dorn, L.D. Psychological and Social Problems in Children with Premature Adrenarche and Precocious Puberty. In When Puberty is Precocious; Pescovitz, O.H., Walvoord, E.C., Eds.; Humana Press: Totowa, NJ, USA, 2007; pp. 309–327. ISBN 978-1-58829-742-6. [Google Scholar]
- Schultz, K.A.P.; Sencer, S.F.; Messinger, Y.; Neglia, J.P.; Steiner, M.E. Pediatric Ovarian Tumors: A Review of 67 Cases. Pediatr. Blood Cancer 2005, 44, 167–173. [Google Scholar] [CrossRef]
- Stefanovska, M.J.; Celebic, A.; Calleja-Agius, J.; Staric, K.D. Ovarian Cancer in Children and Adolescents: A Unique Clinical Challenge. Eur. J. Surg. Oncol. 2024, 51, 108785. [Google Scholar] [CrossRef]
- Shibata, N.; Nyuzuki, H.; Sasaki, S.; Ogawa, Y.; Okada, M.; Nagasaki, K. Peripheral Precocious Puberty in a Girl with an Intracranial hCG-Producing Tumor: Case Report and Literature Review. Endocr. J. 2021, 68, 1463–1467. [Google Scholar] [CrossRef] [PubMed]
- Henley, D.; Korach, K. Physiological Effects and Mechanisms of Action of Endocrine Disrupting Chemicals That Alter Estrogen Signaling. Hormones 2010, 9, 191–205. [Google Scholar] [CrossRef]
- Kunz, G.J.; Klein, K.O.; Clemons, R.D.; Gottschalk, M.E.; Jones, K.L. Virilization of Young Children After Topical Androgen Use by Their Parents. Pediatrics 2004, 114, 282–284. [Google Scholar] [CrossRef]
- Lumbroso, S.; Paris, F.; Sultan, C. European Collaborative Study Activating Gsalpha Mutations: Analysis of 113 Patients with Signs of McCune-Albright Syndrome—A European Collaborative Study. J. Clin. Endocrinol. Metab. 2004, 89, 2107–2113. [Google Scholar] [CrossRef]
- Javaid, M.K.; Boyce, A.; Appelman-Dijkstra, N.; Ong, J.; Defabianis, P.; Offiah, A.; Arundel, P.; Shaw, N.; Pos, V.D.; Underhil, A.; et al. Best Practice Management Guidelines for Fibrous Dysplasia/McCune-Albright Syndrome: A Consensus Statement from the FD/MAS International Consortium. Orphanet J. Rare Dis. 2019, 14, 139. [Google Scholar] [CrossRef] [PubMed]
- Zacharin, M. The Spectrum of McCune Albright Syndrome. Pediatr. Endocrinol. Rev. PER 2007, 4 (Suppl. S4), 412–418. [Google Scholar] [PubMed]
- Profeta, G.; Micangeli, G.; Tarani, F.; Paparella, R.; Ferraguti, G.; Spaziani, M.; Isidori, A.M.; Menghi, M.; Ceccanti, M.; Fiore, M.; et al. Sexual Developmental Disorders in Pediatrics. Clin. Ter. 2022, 173, 475–488. [Google Scholar] [CrossRef]
- Temeck, J.W.; Pang, S.; Nelson, C.; New, M.I. Genetic Defects of Steroidogenesis in Premature Pubarche*. J. Clin. Endocrinol. Metab. 1987, 64, 609–617. [Google Scholar] [CrossRef]
- Cavlan, D.; Bharwani, N.; Grossman, A. Androgen- and Estrogen-Secreting Adrenal Cancers. Semin. Oncol. 2010, 37, 638–648. [Google Scholar] [CrossRef] [PubMed]
- Pant, V.; Baral, S. Van Wyk Grumbach Syndrome with Precocious Puberty and Ovarian Cysts: Value of Thyroid Function Tests. J. Pediatr. Surg. Case Rep. 2019, 43, 32–34. [Google Scholar] [CrossRef]
- Gil, M.; Martino, F.; Besseghine, G.; Ortiz, M.V.R.; Rojas, S.; Rodriguez, M.E.; Arcari, A.; Freire, A. Peripheral Precocious Puberty Secondary to Severe Hypothyroidism. Arch. Argent. Pediatr. 2025, 123, e202410456. [Google Scholar] [CrossRef]
- Vassart, G. A Molecular Dissection of the Glycoprotein Hormone Receptors. Trends Biochem. Sci. 2004, 29, 119–126. [Google Scholar] [CrossRef]
- Cabrera, S.M.; DiMeglio, L.A.; Eugster, E.A. Incidence and Characteristics of Pseudoprecocious Puberty Because of Severe Primary Hypothyroidism. J. Pediatr. 2013, 162, 637–639. [Google Scholar] [CrossRef]
- Van Winter, J.T.; Noller, K.L.; Zimmerman, D.; Melton, L.J. Natural History of Premature Thelarche in Olmsted County, Minnesota, 1940 to 1984. J. Pediatr. 1990, 116, 278–280. [Google Scholar] [CrossRef]
- De Vries, L.; Guz-Mark, A.; Lazar, L.; Reches, A.; Phillip, M. Premature Thelarche: Age at Presentation Affects Clinical Course but Not Clinical Characteristics or Risk to Progress to Precocious Puberty. J. Pediatr. 2010, 156, 466–471. [Google Scholar] [CrossRef] [PubMed]
- Leung, A.K.C.; Lam, J.M.; Hon, K.L. Premature Thelarche: An Updated Review. Curr. Pediatr. Rev. 2024, 20, 500–509. [Google Scholar] [CrossRef] [PubMed]
- Pasquino, A.M.; Pucarelli, I.; Passeri, F.; Segni, M.; Mancini, M.A.; Municchi, G. Progression of Premature Thelarche to Central Precocious Puberty. J. Pediatr. 1995, 126, 11–14. [Google Scholar] [CrossRef] [PubMed]
- Volta, C.; Bernasconi, S.; Cisternino, M.; Buzi, F.; Ferzetti, A.; Street, M.E.; Da Milano, A.M. Isolated Premature Thelarche and Thelarche Variant: Clinical and Auxological Follow-up of 119 Girls. J. Endocrinol. Investig. 1998, 21, 180–183. [Google Scholar] [CrossRef]
- Bizzarri, C.; Spadoni, G.L.; Bottaro, G.; Montanari, G.; Giannone, G.; Cappa, M.; Cianfarani, S. The Response to Gonadotropin Releasing Hormone (GnRH) Stimulation Test Does Not Predict the Progression to True Precocious Puberty in Girls with Onset of Premature Thelarche in the First Three Years of Life. J. Clin. Endocrinol. Metab. 2014, 99, 433–439. [Google Scholar] [CrossRef]
- Khokhar, A.; Mojica, A. Premature Thelarche. Pediatr. Ann. 2018, 47, E12–E15. [Google Scholar] [CrossRef]
- Rosenfield, R.L. Normal and Premature Adrenarche. Endocr. Rev. 2021, 42, 783–814. [Google Scholar] [CrossRef]
- Williams, R.M.; Ward, C.E.; Hughes, I.A. Premature Adrenarche. Arch. Dis. Child. 2012, 97, 250–254. [Google Scholar] [CrossRef]
- Liimatta, J.; Utriainen, P.; Laitinen, T.; Voutilainen, R.; Jääskeläinen, J. Cardiometabolic Risk Profile Among Young Adult Females with a History of Premature Adrenarche. J. Endocr. Soc. 2019, 3, 1771–1783. [Google Scholar] [CrossRef]
- DeSalvo, D.J.; Mehra, R.; Vaidyanathan, P.; Kaplowitz, P.B. In Children with Premature Adrenarche, Bone Age Advancement by 2 or More Years Is Common and Generally Benign. J. Pediatr. Endocrinol. Metab. 2013, 26, 215–221. [Google Scholar] [CrossRef]
- Armengaud, J.-B.; Charkaluk, M.-L.; Trivin, C.; Tardy, V.; Bréart, G.; Brauner, R.; Chalumeau, M. Precocious Pubarche: Distinguishing Late-Onset Congenital Adrenal Hyperplasia from Premature Adrenarche. J. Clin. Endocrinol. Metab. 2009, 94, 2835–2840. [Google Scholar] [CrossRef]
- Pinto, E.M.; Zambetti, G.P.; Rodriguez-Galindo, C. Pediatric Adrenocortical Tumours. Best Pract. Res. Clin. Endocrinol. Metab. 2020, 34, 101448. [Google Scholar] [CrossRef] [PubMed]
- Sopher, A.B.; Jean, A.M.; Zwany, S.K.; Winston, D.M.; Pomeranz, C.B.; Bell, J.J.; McMahon, D.J.; Hassoun, A.; Fennoy, I.; Oberfield, S.E. Bone Age Advancement in Prepubertal Children with Obesity and Premature Adrenarche: Possible Potentiating Factors. Obesity 2011, 19, 1259–1264. [Google Scholar] [CrossRef]
- Ejaz, S.; Lane, A.; Wilson, T. Outcome of Isolated Premature Menarche: A Retrospective and Follow-Up Study. Horm. Res. Paediatr. 2015, 84, 217–222. [Google Scholar] [CrossRef] [PubMed]
- Nella, A.A.; Kaplowitz, P.B.; Ramnitz, M.S.; Nandagopal, R. Benign Vaginal Bleeding in 24 Prepubertal Patients: Clinical, Biochemical and Imaging Features. J. Pediatr. Endocrinol. Metab. 2014, 27, 821–825. [Google Scholar] [CrossRef] [PubMed]
- Pallavee, P.; Samal, R. Precocious Puberty: A Clinical Review. Int. J. Reprod. Contracept. Obstet. Gynecol. 2018, 7, 771. [Google Scholar] [CrossRef]
- Yang, S.; Zhang, L.; Khan, K.; Travers, J.; Huang, R.; Jovanovic, V.M.; Veeramachaneni, R.; Sakamuru, S.; Tristan, C.A.; Davis, E.E.; et al. Identification of Environmental Compounds That May Trigger Early Female Puberty by Activating Human GnRHR and KISS1R. Endocrinology 2024, 165, bqae103. [Google Scholar] [CrossRef]
- Aguirre, R.S.; Eugster, E.A. Central Precocious Puberty: From Genetics to Treatment. Best Pract. Res. Clin. Endocrinol. Metab. 2018, 32, 343–354. [Google Scholar] [CrossRef]
- Tanner, J.M.; Goldstein, H.; Whitehouse, R.H. Standards for Children’s Height at Ages 2-9 Years Allowing for Height of Parents. Arch. Dis. Child. 1970, 45, 755–762. [Google Scholar] [CrossRef]
- Carel, J.-C. Precocious Puberty and Statural Growth. Hum. Reprod. Update 2004, 10, 135–147. [Google Scholar] [CrossRef]
- Neocleous, V.; Fanis, P.; Toumba, M.; Gorka, B.; Kousiappa, I.; Tanteles, G.A.; Iasonides, M.; Nicolaides, N.C.; Christou, Y.P.; Michailidou, K.; et al. Pathogenic and Low-Frequency Variants in Children with Central Precocious Puberty. Front. Endocrinol. 2021, 12, 745048. [Google Scholar] [CrossRef] [PubMed]
- Yoon, J.S.; So, C.H.; Lee, H.S.; Lim, J.S.; Hwang, J.S. Prevalence of Pathological Brain Lesions in Girls with Central Precocious Puberty: Possible Overestimation? J. Korean Med. Sci. 2018, 33, e329. [Google Scholar] [CrossRef]
- Dumitrescu, C.E.; Collins, M.T. McCune-Albright Syndrome. Orphanet J. Rare Dis. 2008, 3, 12. [Google Scholar] [CrossRef] [PubMed]
- Cleemann Wang, A.; Hagen, C.P.; Johannsen, T.H.; Madsen, A.G.; Cleemann, L.H.; Christiansen, P.; Main, K.M.; Juul, A.; Jensen, R.B. Differentiation of Idiopathic Central Precocious Puberty From Premature Thelarche Using Principal Component Analysis. J. Clin. Endocrinol. Metab. 2024, 109, 370–379. [Google Scholar] [CrossRef] [PubMed]
- Neely, E.K.; Hintz, R.L.; Wilson, D.M.; Lee, P.A.; Gautier, T.; Argente, J.; Stene, M. Normal Ranges for Immunochemiluminometric Gonadotropin Assays. J. Pediatr. 1995, 127, 40–46. [Google Scholar] [CrossRef]
- Neely, E.K.; Wilson, D.M.; Lee, P.A.; Stene, M.; Hintz, R.L. Spontaneous Serum Gonadotropin Concentrations in the Evaluation of Precocious Puberty. J. Pediatr. 1995, 127, 47–52. [Google Scholar] [CrossRef]
- Ziqin, L.; Qinwei, S.; Xiaobo, C.; Xiaohui, L. The Utility of Serum Inhibin B, Anti-Müllerian Hormone and Insulin Growth Factor-1 in Predicting a Positive Response to GnRH Analogs for Diagnosing Central Precocious Puberty in Girls. J. Pediatr. Endocrinol. Metab. 2021, 34, 1257–1262. [Google Scholar] [CrossRef]
- Carel, J.-C.; Eugster, E.A.; Rogol, A.; Ghizzoni, L.; Palmert, M.R.; on behalf of the Members of the ESPE-LWPES GnRH Analogs Consensus Conference Group. Consensus Statement on the Use of Gonadotropin-Releasing Hormone Analogs in Children. Pediatrics 2009, 123, e752–e762. [Google Scholar] [CrossRef]
- Carel, J.-C.; Léger, J. Precocious Puberty. N. Engl. J. Med. 2008, 358, 2366–2377. [Google Scholar] [CrossRef]
- De Souza, K.B.F.; Veiga, M.S.P.; Martins, G.R.F.; Da Silva, A.P.; Fujita, L.G.A.; Tomé, J.M.; Palhares, H.M.D.C.; Borges, M.D.F. Assessment of Gonadotropin Concentrations Stimulated by Gonadotropin-Releasing Hormone Analog by Electrochemiluminescence in Girls with Precocious Puberty and Premature Thelarche. Horm. Res. Paediatr. 2021, 94, 433–440. [Google Scholar] [CrossRef]
- Brito, V.N.; Batista, M.C.; Borges, M.F.; Latronico, A.C.; Kohek, M.B.F.; Thirone, A.C.P.; Jorge, B.H.; Arnhold, I.J.P.; Mendonca, B.B. Diagnostic Value of Fluorometric Assays in the Evaluation of Precocious Puberty*. J. Clin. Endocrinol. Metab. 1999, 84, 3539–3544. [Google Scholar] [CrossRef] [PubMed]
- Banker, H.; Cohen, H.L.; Sandhu, P.K. Sonography Pediatric Gynecology Assessment, Protocols, and Interpretation. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- Eksioglu, A.S.; Yilmaz, S.; Cetinkaya, S.; Cinar, G.; Yildiz, Y.T.; Aycan, Z. Value of Pelvic Sonography in the Diagnosis of Various Forms of Precocious Puberty in Girls. J. Clin. Ultrasound 2013, 41, 84–93. [Google Scholar] [CrossRef] [PubMed]
- Badouraki, M.; Christoforidis, A.; Economou, I.; Dimitriadis, A.S.; Katzos, G. Evaluation of Pelvic Ultrasonography in the Diagnosis and Differentiation of Various Forms of Sexual Precocity in Girls. Ultrasound Obstet. Gynecol. 2008, 32, 819–827. [Google Scholar] [CrossRef]
- Greulich, W.W.; Pyle, S.I. Radiographic Atlas of Skeletal Development of the Hand and Wrist, 2nd ed.; Stanford University Press: Stanford, CA, USA, 2011; ISBN 978-0-8047-0398-7. [Google Scholar]
- Tanner, J.M.; Cameron, N. Assessment of Skeletal Maturity and Prediction of Adult Height (TW3 Method), 3rd ed.; Saunders: London, UK, 2001; ISBN 978-0-7020-2511-2. [Google Scholar]
- Cavallo, F.; Mohn, A.; Chiarelli, F.; Giannini, C. Evaluation of Bone Age in Children: A Mini-Review. Front. Pediatr. 2021, 9, 580314. [Google Scholar] [CrossRef]
- Klein, D.A.; Emerick, J.E.; Sylvester, J.E.; Vogt, K.S. Disorders of Puberty: An Approach to Diagnosis and Management. Am. Fam. Physician 2017, 96, 590–599. [Google Scholar] [PubMed]
- Canton, A.P.M.; Latronico, A.C. Brain MRI in Girls with Central Precocious Puberty: A Time for New Approaches. J. Clin. Endocrinol. Metab. 2021, 106, e2806–e2808. [Google Scholar] [CrossRef]
- Lee, H.S. Central Precocious Puberty: Is Routine Brain MRI Screening Necessary for Girls?: Commentary on “Brain Magnetic Resonance Imaging (MRI) Findings in Central Precocious Puberty Patients: Is Routine MRI Necessary for Newly Diagnosed Patients?”. Ann. Pediatr. Endocrinol. Metab. 2023, 28, 155–156. [Google Scholar] [CrossRef]
- Voutilainen, R.; Jääskeläinen, J. Premature Adrenarche: Etiology, Clinical Findings, and Consequences. J. Steroid Biochem. Mol. Biol. 2015, 145, 226–236. [Google Scholar] [CrossRef]
- Martin, D.D.; Wit, J.M.; Hochberg, Z.; Van Rijn, R.R.; Fricke, O.; Werther, G.; Cameron, N.; Hertel, T.; Wudy, S.A.; Butler, G.; et al. The Use of Bone Age in Clinical Practice—Part 2. Horm. Res. Paediatr. 2011, 76, 10–16. [Google Scholar] [CrossRef]
- Fuqua, J.S. Treatment and Outcomes of Precocious Puberty: An Update. J. Clin. Endocrinol. Metab. 2013, 98, 2198–2207. [Google Scholar] [CrossRef]
- Conn, P.M.; Crowley, W.F. Gonadotropin-Releasing Hormone and Its Analogs. Annu. Rev. Med. 1994, 45, 391–405. [Google Scholar] [CrossRef] [PubMed]
- Bangalore Krishna, K.; Fuqua, J.S.; Rogol, A.D.; Klein, K.O.; Popovic, J.; Houk, C.P.; Charmandari, E.; Lee, P.A. Use of Gonadotropin-Releasing Hormone Analogs in Children: Update by an International Consortium. Horm. Res. Paediatr. 2019, 91, 357–372. [Google Scholar] [CrossRef] [PubMed]
- Fuld, K.; Chi, C.; Neely, E.K. A Randomized Trial of 1- and 3-Month Depot Leuprolide Doses in the Treatment of Central Precocious Puberty. J. Pediatr. 2011, 159, 982–987.e1. [Google Scholar] [CrossRef] [PubMed]
- Demirbilek, H.; Alikasifoglu, A.; Gonc, N.E.; Ozon, A.; Kandemir, N. Assessment of Gonadotrophin Suppression in Girls Treated with GnRH Analogue for Central Precocious Puberty; Validity of Single Luteinizing Hormone Measurement after Leuprolide Acetate Injection. Clin. Endocrinol. 2012, 76, 126–130. [Google Scholar] [CrossRef]
- Luo, X.; Liang, Y.; Hou, L.; Wu, W.; Ying, Y.; Ye, F. Long-Term Efficacy and Safety of Gonadotropin-Releasing Hormone Analog Treatment in Children with Idiopathic Central Precocious Puberty: A Systematic Review and Meta-Analysis. Clin. Endocrinol. 2021, 94, 786–796. [Google Scholar] [CrossRef]
- Feuillan, P.; Calis, K.; Hill, S.; Shawker, T.; Robey, P.G.; Collins, M.T. Letrozole Treatment of Precocious Puberty in Girls with the McCune-Albright Syndrome: A Pilot Study. J. Clin. Endocrinol. Metab. 2007, 92, 2100–2106. [Google Scholar] [CrossRef]
- Renaud, E.J.; Sømme, S.; Islam, S.; Cameron, D.B.; Gates, R.L.; Williams, R.F.; Jancelewicz, T.; Oyetunji, T.A.; Grabowski, J.; Diefenbach, K.A.; et al. Ovarian Masses in the Child and Adolescent: An American Pediatric Surgical Association Outcomes and Evidence-Based Practice Committee Systematic Review. J. Pediatr. Surg. 2019, 54, 369–377. [Google Scholar] [CrossRef]
- Emre, Ş.; Özcan, R.; Bakır, A.C.; Kuruğoğlu, S.; Çomunoğlu, N.; Şen, H.S.; Celkan, T.; Tekant, G.T. Adrenal Masses in Children: Imaging, Surgical Treatment and Outcome. Asian J. Surg. 2020, 43, 207–212. [Google Scholar] [CrossRef]
- Qudsiya, Z.; Gupta, V. Peripheral Precocious Puberty. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
| Central Precocious Puberty | Peripheral Precocious Puberty | Premature Thelarche | Premature Adrenarche | Premature Menarche | |
|---|---|---|---|---|---|
| Etiology | Early activation of the HPG axis | Excess sex steroid production independent of gonadotropins | Idiopathic, linked to transient ovarian activity or mini-puberty | Early adrenal androgen production | Isolated uterine bleeding without HPG axis activation |
| Onset Age | Before 8 years | Before 8 years | Typically, <3 years or >6 years | Before 8 years | Before 8 years |
| Clinical Presentation | Progressive breast development, pubic hair, growth acceleration | Breast development, vaginal bleeding, or virilization without pubertal progression | Isolated breast development without other pubertal signs | Appearance of pubic/axillary hair, body odor, mild acne | Isolated vaginal bleeding without breast development or pubic hair |
| Growth Velocity | Accelerated, often above the 95th percentile | Variable, depending on hormonal excess | Normal for age | Normal for age | Normal for age |
| Bone Age | Advanced (>1–2 years above chronological age) | Advanced, depending on hormone levels | Normal | Normal or mildly advanced | Normal |
| Gonadotropin Levels | Elevated basal and/or GnRH-stimulated LH and FSH | Low or suppressed | Prepubertal | Prepubertal | Prepubertal |
| Sex Steroid Levels | Elevated estradiol | Elevated estrogen or androgens | Normal or slightly elevated estradiol | Elevated DHEA-S, androstenedione | Normal |
| Ultrasound Findings | Increased ovarian volume and uterine length | Ovarian cysts or adrenal abnormalities | Prepubertal ovarian and uterine morphology | Normal ovaries and uterus | Normal |
| Associated Conditions | CNS abnormalities (e.g., hypothalamic hamartoma) | Ovarian/adrenal tumors, McCune–Albright syndrome | Mini-puberty, obesity | Metabolic syndrome risk, polycystic ovary syndrome | Functional ovarian cysts |
| Progression | Progressive without treatment | Progressive without treatment | Non-progressive, self-limiting | Non-progressive, self-limiting | Self-limiting, usually single episode |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paparella, R.; Bei, A.; Brilli, L.; Maglione, V.; Tarani, F.; Niceta, M.; Pucarelli, I.; Tarani, L. Precocious Puberty and Benign Variants in Female Children: Etiology, Diagnostic Challenges, and Clinical Management. Endocrines 2025, 6, 29. https://doi.org/10.3390/endocrines6020029
Paparella R, Bei A, Brilli L, Maglione V, Tarani F, Niceta M, Pucarelli I, Tarani L. Precocious Puberty and Benign Variants in Female Children: Etiology, Diagnostic Challenges, and Clinical Management. Endocrines. 2025; 6(2):29. https://doi.org/10.3390/endocrines6020029
Chicago/Turabian StylePaparella, Roberto, Arianna Bei, Lorenzo Brilli, Vittorio Maglione, Francesca Tarani, Marcello Niceta, Ida Pucarelli, and Luigi Tarani. 2025. "Precocious Puberty and Benign Variants in Female Children: Etiology, Diagnostic Challenges, and Clinical Management" Endocrines 6, no. 2: 29. https://doi.org/10.3390/endocrines6020029
APA StylePaparella, R., Bei, A., Brilli, L., Maglione, V., Tarani, F., Niceta, M., Pucarelli, I., & Tarani, L. (2025). Precocious Puberty and Benign Variants in Female Children: Etiology, Diagnostic Challenges, and Clinical Management. Endocrines, 6(2), 29. https://doi.org/10.3390/endocrines6020029






