Endocrine Parameters and Climate Change
Abstract
1. Introduction
2. Materials and Methods
2.1. Summary of Inclusion and Exclusion Criteria
- Primary research investigations;
- Studies published in English;
- Research investigations on endocrine laboratory parameters, climate change, and/or heat;
- Research investigations published in 2014 or thereafter (i.e., the past 10 years);
- Primary studies that employed experimental research methods.
- Nonprimary research investigations;
- Research investigations that were not published in English;
- Research investigations that did not discuss endocrine laboratory parameters, climate change, and/or heat;
- Research investigations published before 2014;
- Primary studies that did not employ experimental research methods.
2.2. Search Strategy
2.3. Study Selection
2.4. Data Extraction
2.5. Data Synthesis
3. Limitations of the Study and Risk of Bias (ROB) Analysis
3.1. Limitations of the Study
3.2. Risk of Bias (ROB) Analysis
4. Results
4.1. Overview of Search Outcomes
4.2. Characteristics of the Included Articles
4.3. Impact of Climate Change on Endocrine Parameters
4.3.1. Human Studies
4.3.2. Animal Studies
4.4. Mechanisms of Endocrine Disruption
5. Discussion
6. Conclusions
Funding
Conflicts of Interest
References
- Rannaud-Bartaire, P.; Demeneix, B.A.; Fini, J.B. Pressures of the urban environment on the endocrine system: Adverse effects and adaptation. Mol. Cell. Endocrinol. 2024, 583, 112125. [Google Scholar] [CrossRef] [PubMed]
- Bonier, F.; Martin, P.R. How can we estimate natural selection on endocrine traits? Lessons from evolutionary biology. Proc. R. Soc. B Biol. Sci. 2016, 283, 20161887. [Google Scholar] [CrossRef] [PubMed]
- Brander, S.M.; White, J.W.; DeCourten, B.M.; Major, K.; Hutton, S.J.; Connon, R.E.; Mehinto, A. Accounting for transgenerational effects of toxicant exposure in population models alters the predicted long-term population status. Environ. Epigenetics 2022, 8, dvac023. [Google Scholar] [CrossRef]
- Chiefari, E.; Pastore, I.; Puccio, L.; Caroleo, P.; Oliverio, R.; Vero, A.; Foti, D.P.; Vero, R.; Brunetti, A. Impact of Seasonality on Gestational Diabetes Mellitus. Endocr. Metab. Immune Disord. -Drug Targets 2017, 17, 246–252. [Google Scholar] [CrossRef] [PubMed]
- Gore, A.C.; Chappell, V.A.; Fenton, S.E.; Flaws, J.A.; Nadal, A.; Prins, G.S.; Toppari, J.; Zoeller, R.T. EDC-2: The Endocrine Society’s second scientific statement on endocrine-disrupting chemicals. Endocr. Rev. 2015, 36, E1–E150. [Google Scholar]
- Russart, K.L.; Nelson, R.J. Light at night as an environmental endocrine disruptor. Physiol. Behav. 2018, 190, 82–89. [Google Scholar] [CrossRef]
- Seebacher, F. Interactive effects of anthropogenic environmental drivers on endocrine responses in wildlife. Mol. Cell. Endocrinol. 2022, 556, 111737. [Google Scholar] [CrossRef] [PubMed]
- Hannan, F.M.; Leow, M.K.; Lee, J.K.; Kovats, S.; Elajnaf, T.; Kennedy, S.H.; Thakker, R.V. Endocrine effects of heat exposure and relevance to climate change. Nat. Rev. Endocrinol. 2024, 20, 673–684. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Roig, M.D.; Pascal, R.; Cahuana, M.J.; García-Algar, O.; Sebastiani, G.; Andreu-Fernández, V.; Martínez, L.; Rodríguez, G.; Iglesia, I.; Ortiz-Arrabal, O.; et al. Environmental exposure during pregnancy: Influence on prenatal development and early life: A comprehensive review. Fetal Diagn. Ther. 2021, 48, 245–257. [Google Scholar] [CrossRef] [PubMed]
- Caporale, N.; Leemans, M.; Birgersson, L.; Germain, P.L.; Cheroni, C.; Borbély, G.; Engdahl, E.; Lindh, C.; Bressan, R.B.; Cavallo, F.; et al. From cohorts to molecules: Adverse impacts of endocrine disrupting mixtures. Science 2022, 375, eabe8244. [Google Scholar] [CrossRef]
- Heindel, J.J. History of the obesogen field: Looking back to look forward. Front. Endocrinol. 2019, 10, 14. [Google Scholar] [CrossRef] [PubMed]
- Cumpston, M.; Li, T.; Page, M.J.; Chandler, J.; Welch, V.A.; Higgins, J.P.; Thomas, J. Updated guidance for trusted systematic reviews: A new edition of the Cochrane Handbook for Systematic Reviews of Interventions. Cochrane Database Syst. Rev. 2019, 10, ED000142. [Google Scholar] [CrossRef] [PubMed]
- Kim, G. How to perform and write a systematic review and meta-analysis. Child Health Nurs. Res. 2023, 29, 161. [Google Scholar] [CrossRef]
- Iddagoda, M.T.; Flicker, L. Clinical systematic reviews—A brief overview. BMC Med. Res. Methodol. 2023, 23, 226. [Google Scholar] [CrossRef]
- Tawfik, G.M.; Dila, K.A.; Mohamed, M.Y.; Tam, D.N.; Kien, N.D.; Ahmed, A.M.; Huy, N.T. A step by step guide for conducting a systematic review and meta-analysis with simulation data. Trop. Med. Health 2019, 47, 46. [Google Scholar] [CrossRef]
- Kolaski, K.; Romeiser Logan, L.; Ioannidis, J.P. Guidance to best tools and practices for systematic reviews. J. Pediatr. Rehabil. Med. 2023, 16, 241–273. [Google Scholar] [PubMed]
- Jpt, H. Cochrane Handbook for Systematic Reviews of Interventions. 2024. Available online: https://training.cochrane.org/handbook/current (accessed on 27 January 2025).
- Schmidt, L.; Mutlu, A.N.; Elmore, R.; Olorisade, B.K.; Thomas, J.; Higgins, J.P. Data extraction methods for systematic review (semi) automation: Update of a living systematic review. F1000Research 2021, 10, 401. [Google Scholar] [CrossRef] [PubMed]
- Al-Khabori, M.; Rasool, W. Introduction to systematic reviews and meta-analyses of therapeutic studies. Oman Med. J. 2022, 37, e428. [Google Scholar] [CrossRef]
- Sukhera, J. Narrative reviews: Flexible, rigorous, and practical. J. Grad. Med. Educ. 2022, 14, 414–417. [Google Scholar] [CrossRef]
- Nishimura, T.; Motoi, M.; Toyoshima, H.; Kishida, F.; Shin, S.; Katsumura, T.; Nakayama, K.; Oota, H.; Higuchi, S.; Watanuki, S.; et al. Endocrine, inflammatory and immune responses and individual differences in acute hypobaric hypoxia in lowlanders. Sci. Rep. 2023, 13, 12659. [Google Scholar] [CrossRef] [PubMed]
- Tang, C.; Liang, Y.; Guo, J.; Wang, M.; Li, M.; Zhang, H.; Arbab, A.A.; Karrow, N.A.; Yang, Z.; Mao, Y. Effects of seasonal heat stress during late gestation on growth performance, metabolic and immuno-endocrine parameters of calves. Animals 2022, 12, 716. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Li, C.; Fang, T.; Yao, J.; Gu, X. Effects of heat stress on endocrine, thermoregulatory, and lactation capacity in heat-tolerant and-sensitive dry cows. Front. Vet. Sci. 2024, 11, 1405263. [Google Scholar]
- Lyrio, L.L.; Lazaro, M.A.; Sonegheti, R.; Moulin, L.; Coslop, L.; Sarto, C.G.; Loureiro, B.; Favoreto, M.G. Effects of heat stress on sperm quality of French Bulldogs. Theriogenology 2023, 199, 131–137. [Google Scholar] [CrossRef] [PubMed]
- Mizukami, H.; Suzuki, T.; Nambo, Y.; Ishimaru, M.; Naito, H.; Korosue, K.; Akiyama, K.; Miyata, K.; Yamanobe, A.; Nagaoka, K.; et al. Comparison of growth and endocrine changes in Thoroughbred colts and fillies reared under different climate conditions. J. Equine Sci. 2015, 26, 49–56. [Google Scholar] [CrossRef]
- Behringer, V.; Heistermann, M.; Malaivijitnond, S.; Schülke, O.; Ostner, J. Developmental and environmental modulation of fecal thyroid hormone levels in wild Assamese macaques (Macaca assamensis). Am. J. Primatol. 2023, 85, e23530. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Wang, W.; Sun, P.; Huang, S.; Gao, R.; Kong, D.; Ru, W.; Wronski, T.; Zhang, G. Extrinsic factors, endocrine mechanisms, and behavioral indicators of migratory restlessness in wintering whooper swans (Cygnus cygnus). Sci. Rep. 2021, 11, 12636. [Google Scholar] [CrossRef] [PubMed]
- Fu, J.; Zhang, G.; Xu, P.; Guo, R.; Li, J.; Guan, H.; Li, Y. Seasonal changes of thyroid function parameters in women of reproductive age between 2012 and 2018: A retrospective, observational, single-center study. Front. Endocrinol. 2021, 12, 719225. [Google Scholar] [CrossRef]
- Samir, H.; Nyametease, P.; Elbadawy, M.; Fathi, M.; Mandour, A.S.; Radwan, F.; Nagaoka, K.; Sasaki, K.; Watanabe, G. Assessment of correlations and concentrations of salivary and plasma steroids, testicular morphometry, and semen quality in different climatic conditions in goats. Theriogenology 2020, 157, 238–244. [Google Scholar] [CrossRef]
- Tangyuenyong, S.; Sato, F.; Nambo, Y.; Murase, H.; Endo, Y.; Tanaka, T.; Nagaoka, K.; Watanabe, G. Comparison of physical body growth and metabolic and reproductive endocrine functions between north and south climates of Japan in trained Thoroughbred yearling horses. J. Equine Sci. 2017, 28, 77–86. [Google Scholar] [CrossRef] [PubMed]
- Ishimaru, M.; Tsuchiya, T.; Endo, Y.; Matsui, A.; Ohmura, H.; Murase, H.; Korosue, K.; Sato, F.; Taya, K. Effects of different winter paddock management of Thoroughbred weanlings and yearlings in the cold region of Japan on physiological function, endocrine function and growth. J. Vet. Med. Sci. 2024, 86, 756–768. [Google Scholar] [CrossRef] [PubMed]
- Shi, R.; Dou, J.; Liu, J.; Sammad, A.; Luo, H.; Wang, Y.; Guo, G.; Wang, Y. Genetic parameters of hair cortisol as an indicator of chronic stress under different environments in Holstein cows. J. Dairy Sci. 2021, 104, 6985–6999. [Google Scholar] [CrossRef] [PubMed]
- Blond, B.; Majkić, M.; Spasojević, J.; Hristov, S.; Radinović, M.; Nikolić, S.; Anđušić, L.; Čukić, A.; Došenović Marinković, M.; Vujanović, B.D.; et al. Influence of Heat Stress on Body Surface Temperature and Blood Metabolic, Endocrine, and Inflammatory Parameters and Their Correlation in Cows. Metabolites 2024, 14, 104. [Google Scholar] [CrossRef] [PubMed]
- Pragna, P.; Sejian, V.; Soren, N.M.; Bagath, M.; Krishnan, G.; Beena, V.; Devi, P.I.; Bhatta, R. Summer season induced rhythmic alterations in metabolic activities to adapt to heat stress in three indigenous (Osmanabadi, Malabari and Salem Black) goat breeds. Biol. Rhythm. Res. 2018, 49, 551–565. [Google Scholar] [CrossRef]
- Shilja Shaji, S.S.; Sejian, V.; Bagath, M.; Manjunathareddy, G.B.; Kurien, E.K.; Girish Varma, G.V.; Raghavendra Bhatta, R.B. Summer season related heat and nutritional stresses on the adaptive capability of goats based on blood biochemical response and hepatic HSP70 gene expression. Biol. Rhythm. Res. 2017, 48, 65–83. [Google Scholar] [CrossRef]
- Bruzzio, H.L. Effects of Ocean Acidification and Hypoxia on Stress and Growth Hormone Responses in Juvenile Blue Rockfish (Sebastes mystinus). Capstone Projects and Master’s Theses. Capstone Projects and Master’s Thesis; Moss Landing Marine Laboratories: Moss Landing, CA, USA, 2022. Available online: https://digitalcommons.csumb.edu/caps_thes_all/1333 (accessed on 1 January 2024).
- Hadinia, S.H.; Carneiro, P.R.; Fitzsimmons, C.J.; Bédécarrats, G.Y.; Zuidhof, M.J. Post-photostimulation energy intake accelerated pubertal development in broiler breeder pullets. Poult. Sci. 2020, 99, 2215–2229. [Google Scholar] [CrossRef] [PubMed]
- Lane, S.J.; Emmerson, M.G.; VanDiest, I.J.; Hucul, C.; Beck, M.L.; Davies, S.; Gilbert, E.R.; Sewall, K.B. Hypothalamic-pituitary-adrenal axis regulation and organization in urban and rural song sparrows. Gen. Comp. Endocrinol. 2021, 310, 113809. [Google Scholar] [CrossRef]
- Li, Z.; Li, Y.; Zhou, X.; Cao, Y.; Li, C. Preventive effects of supplemental dietary zinc on heat-induced damage in the epididymis of boars. J. Therm. Biol. 2017, 64, 58–66. [Google Scholar] [CrossRef] [PubMed]
- Halder, S.; Sarkar, M.; Dey, S.; Bhunia, S.K.; Koley, A.R.; Giri, B. Protective effects of red grape (Vitis vinifera) juice through restoration of antioxidant defense, endocrine swing and Hsf1, Hsp72 levels in heat stress induced testicular dysregulation of Wister rat. J. Therm. Biol. 2018, 71, 32–40. [Google Scholar] [CrossRef]
- de Souza, V.V.; Moreira, D.P.; Braz-Mota, S.; Valente, W.; Cotta, G.C.; da Silva Rodrigues, M.; Nóbrega, R.H.; Corrêa, R.D.; de Melo Hoyos, D.C.; Sanches, E.A.; et al. Simulated climate change and atrazine contamination can synergistically impair zebrafish testicular function. Sci. Total Environ. 2024, 946, 174173. [Google Scholar] [CrossRef] [PubMed]
- Dovolou, E.; Giannoulis, T.; Nanas, I.; Amiridis, G.S. Heat stress: A serious disruptor of the reproductive physiology of dairy cows. Animals 2023, 13, 1846. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.D.; Kim, Y.J.; Jang, M.; Bae, S.G.; Yun, S.H.; Lee, M.R.; Seo, Y.R.; Cho, J.K.; Kim, S.J.; Lee, W.J. Heat stress during summer attenuates expression of the hypothalamic kisspeptin, an upstream regulator of the hypothalamic–pituitary–gonadal axis, in domestic sows. Animals 2022, 12, 2967. [Google Scholar] [CrossRef] [PubMed]
- Nanas, I.; Chouzouris, T.M.; Dadouli, K.; Dovolou, E.; Stamperna, K.; Barbagianni, M.; Valasi, I.; Tsiaras, A.; Amiridis, G.S. A study on stress response and fertility parameters in phenotypically thermotolerant and thermosensitive dairy cows during summer heat stress. Reprod. Domest. Anim. 2020, 55, 1774–1783. [Google Scholar] [CrossRef]
- Khan, A.; Dou, J.; Wang, Y.; Jiang, X.; Khan, M.Z.; Luo, H.; Usman, T.; Zhu, H. Evaluation of heat stress effects on cellular and transcriptional adaptation of bovine granulosa cells. J. Anim. Sci. Biotechnol. 2020, 11, 25. [Google Scholar] [CrossRef] [PubMed]
- Nanas, I.; Barbagianni, M.; Dadouli, K.; Dovolou, E.; Amiridis, G.S. Ultrasonographic findings of the corpus luteum and the gravid uterus during heat stress in dairy cattle. Reprod. Domest. Anim. 2021, 56, 1329–1341. [Google Scholar] [CrossRef] [PubMed]
- Wolfenson, D.; Roth, Z. Impact of heat stress on cow reproduction and fertility. Anim. Front. 2019, 9, 32–38. [Google Scholar] [CrossRef] [PubMed]
- Nanas, I.; Chouzouris, T.M.; Dovolou, E.; Dadouli, K.; Stamperna, K.; Kateri, I.; Barbagianni, M.; Amiridis, G.S. Early embryo losses, progesterone and pregnancy associated glycoproteins levels during summer heat stress in dairy cows. J. Therm. Biol. 2021, 98, 102951. [Google Scholar] [CrossRef] [PubMed]
- Țogoe, D.; Mincă, N.A. The impact of heat stress on the physiological, productive, and reproductive status of dairy cows. Agriculture 2024, 14, 1241. [Google Scholar] [CrossRef]
- Sejian, V.; Bhatta, R.; Gaughan, J.B.; Dunshea, F.R.; Lacetera, N. Adaptation of animals to heat stress. Animal 2018, 12, s431–s444. [Google Scholar] [CrossRef] [PubMed]
- Giannone, C.; Bovo, M.; Ceccarelli, M.; Torreggiani, D.; Tassinari, P. Review of the heat stress-induced responses in dairy cattle. Animals 2023, 13, 3451. [Google Scholar] [CrossRef]
- Cartwright, S.L.; Schmied, J.; Karrow, N.; Mallard, B.A. Impact of heat stress on dairy cattle and selection strategies for thermotolerance: A review. Front. Vet. Sci. 2023, 10, 1198697. [Google Scholar] [CrossRef] [PubMed]
- Napolitano, F.; De Rosa, G.; Chay-Canul, A.; Álvarez-Macías, A.; Pereira, A.M.; Bragaglio, A.; Mora-Medina, P.; Rodríguez-González, D.; García-Herrera, R.; Hernández-Ávalos, I.; et al. The challenge of global warming in water buffalo farming: Physiological and behavioral aspects and strategies to face heat stress. Animals 2023, 13, 3103. [Google Scholar] [CrossRef]
- Ko, S.H. Effects of heat stress-induced sex hormone dysregulation on reproduction and growth in male adolescents and beneficial foods. Nutrients 2024, 16, 3032. [Google Scholar] [CrossRef] [PubMed]
- Shakeel, M.; Yoon, M. Heat stress and stallion fertility. J. Anim. Sci. Technol. 2023, 65, 683. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Guzmán, J.A.; Parra-Bracamonte, G.M.; Velazquez, M.A. Impact of heat stress on oocyte developmental competence and pre-implantation embryo viability in cattle. Animals 2024, 14, 2280. [Google Scholar] [CrossRef]
- Zeitoun, M.M.; Derar, D.R.; Ali, A.; Alharbi, Y.M. Expression of hormones, cytokines, and antioxidants in heat-stressed subfertile female dromedaries. Animals 2022, 12, 2125. [Google Scholar] [CrossRef] [PubMed]
- Wachida, N.; Dawuda, P.; Ate, I.U.; Rekwot, P.I. Impact of environmental heat stress on ovarian function of Zebu cows. J. Anim. Health Prod. 2022, 10, 412–419. [Google Scholar] [CrossRef]
- Contreras-Méndez, L.A.; Medrano, J.F.; Thomas, M.G.; Enns, R.M.; Speidel, S.E.; Luna-Nevárez, G.; López-Castro, P.A.; Rivera-Acuña, F.; Luna-Nevárez, P. The anti-müllerian hormone as endocrine and molecular marker associated with reproductive performance in holstein dairy cows exposed to heat stress. Animals 2024, 14, 213. [Google Scholar] [CrossRef]
- Bidne, K.L.; Romoser, M.R.; Ross, J.W.; Baumgard, L.H.; Keating, A.F. Heat stress during the luteal phase decreases luteal size but does not affect circulating progesterone in gilts. J. Anim. Sci. 2019, 97, 4314–4322. [Google Scholar] [CrossRef] [PubMed]
- Egbuniwe, I.C.; Akogwu, M.S.; Obetta, T.U. Mechanisms underlying reproductive responses of Japanese quails to heat stress conditions. Int. J. Biometeorol. 2024, 68, 2173–2184. [Google Scholar] [CrossRef]
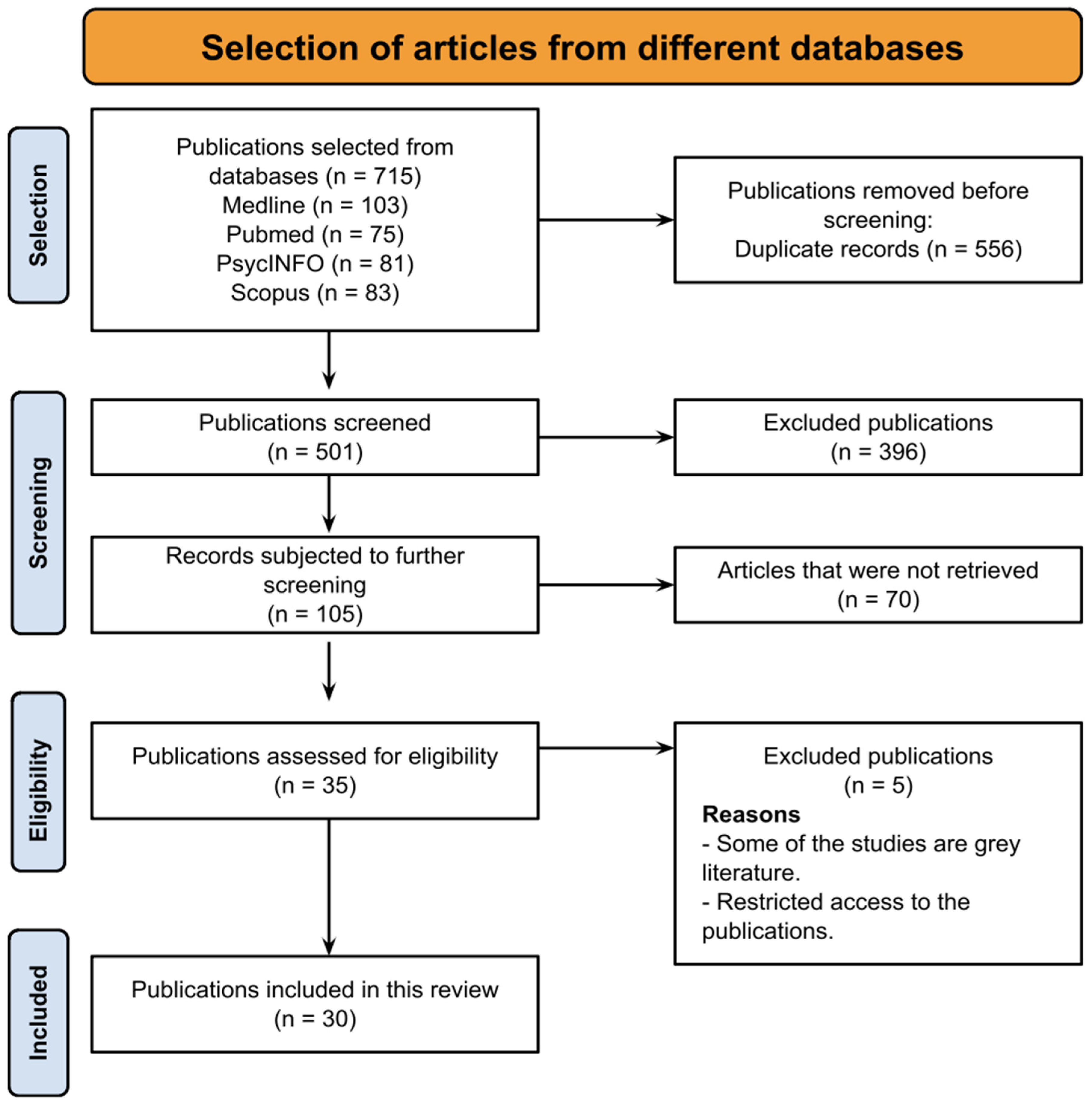
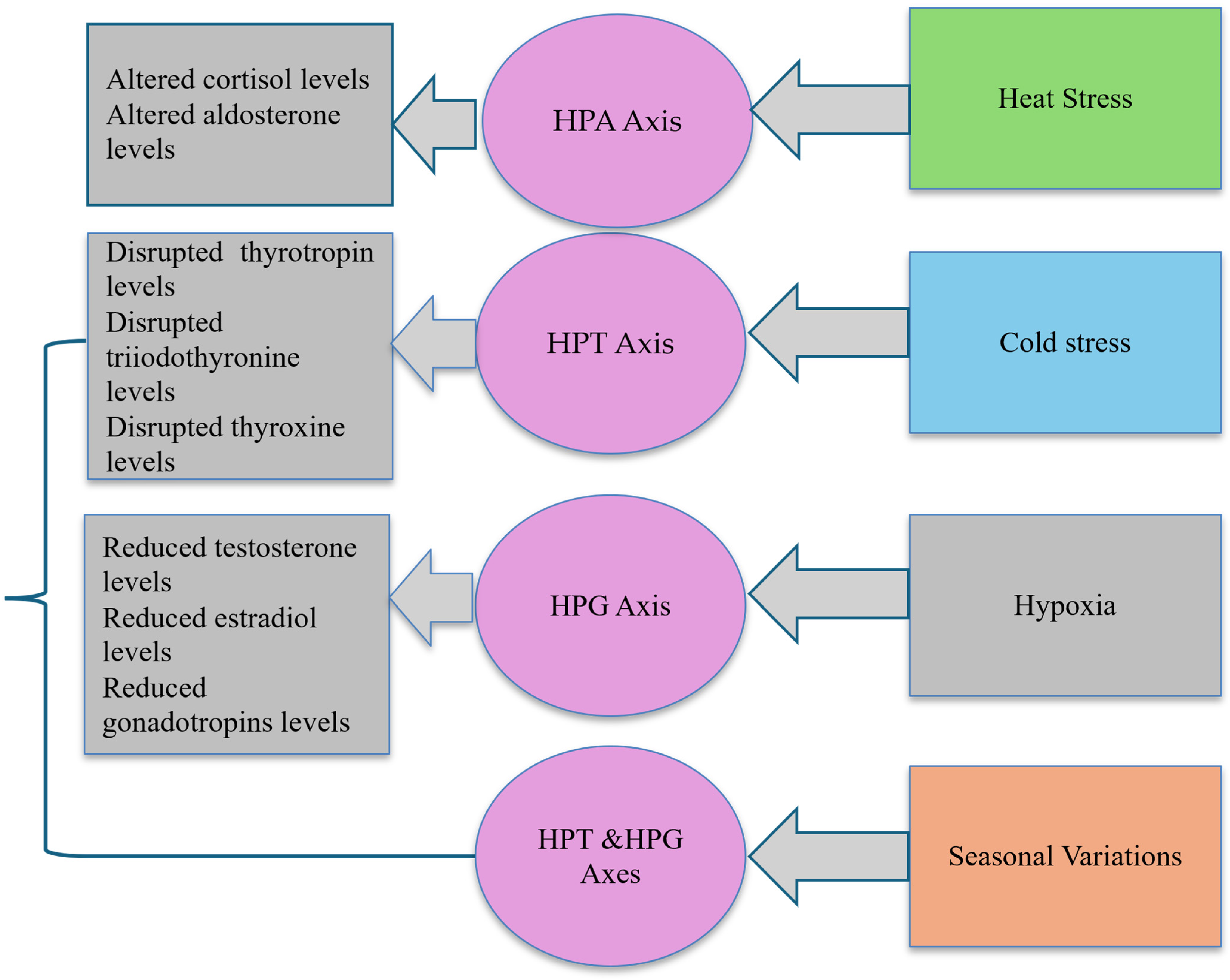
| Author(s) (Year) | Research Objective | Test Subjects | Type of Exposure | Exposure Conditions | Effect on Endocrine Laboratory Parameters |
|---|---|---|---|---|---|
| Nishimura et al. [21] | To explore endocrine, inflammatory, and immunological responses. | Healthy male students | Chronic (climatic chamber) | Hypobaric hypoxia, 75 min of exposure to settings mimicking 3500 m. | Decrease in aldosterone and cortisol levels. |
| Tang et al. [22] | To investigate how seasonal heat stress during late gestation affects calf development, metabolism, and the functions of the immune and endocrine systems. | Female calves | Acute, seasonal | 33 days of summer and winter stress, respectively | Decrease in adrenocorticotropin |
| Chen et al. [23] | The purpose of this study was to look into the effects of heat stress on endocrine, thermoregulatory, and lactation parameters in heat-tolerant and heat-sensitive dry cows. | Dry cows | Acute, seasonal | 40 days of heat stress | Reduction in plasma cortisol. Increase in plasma triiodothyronine and growth hormone levels. |
| Lyrio et al. [24] | To assess the implications of heat stress on the quality of semen in French Bulldogs, as well as the time frame of these impacts. | French Bulldogs | Acute (electrical heat pad) | 11 min of exposure to 40 °C for 60 days. | No difference in testosterone levels in the semen. |
| Mizukami et al. [25] | To evaluate the growth and hormonal changes in Thoroughbred colts and fillies raised at two Racing. | Thoroughbred colts and fillies | Acute (seasonal). | 7 months of summer and winter stress. | |
| Behringer et al. [26] | To explore the influence of demographic and environmental variables on immunoreactive fecal total triiodothyronine levels in young Assamese macaques. | Young Assamese macaques | Acute (seasonal). | 7 months of summer and winter stress | Increase in the concentrations of immunoreactive fecal total triiodothyronine in immature and female Assamese macaques. Rise in immunoreactive fecal glucocorticoid levels in developing immature Assamese macaques. |
| Yang et al. [27] | To explore the impact of two extrinsic stimuli (day length data and daily extrinsic temperatures) on the endocrine metabolism and behavior of whooper swans before their spring migration. | Whooper swans | Acute (seasonal). | 5 months of winter stress | High levels of fecal glucocorticoid metabolite |
| Fu et al. [28] | To assess the seasonal fluctuations in the quantities of thyrotropin, free triiodothyronine, free thyroxine, and thyrotropin index in women of reproductive maturity. | Women aged 20 to 49 years. | Acute (seasonal). | 11 months of summer and winter stress | Increase in thyrotropin, free triiodothyronine, free thyroxine, and thyrotropin index in the winter months. |
| Samir et al. [29] | To ascertain if the quantities of steroids (T, E2, and cortisol) in male goat saliva correspond to their levels in the bloodstream. | 7 male Shiba goats | Acute (seasonal). | 6 months of spring and summer stress | Higher concentrations of salivary cortisols in summer compared to spring. No significant difference in the levels of testosterone, estradiol, and plasma cortisol between spring and summer. |
| Tangyuenyong et al. [30] | To examine the effects of artificial light supplementation on the concentrations of thyroxine, body growth hormone, and reproductive hormones in trained thoroughbred yearling horses from Hokkaido and Miyazaki. | Trained thoroughbred yearling horses | Acute (artificial light supplementation). | 7 months of summer stress (14.5 h of daylight and 9.5 h of darkness). | In natural settings, Hokkaido yearlings had higher total thyroxine concentrations but lower insulin-like growth factor-I (colt) and prolactin levels compared to Miyazaki yearlings. Under light supplementation, the quantities of prolactin and progesterone increased in Hokkaido and Miyazaki, resulting in early ovarian activity compared to controls. |
| Ishimaru et al. [31] | To compare the physiological, endocrine, and developmental parameters of thoroughbred weanlings and yearlings in Hokkaido, Japan, to those kept outdoors for 22 h in the paddock (22 h group) and 7 h in the daytime with walking exercise for 1 h using a horse-walker (7 h + W group). | Thoroughbred colts and fillies | Acute (seasonal) | 3 months of winter stress only (22 h outdoors) 3 months of winter stress and walking exercise (7 h outdoors in the daytime with walking exercise) | No significant change in the levels of circulating cortisol and thyroxine in the 22 h group. Increase in the concentrations of circulating cortisol, thyroxine, prolactin, and insulin-like growth factor in the 7 h + W group. |
| Shi et al. [32] | To examine the relationships between three hair cortisol features (hair cortisol concentration, protein concentration, and the ratio of hair cortisol concentration to protein concentration) with the sampling year and season and hair color in dairy cows. | Holstein dairy cows | Chronic stress | 18 months of heat stress | Rise in the concentration of hair cortisol. |
| Blond et al. [33] | To determine if heat stress influences the values of metabolic, endocrinological, and inflammatory markers, as well as body surface temperature in cows throughout the early and middle phases of lactation. | Holstein-Friesian breed of cows | Acute (seasonal) | 3 months of heat stress. | Increased levels of cortisol and insulin. Decrease in triiodothyronine (T3) concentration. |
| Author(s) (Year) | Type of Exposure | Mechanism of Endocrine Disruption | Effect on Endocrine Laboratory Parameters |
|---|---|---|---|
| Pragna et al. [34] | Heat stress | Alterations in the metabolic activities of the Osmanabadi, Malabari and Salem black goats. | An increase in the production of plasma triiodothyronine, and a decline in the synthesis of thyroid stimulating hormone. |
| Shilja et al. [35] | Heat stress | The activation of the hypothalamic–pituitary–adrenal axis, which resulted in an increase in the expression of adrenal and hepatic Heat Shock Protein 70, and messenger ribonucleic acid (mRNA) in goats. | Rise in the release of plasma cortisol and aldosterone in goats. |
| Bruzzio et al. [36] | Acidification of the ocean and hypoxia. | The acidification of the ocean affected hormonal stress physiology in juvenile blue rockfish. The dissolved oxygen levels also led to differences in metabolic processes, physiological state, and behavioral stress in juvenile blue rockfish. | Increase in the circulation of cortisol and decrease in the production of insulin-like growth factor 1. |
| Hadinia et al. [37] | Artificial light | Activation of the hypothalamic–pituitary–gonadal axis, which resulted in increased body lipid accumulation, and enhanced levels of reproductive hormones in broiler breeder pullets. | Rise in the circulation of gonadotropin releasing hormone 1, luteinizing hormone, follicle stimulating-hormone, and 17-beta-estradiol. |
| Lane et al. [38] | Lack of nature | The stimulation of the hypothalamic–pituitary–gonadal axis leads to a glucocorticoid stress response. This response is stopped by a negative feedback mechanism, which occurs predominantly via the attachment of receptors in the hippocampus and hypothalamus, resulting in lowered production and release of hormones from the adrenal gland. | Decline in corticosterone levels and synthesis of glucocorticoids. |
| Li et al. [39] | Heat stress | Exposure to heat elevated blood testosterone levels and the expression of glucocorticoid receptors in the nuclei of primary and basal cells from Bama miniature pigs. | Rise in the level of testosterone in the serum of Bama miniature pigs. |
| Halder et al. [40] | Heat stress | Prolonged exposure to high temperatures in male rats resulted in oxidative stress and a substantial reduction in superoxide dismutase, catalase, and glutathione in the testicles. However, there was a significant increase in the synthesis of testicular lipid peroxidase, enzyme caspase-3, and expressions of testicular heat shock protein 72 and heat shock factor 1. In addition, extended exposure to high temperatures also led to a drop in the formation of 17β-hydroxysteroid dehydrogenase-3. | Low serum testosterone levels. Increase in the concentration of corticosteroids in the serum |
| de Souza et al. [41] | Climate change | Amplification of the impact of pollutants on fish, which impairs the expression of testicular genes. Over time, the aforementioned disorder leads to serious testicular injury and subfertility in zebrafish. | Impedes the formation of 11-ketotestosterone and 17β-estradiol in zebrafish. |
| Dovolou et al. [42] | Heat stress | The hypothalamus–pituitary–gonadal axis communication is disrupted, leading to an endless cycle of decreased gonadotrophic support and inefficient biosynthesis of steroid hormones in the ovary. | Inhibits the secretion of luteinizing hormone and gonadotropin. |
| Kim et al. [43] | Heat stress | Summer heat stress lowered the expression of kisspeptin and neuronal activity in the hypothalamus, thereby suppressing the activation of the hypothalamus–pituitary–gonadal axis. | Low levels of circulatory estrogen and gonadotropin. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arneth, B. Endocrine Parameters and Climate Change. Endocrines 2025, 6, 5. https://doi.org/10.3390/endocrines6010005
Arneth B. Endocrine Parameters and Climate Change. Endocrines. 2025; 6(1):5. https://doi.org/10.3390/endocrines6010005
Chicago/Turabian StyleArneth, Borros. 2025. "Endocrine Parameters and Climate Change" Endocrines 6, no. 1: 5. https://doi.org/10.3390/endocrines6010005
APA StyleArneth, B. (2025). Endocrine Parameters and Climate Change. Endocrines, 6(1), 5. https://doi.org/10.3390/endocrines6010005






