Abstract
Through several pathological mechanisms, chronic stress contributes to the development of “osteosarcopenic obesity”, a clinical syndrome that includes impairments in the structure and function of a patient’s bones, skeletal muscles, and adipose tissue. This syndrome, which could be alternatively called “chronic stress and inflammation syndrome”, has its genesis in early life and, by the age of 50–60 years, affects up to two-thirds of Western populations. Chronic psycho-socioeconomic stress and lifestyle factors, such as a sedentary life, poor quality nutrition, irregular daily schedules, and inadequate sleep, which all act on a genetic and epigenetic predisposition background, play essential pathogenic roles in the development of this widespread syndrome. Key pathogenic mediators are those of the stress system and inflammatory reaction. Lifestyle changes, in combination with stress management, can prevent, arrest, or reverse this debilitating syndrome.
1. Introduction
Osteosarcopenic obesity (OSO), otherwise known as “osteosarcopenic adiposity”, is a syndrome which clinical phenotype combines impairments in the structure and function of a patient’s bones, skeletal muscles, and adipose tissue [1]. The etymology of the first term originates from three Greek words (osteo- meaning bone-, sarco- meaning flesh, and penia- meaning deficiency). In contrast, the second term has a Latin origin. Chronic stress, i.e., prolonged impairment of homeostasis, results in the coexistence of bone loss (osteoporosis); sarcopenia/dynapenia (decreased muscle performance); and increased adiposity, either as overt, BMI-defined overweight/obesity or because of tissue accumulation and organ infiltration with fat (liver, skeletal muscle, and bone) [1,2]. This condition is becoming more prevalent in aging populations. This review aims to provide an overview of current research on the association between stress and OSO, including the underlying mechanisms and potential treatment options.
1.1. Obesity, Osteoporosis, Sarcopenia, and Stress
Obesity, a disorder characterized by excess body weight linked to increased fat mass, is associated with many adverse health consequences and a reduced life expectancy [3]. The worldwide rise in the prevalence of obesity has had a significant, multifaceted impact on individuals and society. People’s diet often surpass caloric need leading to obesitySeveral conditions, including psychological distress, nonalcoholic fatty liver, respiratory difficulties, sleep apnea/sleep disturbance, joint and mobility problems, type 2 diabetes mellitus (T2D), dyslipidemia, arterial hypertension, cardiovascular morbidity, and neoplasmatic diseases, have all been associated with obesity. In recent years, researchers have debated whether OSO affects only older people or involves a lifelong process with onset in youth [4].
Osteoporosis is a bone-related metabolic disorder which main symptoms include decreased bone mass, impaired bone tissue, and a disarrangement in the osteal microarchitecture. Bone mass grows from birth to adulthood and reaches peak bone mass in young adulthood, which is followed by a gradual bone loss [5]. In addition, lifestyle and dietary habits, such as smoking, alcohol consumption, and sedentary life, are associated with an increased future hazard in fracture risk, which is J-shaped, according to a cohort study from the Swedish National Patient Registry [6].
Bone tissue loss occurs naturally through resorption due to increased osteoclast activity, while bone tissue is rebuilt through the formation process due to osteoblastic function. Thus, bone loss is associated with an increased resorption along with a decreased formation rate. Osteoporosis may lead to weaker bones being vulnerable to increased fracture risk [7]. Fragility fractures, especially in the hips, vertebrae, or distal radius, constitute a significant cause of morbidity in the population, are associated with pain, and often have incomplete rehabilitation. Moreover, hip fractures are associated with increased mortality, usually occurring 3–6 months after a fracture [8].
The function and homeostasis of skeletal muscles are linked with a complex equilibrium between regeneration and degeneration through poorly explained mechanisms and anabolic and catabolic pathways. The European Working Group on Sarcopenia in Older People (EWGSOP2) proposed the definition of primary sarcopenia as a progressive age-related disease of skeletal muscles that involves increased loss of muscle mass and function associated with disability, often referred to as dynapenia. Primary sarcopenia has been linked to an increased risk of physical disability, falls, frailty, low quality of life, and several adverse outcomes and death. Sarcopenia might appear suddenly, following a disease or a period of immobility, or in a more chronic gradual manner [9,10]. Muscle mass and strength, along with bone mineral density, have their maximum values up to the 25th year of age, and after a plateau period, their values decrease with age. Sarcopenia may occur in both lean and obese people.
Secondary sarcopenia may be caused by cancer, which is associated with cachexia and muscle loss. It is linked to frequent accidents, such as falls, declining ability to function in certain areas, symptoms of weakness, and even death [10]. It is most often seen in older populations. However, middle-aged people may present sarcopenia resulting from a combination of risk factors, including genetic and lifestyle-related parameters [10]. The data from the English Longitudinal Study of Aging showed that the risk of all-cause mortality was significantly greater in people with weight loss and reduced handgrip strength [11]. A recent meta-analysis described an association between sarcopenia and increased risk of falls and high incidence of hospitalization, as well as increased mortality risk, with a pooled odds ratio of 3.59 (95% CI 2.96–4.27) in people aged 79 years or older [12]. Research has focused on the link between lean body mass and adipose tissue in recent years. Studies in older women with OSO showed lower handgrip strength, abnormal balance, and an increased risk of falls, bone fractures, and immobility compared to obese-only women of the same age [13]. Research data regarding the Chinese population showed that men with OSO had a high prevalence of vertebral, hip, and ankle fractures; however, women with OSO had only a high prevalence of ankle fractures [14]. Another study found that nonobese people with sarcopenia were more vulnerable to hip/femur fractures, inflammation, edema, and malnutrition than persons with sarcopenic obesity, who had fewer adverse outcomes, probably because of the “obesity paradox” [15,16]. One study found that people with osteosarcopenia who acquired a fracture had an increased postoperative mortality rate [17]. Several studies have been conducted to prevent falls. A recent study found that fractures were more common in patients with osteoporosis or sarcopenia than in healthy age- and sex-matched people [18,19].
Stress is a state in which an individual’s homeostasis is threatened or disturbed by a stressor, which can be psychosocial or physical [20]. Changes in people’s lives, including advances in technology, occupational stressors, as well as changes in peoples’ nutritional options and choices, have dramatically increased the level of stress experienced on an everyday basis [21]. The triple etiology of nutritional, oxidative, and inflammatory factors represents the basis for stress origins. Prolonged dyshomeostasis or cacostasis caused by chronic stress and the resultant changes in stress system activity and low-grade, chronic inflammation (LGCI) or “para-inflammation” are indisputably related to various chronic diseases, including osteoporosis, sarcopenia/dynapenia, and obesity [4,21,22].
1.2. Diagnostic Criteria
The diagnostic criteria for diagnosing OSO is a combination of osteopenia or osteoporosis, sarcopenia, and obesity, i.e., bone, muscle, and fat impairments [13]. As this syndrome is a new entity, we suggest the following diagnostic criteria:
- The presence of osteopenia or osteoporosis. The diagnosis of osteopenia or osteoporosis is confirmed when bone mineral density is between 1 and 2.5 and less than 2.5 SD or more below the expected value, respectively. The IOF, ESCEO, ISCD, WHO, and NOF have recommended calculating the T-score using dual-energy X-ray absorptiometry (DXA) according to the NHANES III reference database for femoral neck and/or lumbar spine (L1–L4) measurements in women aged 20–29 years [8].
- Loss of muscle mass and function associated with disability [23].
- Body mass index ≥ 30 kg/m2 [24].
2. Stress-Induced Osteosarcopenic Obesity
2.1. Etiology and Pathophysiology
The prevalence of osteosarcopenic obesity in community-dwelling adults has been reported to be 10.7% [25]. Several risk factors have been associated with stress-induced osteopenia or osteoporosis, sarcopenia, and obesity All these factors may interconnect and lead to disease.
Chronic stress and the inflammation with which it is associated contribute to OSO and several related pathological phenomena [26,27], depending on an individual’s genetic and epigenetic background, as distinct pathologies.
Recent research suggests that in parallel with an increased BMI, an alteration of body composition is seen, with an increase in fat mass from 10 to 25 percent in men and from 20 to 35 percent in women, and a decrease in muscle mass from 50 to less than 40% in men and from 35 to less than 30% in women [28]. This shift in body composition linked to obesity represents an additional risk factor for insulin resistance, sarcopenia, osteoporosis, and cardiometabolic consequences [28]. In addition, mesenchymal stem cells may be diverted from the production of bone and muscle toward adipose tissue [29].
To confront the state of dyshomeostasis, multiple processes occur in the body, resulting in the activation of the sympathetic nervous system (SNS) and the hypothalamic–pituitary–adrenal axis (HPA) [30].
These systems release chemicals to help restore balance, such as catecholamines released to increase heart rate and blood pressure, allowing the body to address the fight against the trigger of change [31]. These processes are pivotal in the survival and adaptation of the human organism [31].
However, chronic cacostasis has been linked to pathological consequences in the endocrine, immune, nervous, and cardiovascular systems and one’s body composition [32]. In addition, prolonged stress is a risk factor for developing several physical and psychiatric disorders [32]. Stress-related diseases may be cardiovascular, metabolic, psychiatric, neurodegenerative diseases, or cancer [33].
2.1.1. Stress and Inflammation
Given its subclinical nature, low-grade chronic inflammation is challenging to diagnose [34]. It is defined by the presence of an increased number of circulating pro-inflammatory cytokines and an unsubsiding presence of excess immune cells in one’s circulatory system [34]. In addition, due to the extensive adverse effects, subclinical inflammation has a remarkable impact on the body’s metabolic processes [35].
Several lines of evidence have shown that metabolic syndrome and obesity are associated with oxidative stress. For example, impaired high-density lipoprotein (HDL)-enabled antioxidative mechanism, increased lipid peroxidation, carbonylation of cellular proteins, and NADPH oxidase activity are associated with obesity, leading to enhanced ROS formation and advanced mitochondrial oxidative products [36]. In addition, recent studies found that alterations in peripheral interleukin-6 circadian rhythm, as a sequel of combat deployment, were associated with cases of posttraumatic stress [34,37].
2.1.2. Endocrine Factors
Chronically elevated cortisol secretion, low-grade chronic inflammation, and the associated insulin resistance synergistically impair bone, muscle, and adipose tissue functions [38]. In the presence of glucocorticoids, mesenchymal stem cells involved in the production of bone, muscle, and adipose tissue show preferential differentiation into adipose tissue over bone or muscle tissue [39]. Excess adiposity, primarily abdominal or visceral adiposity, is associated with the secretion of inflammatory cytokines and other mediators, which appear to participate in a “positive feedback loop” between inflammation and excess body fat. Thus, progressively increasing fat accumulation is associated with gradually increasing secretion of inflammatory molecules, worsening insulin sensitivity, and increasing cortisol secretion, with these changes leading to further adiposity [26]. Modern-day Western lifestyles that are more passive and nutritionally deficient and more fast-paced and stressful than our evolutionary paradigms may lead to pathologies, including OSO [40,41].
Poor nutritional choices underscore the discordance between modern diets and diets followed during paleolithic times and are linked to disturbances in one’s circadian rhythms [21,26,27,42]. Modern humans’ ways of organizing their sleep and eating schedules based on either choice or necessity, instead of a regular schedule aligning with the time of day, are significant causes of circadian dysrhythmia [43,44]. Misalignments of one’s circadian rhythms, between the central circadian clock (i.e., the suprachiasmatic nucleus of the hypothalamus) and the peripheral clocks of adipose, liver, muscle, or other tissues, affect the expression of many genes linked to metabolic and other functions. Increased diurnal cortisol secretion, with lower hormone levels at 8 am and increased levels at 8 pm, or low hormone values during the day, provides inadequate levels and is often associated with low-grade inflammation. The consequences of increased exposure of tissues to glucocorticoids resulted from activated HPA axis and misalignment of cortisol secretion with the tissues’ glucocorticoid-sensitivity circadian rhythms include an increase in immune-related local and/or systemic inflammatory mediators, such as tumor necrosis factor (TNF)-alpha and several interleukins (IL), including IL-1and IL-6, as well as metabolic and psychological changes.
Altered levels of these mediators due to the disruption of natural schedules catering to one’s sleep and energy needs are associated with many physical and psychiatric disorders, including depression, cardiovascular diseases, type 2 diabetes mellitus, and metabolic syndrome [45]. Research has shown that young girls with social jet lag or who slept very late at night had a higher risk for greater waist circumference and obesity than those with the morning chronotype, even if sleep duration was similar. However, the results were not replicated in boys [46].
Adipocytes produce adipokines; by secreting pro-inflammatory adipokines, adipocytes contribute to the inflammatory status of the human organism [10].
Indeed, bodyweight gain may play a key role in inducing OSO. An adipokine, leptin, acts as an afferent signal in a negative feedback loop, controlling adiposity and body weight by suppressing food intake and stimulating the basal metabolic rate. Although obese individuals secrete large amounts of circulating leptin, their central nervous system is typically insensitive to leptin presence, which is called leptin resistance [47]. Leptin resistance may reduce fatty acid (FA) oxidation in muscles, followed by hepatic, myosceletal, and heart fat deposition, thereby decreasing muscle quality in older obese people [47]. Another adipokine, adiponectin, is an anti-inflammatory, insulin-sensitizing peptide that is negatively associated with muscle mass because of its inability to control NF-kB [48]. Another endogenous peptide secreted by adipocytes and induced by muscle contraction is apelin, which production declines with age through several pathways.
An inflammatory state is causative in insulin resistance and promotes skeletal muscle catabolism. Additionally, hypertrophic adipocytes increase the production of free fatty acids, which accumulate ectopically between muscle fibers and inside other non-adipose tissues in the form of triacylglycerol. They cause mitochondrial dysfunction, beta-oxidation of fatty acids, and increased production of reactive oxygen species. In addition, due to the cell saturation of triacylglycerol, they produce toxic reactive lipid species and stimulate apoptosis. These reactive lipids may accumulate in skeletal muscles, contributing to the development of sarcopenia [49,50].
Earlier studies suggested that myostatin, irisin, and osteocalcin mediate the crosstalk between muscles and bones. However, the role of these endocrine factors in the pathogenesis of OSO has not been fully understood. Research data showed that muscle loss in older people may occur through increased levels of myostatin, which is well established for its overexpression in inducing protein degradation in muscles along with inhibiting osteoblastic differentiation in bones; myostatin is a negative regulator of muscle mass that causes muscle atrophy, and decreased concentrations of myosin, the major muscle motor protein, contribute to decreased muscle function [51].
The pathogenic interrelations between adipose tissues and muscles are also crucial in sarcopenia. Myokines are specific peptides secreted by skeletal muscles that mediate some of the known positive effects of physical exercise. Myokines may affect myocytes and immune cells because of autocrine and/or paracrine actions and cells such as adipocytes and hepatocytes via endocrine effects [49]. IL-6 is the most well-studied myokine. Exercise may enhance IL-6 secretion from muscles and increases plasma IL-6 levels up to 100-fold [49]. In addition, the myokine irisin, which is produced during exercise, plays a significant role in controlling fat gain by mediating the trans-differentiation of brown into white adipose tissue, activating non-shivering thermogenesis, and inducing myocyte differentiation and growth via increases in the expression and secretion of insulin-like growth factor 1 (IGF-1), which promotes osteoblastogenesis and myogenesis, along with reducing adipogenesis [52].
Less physical activity with aging may be associated with a decline in muscle mass and irisin production, which leads to an increased adipose tissue mass and, thus, sarcopenic obesity. Furthermore, a recent experimental study showed that irisin stimulates cortical bone mass and bone strength in mice by increasing osteoblastic bone formation, up-regulating pro-osteoblastic genes, and decreasing osteoblast inhibitors [53]. Therefore, reduced irisin production may lead to OSO [52,53]. In addition, immobilization is another cause of the maximum shortening velocity of single muscle fibers [54].
Osteocalcin is a protein hormone secreted by osteoblasts. It has essential roles in bone matrix building and glucose metabolism by playing a key role in insulin sensitivity and adiponectin secretion by adipocytes; it possibly has anabolic actions on muscles by inducing testosterone production [55].
The key factors of osteoblastogenesis, including osteoblast maturation and differentiation, are runt-related transcription factor 2 (RUNX2), which is regulated by estrogen and prostaglandin eicosanoids 2, and musculoaponeurotic fibrosarcoma oncogene homolog (Maf); it has been suggested that both play an inhibitory role in myogenesis, along with hindering adipogenesis and extending osteoblastogenesis [56].
2.2. Purines
The physiological role of ATP at the skeletal neuromuscular junction has been previously reported. Extracellular nucleotides may act as modulators of bone cell differentiation, function, and survival [57]. In the extracellular space, ATP is hydrolyzed into adenosine by one or more ectonucleotidases in human primary osteoblast cells (HPOC), and nucleoside is the end-product of the cascade [58]. Adenosine accumulates in the extracellular area in response to metabolic or oxidative stress, tissue injury, hypoxia, or inflammation. Purinergic signaling has been implicated in bone since extracellular ATP may disturb bone formation while promoting bone resorption. Still, it also has a crucial role in modulating osteal cells’ differentiation, function, and survival after its physiological secretion in response to mechanical stress [59]. ATP has also been suggested as an endogenous inhibitor of bone mineralization by blocking the mineralization of collagenous matrix [60]. Purinergic signaling via P2 receptors can interact with other intracellular pathways to regulate osteoblast function.
Moreover, ATP has been found to stimulate human osteoclast activity indirectly by inducing upregulation of osteoblast-expressed RANKL via P2X7 receptors [61]. The P2X7 receptor gene, P2RX7, is located on chromosome 12q24.31 and is characterized by numerous polymorphisms [62]. The purinergic P2X7 receptor plays a vital role in regulating osteoblast and osteoclast activity, and every change in receptor function may be reflected in bone mass [63]. The Danish Osteoporosis Prevention Study studied 1694 women who were followed for 10 years and genotyped for 12 functional P2X7 receptor variants. Increased bone loss with fractures was associated with Arg307Gln amino acid substitution, and heterozygous individuals for this polymorphism had a 40% increased rate of bone loss. Low-risk alleles were linked to a low rate of bone loss, and high-risk alleles were associated with increased bone loss [63]. Among the P2 receptor subtypes expressed by bone cells, the P2X7 receptor has been correlated with the treatment of osteoporosis. This receptor has been implicated in regulating the release of inflammatory cytokines IL-6, TNF-α, and plasminogen activator inhibitor-1, at least in part via inflammasome activation [64]. In addition, endogenously released adenosine, which signals via four adenosine receptor subtypes acting on G-protein-coupled receptors, A1, A2A, A2B, and A3, on osteoclasts and their precursor cells, acts as a mediator on bone turnover and trans-differentiation of osteoblasts to adipocytes, showing its key role in the pathogenesis of osteoporosis. The most abundant A2B receptor in bone marrow MSCs seems to have a consistent role in cell differentiation, which may be balanced through the relative strength of A1 or A2A receptor, thus determining whether osteoblasts are driven into proliferation or differentiation [65].
Moreover, A1 receptor has been associated with the lipogenic activity of adipocytes, and A1R is also implicated in adipogenesis and leptin production. A1R regulates lipolysis and serum-free fatty acid levels, playing a crucial role in the pathogenesis of insulin resistance, diabetes, and cardiovascular diseases by controlling the proliferation and regeneration of β cells in inflammatory microenvironments [66]; in addition, it activates lipolysis and the thermogenic program in brown and white human adipocytes [66]. Additionally, preclinical trials suggest that pharmacological manipulation of the purinergic cascade in adipocytes and other adipose tissue cells may be used to treat obesity and type 2 diabetes [67]. Purines act synergistically with hormones (e.g., PTH), playing a fundamental role in mesenchymal stem cell proliferation and differentiation (adipogenesis, myogenesis, and osteogenesis) [68].
Both nutritional stress and the genetic model of obesity are linked to an increased expression of P2X7 that is correlated with increased macrophage infiltration within adipose tissues and increased expressions of CD39 and CD73. Purinergic signaling cascade in adipocytes and other cellular players of fatty tissues may be a valuable target for treating obesity and metabolic disorders. Further experimental studies showed that P2X7R stimulation directs the differentiation of MSCs toward the osteoblast lineage rather than toward adipocytes. In addition, animal studies found that ATP and BzATP reduce leptin mRNA levels and inhibit insulin-induced leptin secretion. Furthermore, adenosine has been implicated in inflammation and sustaining IL-1β production in metabolically unhealthy obese individuals; moreover, its receptor expression is correlated with increased body mass index [69]. However, the exact pathophysiological pathways between purine metabolism and OSO remain unclear and offer an exciting field for further research.
2.2.1. Vitamin D
Since 1969, when Haussler described Vitamin D and its receptor (VDR) and confirmed its presence in various tissues, many researchers have focused on the mechanistic link between vitamin D and the occurrence of various diseases, such as obesity, a chronic, low-grade inflammatory state that leads to impaired metabolic processes in adipose and lean tissues; the pathogenesis of insulin resistance; metabolic syndrome; type 2 diabetes mellitus; and cancer prevention [70,71,72]. The presence of VDR has been reported in skeletal muscle tissues [73]; therefore, numerous studies have focused on musculoskeletal disorders and the mechanical properties of vitamin D function. In adults and older adults, moderate vitamin D deficiency is often associated with increased serum parathormone (PTH) concentration, leading to high bone turnover and decreased BMD. In the MORE study, postmenopausal women with vitamin D deficiency had significantly higher serum PTH, more elevated serum alkaline phosphatase, and lower BMD of the trochanter compared with those with adequate 25OHD levels [74]. A multicenter bazedoxifene trial involving 29 countries, with 7441 postmenopausal women as participants, reported similar outcomes [71]. This study showed that worldwide, a negative correlation between 25(OH)D and mean PTH, osteocalcin (OC), and C-terminal cross-linked telopeptides of type I collagen (CTX) was observed. With increasing 25(OH)D categories, the BMD of all sites increased. Another study with 1319 elderly participants (643 men and 676 women) confirmed that these thresholds for PTH and BMD, as well as skeletal mass and physical performance in older people, seemed to improve when serum 25(OH)D were increased above 50–60 nmol/L [71]. A Hungarian study found that males with vitamin D deficiency had significantly lower BMD and a higher 10-year hip and osteoporotic fracture probability using a country-specific FRAX algorithm [75]. A significant positive effect of vitamin D supplementation on global muscle strength in older adults was previously shown, but no impact on lean mass was reported [12]. Additionally, vitamin D supplements increase lower limb muscle strength in athletes [76].
Moreover, vitamin D deficiency has been linked with an increased risk of falls, and past studies have shown benefits after vitamin D and calcium supplementation in elderly recurrent fallers, mainly during winter. A recent meta-analysis reported that when vitamin D was supplemented with calcium.there was a significant reduction in fall incidence and fracture rates [77]. However, high annual or monthly single oral doses of vitamin D increased the risk of falling in a U-shaped model. The authors suggested that the lower value of fall rates occurred in the dose range of 1600 to 3200 IU/d [78]. The association between obesity and vitamin D deficiency has been previously documented [79]. A population-based prospective cohort study with 1501 participants from Norway reported a negative correlation between season-standardized serum 25(OH)D level and risk of clinical weight gain [80]. However, whether a causal link between vitamin D deficiency and obesity and metabolic syndrome exists or whether increased adiposity has a causal role for low vitamin D status is still not well established [78].
Most intervention studies found no statistically significant benefits for metabolic syndrome and related diseases. However, a serious concern is whether vitamin D supplementation is associated with the risk of developing kidney stones, especially when combined with calcium prescription. A recent review has hypothesized that some predisposed individuals, who transform 25-hydroxyvitamin D into calcitriol more efficiently, with a probably reduced capacity for degrading calcitriol, have a higher risk of developing kidney stones after supplementation with vitamin D [81].
2.2.2. Genetic and Epigenetic Factors
Family history is a well-established risk factor for osteoporosis, sarcopenia, and obesity; genotype is, in fact, a significant influencer of altered energy balance.
The hypothalamus plays a critical role in energy expenditure and balance. Recent studies suggested that predisposing genetic traits related to appetite and satiety may be linked to behavioral traits through appetitive neural pathways influencing control over overeating or eating in response to negative emotions [82]. Additionally, the hippocampus and the rest of the limbic system have been associated with cognition, memory, emotion, and control of BMI [82].
Monogenic or oligogenic autosomal or X-linked patterns of inheritance have been described; however, obesity is primarily polygenic and is a result of multiple gene defects interacting with the environment [83].
Homozygous mutation in the leptin (LEP) gene and loss-of-function mutations in SH2B1 (SH2B adaptor protein 1, a key regulator of leptin signaling by both stimulating Janus kinase 2 (JAK2) activity and assembling a JAK2/IRS1/2 signaling complex) are associated with rapid weight gain within the first years of life, severe hyperphagia, and behavioral problems [83]. Other homozygous/compound heterozygous loss-of-function mutations in monogenic obesity genes in the leptin/melanocortin pathway or heterozygous loss-of-function mutations in POMC, including loss-of-function missense mutation in β-MSH, partial loss-of-function heterozygous mutations in PCSK1, heterozygous loss-of-function coding mutations in the melanocortin 3 receptor (MC3R) gene, and heterozygous mutations in melanocortin receptor accessory protein 2 (MRAP2), have all been associated with either childhood or adult obesity. As with obesity, osteoporosis and sarcopenia have a vital genetic component, with heritability ranging over 50%.
On the other hand, mutations or polymorphisms of the gene family 210 member A (FAM210A), growth/differentiation factor 8 (GDF8) (also known as myostatin), methyltransferase-like 21C (METTL21C), and sterol regulatory element-binding transcription factor 1/target of myb1-like 2 (SREBF1/TOM1L2) have been linked to genetic predisposition for sarcopenia and osteoporosis [84]. Moreover, smoking-related postmenopausal osteoporosis has been recently associated with mutations in HNRNPC, PFDN2, PSMC5, RPS16, TCEB2, and UBE2V2 genes [85]. Recently, several genome-wide association studies (GWASs) and meta-analyses have focused on genetic alterations associated with BMD in osteoporosis. Among them, the most featured loci are SMOC1, CLDN14, ZBTB40, GPR177, FGFRL1, MEPE, MEF2C, ESR1, SHFM1, WNT16, OPG, SOX6, LRP5, AKAP11, and FOXL1 [86]. Polymorphisms of MTHFR, ACTN3, NRF2, VDR FokI, ADRB2, and NPAS4 loci have been linked with sarcopenia in older adults. A recent study suggested a genetic risk score regarding sarcopenia onset [87]. Moreover, new data were recently reported regarding 10 genes (CFB, CXCL2, HSD11B1, IGF1, IL6, NTN1, PCSK1, PPARGC1A, SOD2, and TF) that were associated with osteoporosis, sarcopenia, and obesity-induced diabetes [88].
DNA methylation has been suggested to assess epigenetic patterns and candidate gene approaches, and epigenome-wide association studies (EWASs) correlating gene methylation levels and obesity have been performed. Additionally, specific alterations of the gut microbiota have been linked to obesity, and newer research has focused on whether an increase in Firmicutes and/or a decrease in Bacteroides plays a significant role in changes in energy balance or changes in energy stores [89].
2.2.3. Aging
The skin has a crucial role in producing the prohormone vitamin D and transforming it into inactive metabolites [68]. It is well documented that advancing age reduces the skin’s capability to synthesize pre-vitamin D3 [90]. It has been reported that the concentration of the precursor of vitamin D3 in the skin, 7-dehydrocholesterol(7-DHC), declines by about 50% from age 20 to 80. Moreover, another study found that older age was a significant predictor of decreased VDR expression [91]. Therefore, vitamin D deficiency, taking into consideration the above-described vitamin D actions, could play a significant synergistic role in OSO following advancing age.
Several cellular changes occur in sarcopenic muscles, including decreased size and reduction in several mostly type II myofibers. Aging promotes the transition of muscle fibers from type II to type I, along with intramuscular and intermuscular fat infiltration (myosteatosis). Cross-sectional studies of single muscle fibers from healthy people found a reduction in muscle fiber quality in fibers expressing type I or IIA myosin heavy chain and, over time, a significant decrease in single muscle fiber peak power [51]. Other biological effects of aging are maximum unloaded shortening velocity, decreased elasticity, and greater stiffness per force unit in older men [51]. Subclinical inflammation also plays an essential role in high muscle catabolism in older people [51]. A decline in satellite cells associated with type IIA fibers suggests a decrease in the regenerative capacity of muscle fibers and a failure to recompense the loss of fibers [51]. The loss of lean mass in sarcopenia is associated with increased accumulated adipose tissue in muscle, increasing myosteatosis with advancing age [92].
Mesenchymal stem cells (MSCs) can differentiate into bone cells, but in elderly individuals, these cells are decreased, resulting in osteoporosis. Adenine and uracil nucleotides are important local regulators of osteogenic differentiation of MSCs in bone marrow [93]. In addition, previous studies showed that cysteine (C)-X-C chemokine receptor-4 (CXCR4), the primary transmembrane receptor for stromal cell-derived factor-1, has decreased expression with aging in bone marrow-derived mesenchymal stromal stem cells (BMSCs), and this CXCR4-deficiency alters the osteogenic differentiation potency of older BMSCs. Recent studies using osteoprogenitor cell lines, animal models, and non-modified MSCs from postmenopausal women have focused on the purinergic signaling pathway and specific targets to regenerate the ability of aged MSCs to differentiate into active osteoblasts [93].
In addition, aged satellite cells, considered the key elements of sarcopenia by altering the regeneration of myofibers, have been shown to translocate across the basal lamina following age-dependent extracellular wall stiffness [94]. Furthermore, decreased muscle fiber vascularization is associated with impaired regulation of satellite cells in older adults [94]. With advanced age, the multiplication of bone marrow stem cells is diminished. Several studies indicated that both sarcopenia and osteoporosis are associated with stem cell senescence and exhaustion along with implicated pathophysiological mechanisms, such as impaired telomere and high levels of reactive oxygen species [84].
3. Lifestyle Intervention
Lifestyle changes remain fundamental for chronic stress-induced obesity and osteosarcopenia, while, occasionally, added pharmacotherapy may be necessary. The management of obesity requires a multidisciplinary team, including physicians, psychologists, dietitians, and ergotherapists. Interventions for osteoporosis include primarily a secondary disease approach, with treat-to-target anti-osteoporotic agents to improve the pre-fracture functional level, and reduction of subsequent fracture risk [17]; weight exercises that focus on strengthening one’s muscles [95]; abstinence from substance use, including alcohol and cigarette smoking [6]; as well as an emphasis on preventing the possibility of a fall [96]. A sedentary lifestyle over time contributes to osteosarcopenia [84].
To prevent OSO, the identification and treatment of secondary and modifiable risk factors, such as endocrine disorders, is essential. In addition, the risk of developing osteosarcopenia can be modified through healthy lifestyle changes that optimize peak bone mass and maintain musculoskeletal health. The Framingham study showed that women and men with relatively lower protein intake had increased bone loss, suggesting that protein intake is vital in maintaining or diminishing bone loss in older people. Protein intake is the most potent dietary strategy to increase muscle protein synthesis [97]. Whey protein contains leucine, which is crucial in stimulating mTORC1 in skeletal muscle. The positive effects of protein intake on bone health may be beneficial under conditions of adequate calcium intake.
The relation between musculoskeletal tissue and dietary protein intake is influenced by advancing age. Therefore, adults older than 65 require higher intake than the recommended protein intake for younger populations (0.8 g/kg/day). Recently, expert consensus groups recommended at least 1.2–1.5 g/kg/day of protein intake (leucine portion of 2.5–3 g for every meal) [52]. The European Society for Clinical and Economic Aspects of Osteoporosis and Osteoarthritis (ESCEO) suggested dietary protein intake of 1.0–1.2 g/kg body weight daily, with at least 20–25 g of high-quality protein at each main meal, dietary calcium intake of 1000–1300 mg, along with physical activity/exercise 3–5 times/week [98]. Additionally, bodyweight loss with specific calorie-reduced modified diets, such as the Mediterranean diet, is essential in treating obesity [24].
Protein quality and intake timing might play some therapeutic role, but the evidence does not support any recommendations. A study showed that vitamin D supplementation and leucine-enriched whey protein increased appendicular lean mass and decreased inflammatory markers in older sarcopenic persons with mobility limitations. Moreover, protein intake for sarcopenia is more effective at increasing muscle mass in older adults when circulating 25-hydroxyvitamin D levels are in an optimal range. Adequate dosing of vitamin D of 800 IU to maintain serum 25-hydroxyvitamin D levels >50 nmol/L, and 2000 IU for people with nutritional rickets, helps maintain muscle mass and strength, as well as bone density [98]. Not too long ago, weekly high doses of vitamin D were considered attractive to restore and maintain normal vitamin D serum levels. However, newer studies showed an unexplained association between bolus dosing and high fall and fracture risk. Past studies have demonstrated the role of calcium in skeletal muscle, specifically in facilitating its contractile force by maintaining normal calcium kinetics.
The use of DHA and EPA in osteosarcopenic populations is not globally recommended due to inconclusive research data, but theoretically, they should be helpful, as has been shown in other groups. In addition, administering prebiotics, such as dietary inulin-type fructo-oligosaccharides, seems to induce the growth of health-promoting Bifidobacterium, Lactobacillus, Roseburia, and Faecalibacterium species in humans [99].
Nutritional interventions and muscle-focusing exercises promote the prevention and treatment of sarcopenia, even though adherence to weight loss and physical activity intervention programs is poor [4]. Anti-obesity medications and bariatric surgery may also be utilized in severe cases of obesity in late adolescents and adults [20]. Physical activity can dramatically reduce genetic factors’ influence on BMI in young and older adults. Decreased sleep duration has been associated with high BMI and increased genetic effects on BMI in heritability studies, suggesting that shorter sleep duration increases the expression of genetic risks for increased BMI. Moreover, improving sleep schedules may be helpful for obesity prevention, especially in girls [46]. A new study suggested that families should advise young students to maintain consistency in sleep schedules and sleep hygiene by limiting electronic media, especially in the evening, before going to bed, as well as chrono-interventions, such as bright-light therapy in the morning and regular meals for girls and boys with severe social jet lag [46]. Behavioral modifications have been essential to weight-loss programs in recent years. Two large randomized clinical trials, the Look AHEAD trial and the Diabetes Prevention Program, have shown these methods’ efficacy and suggested individualizing therapy and frequent face-to-face interventions [24].
Physical activity is the primary physiological stimulus for bone anabolism through its direct loading effect, which acts directly on skeletal muscle. Daily exercise continuously modulates skeletal muscle’s metabolic activity and, hence, its endocrine function and the homeostatic response of all body organs, including bone [100]. Various types of exercise have distinct effects on either bone or muscle. Therefore, not all activities are effective in improving OSO. Aerobic and low-impact exercises, such as cycling or walking, have shown no efficacy in improving BMD, but they are beneficial in improving metabolism.
Pilates and walking have been suggested as crucial mediators in improving quality of life and mitigating depressive and anxious feelings. They may represent excellent means to improve mood disorders in overweight/obese individuals. A Spanish study of community-dwelling Spanish postmenopausal women older than 60 years showed that a twelve-week Pilates exercise intervention improved sleep quality and feelings of anxiety, depression, and fatigue. Resistance and high-impact aerobic training, such as running, may also be beneficial in maintaining or increasing BMD in pre- and postmenopausal women. Subclinical para-inflammation associated with sarcopenia in older people is partially reversible with exercise training [51].
Moreover, it was previously shown that resistance training improved muscle mass and strength and decreased adiposity in older women with OSO. However, bone density remained unchanged after 12 weeks of intervention. In case a patient is characterized by frailty or unwillingness to follow the exercise “prescription,” alternative exercises, such as yoga, Pilates, or Tai Chi, could improve balance, flexibility, and strength. Notwithstanding, passive electrical muscle stimulation has been studied as an adjuvant to standard resistance training with sound effects.
4. Conclusions and Future Prospects
A genetic and epigenetic predisposition, along with chronic psycho-socioeconomic stress and lifestyle factors, such as a sedentary life, poor quality nutrition, irregular daily schedules, and poor sleep, can play a pathogenic role in the development of OSO. In addition, stress and lifestyle factors can lead to inflammation that triggers or aggravates the disease (Figure 1). Treatment modalities vary and include multidisciplinary assessment along with lifestyle, pharmacological, psychological, and nutritional interventions. Future studies could benefit from focusing on early prevention and intervention strategies for OSO.
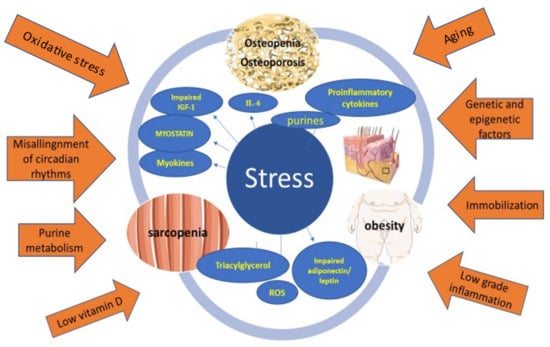
Figure 1.
A genetic and epigenetic predisposition, along with chronic psycho-socioeconomic stress and lifestyle factors, such as a sedentary life, poor quality nutrition with low vitamin D intake, irregular daily schedules, and poor sleep leading to misalignment of circadian rhythms, can play a pathogenic role in the development of OSO. In addition, stress and lifestyle factors can lead to inflammation that triggers or aggravates the disease.
Author Contributions
N.P.-M. conceived the idea, collected the data and wrote the paper. A.P., collected the data and G.P.C. conceived the idea, and wrote the paper. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
Nektaria Papadopoulou-Marketou, Anna Papageorgiou, and George Chrousos declare that they have no conflicts of interest.
References
- Perna, S.; Spadaccini, D.; Nichetti, M.; Avanzato, I.; Faliva, M.A.; Rondanelli, M. Osteosarcopenic Visceral Obesity and Osteosarcopenic Subcutaneous Obesity, Two New Phenotypes of Sarcopenia: Prevalence, Metabolic Profile, and Risk Factors. J. Aging Res. 2018, 2018, 6147426. [Google Scholar] [CrossRef] [PubMed]
- Kelly, O.J.; Gilman, J.C.; Boschiero, D.; Ilich, J.Z. Osteosarcopenic obesity: Current knowledge, revised identification criteria and treatment principles. Nutrients 2019, 11, 747. [Google Scholar] [CrossRef] [PubMed]
- Fontaine, K.R.; Redden, D.T.; Wang, C.; Westfall, A.O.; Allison, D.B. Years of life lost due to obesity. J. Am. Med. Assoc. 2003, 289, 187–193. [Google Scholar] [CrossRef] [PubMed]
- Stefanaki, C.; Peppa, M.; Boschiero, D.; Chrousos, G.P. Healthy overweight/obese youth: Early osteosarcopenic obesity features. Eur. J. Clin. Investig. 2016, 46, 767–778. [Google Scholar] [CrossRef]
- Cosman, F.; de Beur, S.J.; LeBoff, M.S.; Lewiecki, E.M.; Tanner, B.; Randall, S.; Lindsay, R. Clinician’s Guide to Prevention and Treatment of Osteoporosis. Osteoporos. Int. 2014, 25, 2359–2381. [Google Scholar] [CrossRef]
- Prieto-Alhambra, D.; Turkiewicz, A.; Reyes, C.; Timpka, S.; Rosengren, B.; Englund, M. Smoking and Alcohol Intake but Not Muscle Strength in Young Men Increase Fracture Risk at Middle Age: A Cohort Study Linked to the Swedish National Patient Registry. J. Bone Miner. Res. 2020, 35, 498–504. [Google Scholar] [CrossRef]
- Bonnick, S.L. Osteoporosis in men and women. Clin. Cornerstone 2006, 8, 28–39. [Google Scholar] [CrossRef]
- Kanis, J.A.; Cooper, C.; Rizzoli, R.; Reginster, J.Y. European guidance for the diagnosis and management of osteoporosis in postmenopausal women. Osteoporos. Int. 2019, 30, 3–44. [Google Scholar] [CrossRef]
- Cruz-Jentoft, A.J.; Sayer, A.A. Sarcopenia. In The Lancet; Lancet Publishing Group: London, UK, 2019; Volume 393, pp. 2636–2646. [Google Scholar]
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyère, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A.; et al. Sarcopenia: Revised European consensus on definition and diagnosis. In Age and Ageing; Oxford University Press: Oxford, UK, 2019; Volume 48, pp. 16–31. [Google Scholar]
- Hamer, M.; O’Donovan, G. Sarcopenic obesity, weight loss, and mortality: The English Longitudinal Study of Ageing. Am. J. Clin. Nutr. 2017, 106, 125–129. [Google Scholar] [CrossRef]
- Beaudart, C.; Zaaria, M.; Pasleau, F.; Reginster, J.Y.; Bruyère, O. Health outcomes of sarcopenia: A systematic review and meta-analysis. PLoS ONE 2017, 12, e0169548. [Google Scholar] [CrossRef]
- Ilich, J.Z.; Inglis, J.E.; Kelly, O.J.; McGee, D.L. Osteosarcopenic obesity is associated with reduced handgrip strength, walking abilities, and balance in postmenopausal women. Osteoporos. Int. 2015, 26, 2587–2595. [Google Scholar] [CrossRef]
- Hong, W.; Cheng, Q.; Zhu, X.; Zhu, H.; Li, H.; Zhang, X.; Zheng, S.; Du, Y.; Tang, W.; Xue, S.; et al. Prevalence of sarcopenia and its relationship with sites of fragility fractures in elderly Chinese men and women. PLoS ONE 2015, 10, e0138102. [Google Scholar] [CrossRef]
- Fassio, A.; Idolazzi, L.; Rossini, M.; Gatti, D.; Adami, G.; Giollo, A.; Viapiana, O. The obesity paradox and osteoporosis. In Eating and Weight Disorders; Springer International Publishing: Berlin/Heidelberg, Germany, 2018; Volume 23, pp. 293–302. [Google Scholar]
- Perna, S.; Peroni, G.; Faliva, M.A.; Bartolo, A.; Naso, M.; Miccono, A.; Rondanelli, M. Sarcopenia and sarcopenic obesity in comparison: Prevalence, metabolic profile, and key differences. A cross-sectional study in Italian hospitalized elderly. Aging Clin. Exp. Res. 2017, 29, 1249–1258. [Google Scholar] [CrossRef]
- Bae, G.C.; Moon, K.H. Effect of Osteosarcopenia on Postoperative Functional Outcomes and Subsequent Fracture in Elderly Hip Fracture Patients. Geriatr. Orthop. Surg. Rehabil. 2020, 11, 215145932094056. [Google Scholar] [CrossRef]
- Yoo, J.I.; Kim, H.; Ha, Y.C.; Kwon, H.B.; Koo, K.H. Osteosarcopenia in patients with hip fracture is related with high mortality. J. Korean Med. Sci. 2018, 33, 37–45. [Google Scholar] [CrossRef]
- Thomas, T.; Casado, E.; Geusens, P.; Lems, W.F.; Timoshanko, J.; Taylor, D.; Hofbauer, L.C. Is a treat-to-target strategy in osteoporosis applicable in clinical practice? Consensus among a panel of European experts. Osteoporos. Int. 2020, 31, 2303–2311. [Google Scholar] [CrossRef]
- Tsigos, C.; Kyrou, I.; Kassi, E.; Chrousos, G.P. Stress, Endocrine Physiology and Pathophysiology; MDText.com, Inc.: South Dartmouth, MA, USA, 2000. [Google Scholar]
- Ilich, J.Z.; Gilman, J.C.; Cvijetic, S.; Boschiero, D. Chronic stress contributes to osteosarcopenic adiposity via inflammation and immune modulation: The case for more precise nutritional investigation. Nutrients 2020, 12, 989. [Google Scholar] [CrossRef]
- Bano, G.; Trevisan, C.; Carraro, S.; Solmi, M.; Luchini, C.; Stubbs, B.; Manzato, E.; Sergi, G.; Veronese, N. Inflammation and sarcopenia: A systematic review and meta-analysis. In Maturitas; Elsevier Ireland Ltd.: Amsterdam, The Netherlands, 2017; Volume 96, pp. 10–15. [Google Scholar]
- Bauer, J.; Morley, J.E.; Schols, A.M.W.J.; Ferrucci, L.; Cruz-Jentoft, A.J.; Dent, E.; Baracos, V.E.; Crawford, J.A.; Doehner, W.; Heymsfield, S.B.; et al. Sarcopenia: A Time for Action. An SCWD Position Paper. J. Cachexia Sarcopenia Muscle 2019, 10, 956–961. [Google Scholar] [CrossRef]
- Bray, G.A.; Heisel, W.E.; Afshin, A.; Jensen, M.D.; Dietz, W.H.; Long, M.; Kushner, R.F.; Daniels, S.R.; Wadden, T.A.; Tsai, A.G.; et al. The science of obesity management: An endocrine society scientific statement. Endocr. Rev. 2018, 39, 79–132. [Google Scholar] [CrossRef]
- Kolbaşı, E.N.; Demirdağ, F. Prevalence of osteosarcopenic obesity in community-dwelling older adults: A cross-sectional retrospective study. Arch. Osteoporos. 2020, 15, 1–9. [Google Scholar] [CrossRef]
- Ilich, J.Z.; Kelly, O.J.; Kim, Y.; Spicer, M.T. Low-grade chronic inflammation perpetuated by modern diet as a promoter of obesity and osteoporosis. In Arhiv za Higijenu Rada i Toksikologiju; Institute for Medical Research and Occupational Health: Zagreb, Croatia, 2014; Volume 65, pp. 139–148. [Google Scholar]
- Ilich, J.Z.; Kelly, O.J.; Inglis, J.E.; Panton, L.B.; Duque, G.; Ormsbee, M.J. Interrelationship among muscle, fat, and bone: Connecting the dots on cellular, hormonal, and whole body levels. In Ageing Research Reviews; Elsevier Ireland Ltd.: Amsterdam, The Netherlands, 2014; Volume 15, pp. 51–60. [Google Scholar]
- Eaton, S.B.; Eaton, S.B. Physical Inactivity, Obesity, and Type 2 Diabetes: An Evolutionary Perspective. In Research Quarterly for Exercise and Sport; Routledge: Abingdon, UK, 2017; Volume 88, pp. 1–8. [Google Scholar]
- Caplan, A.I. Mesenchymal stem cells: Time to change the name! Stem Cells Transl. Med. 2017, 6, 1445–1451. [Google Scholar] [CrossRef] [PubMed]
- Foley, P.; Kirschbaum, C. Human hypothalamus-pituitary-adrenal axis responses to acute psychosocial stress in laboratory settings. Neurosci. Biobehav. Rev. 2010, 35, 91–96. [Google Scholar] [CrossRef] [PubMed]
- Chrousos, G.P. Stress and disorders of the stress system. Nat. Rev. Endocrinol. 2009, 5, 374–381. [Google Scholar] [CrossRef] [PubMed]
- Tamashiro, K.L.K.; Hegeman, M.A.; Nguyen, M.M.N.; Melhorn, S.J.; Ma, L.Y.; Woods, S.C.; Sakai, R.R. Dynamic body weight and body composition changes in response to subordination stress. Physiol. Behav. 2007, 91, 440–448. [Google Scholar] [CrossRef]
- Cohen, S.; Janicki-Deverts, D.; Miller, G.E. Psychological stress and disease. JAMA 2007, 298, 1685–1687. [Google Scholar] [CrossRef]
- Agorastos, A.; Hauger, R.L.; Barkauskas, D.A.; Lerman, I.R.; Moeller-Bertram, T.; Snijders, C.; Haji, U.; Patel, P.M.; Geracioti, T.D.; Chrousos, G.P.; et al. Relations of combat stress and posttraumatic stress disorder to 24-h plasma and cerebrospinal fluid interleukin-6 levels and circadian rhythmicity. Psychoneuroendocrinology 2019, 100, 237–245. [Google Scholar] [CrossRef]
- Hotamisligil, G.S. Inflammation and metabolic disorders. Nature 2006, 444, 860–867. [Google Scholar] [CrossRef]
- Rani, V.; Deep, G.; Singh, R.K.; Palle, K.; Yadav, U.C.S. Oxidative stress and metabolic disorders: Pathogenesis and therapeutic strategies. Life Sci. 2016, 148, 183–193. [Google Scholar] [CrossRef]
- Chrousos, G.P. Stress, chronic inflammation, and emotional and physical well-being: Concurrent effects and chronic sequelae. J. Allergy Clin. Immunol. 2000, 106 (Suppl. 5), S275–S291. [Google Scholar] [CrossRef]
- Lecka-Czernik, B. Diabetes, bone and glucose-lowering agents: Basic biology. Diabetologia 2017, 60, 1163–1169. [Google Scholar] [CrossRef]
- Wu, C.L.; Diekman, B.O.; Jain, D.; Guilak, F. Diet-induced obesity alters the differentiation potential of stem cells isolated from bone marrow, adipose tissue and infrapatellar fat pad: The effects of free fatty acids. Int. J. Obes. 2013, 37, 1079–1087. [Google Scholar] [CrossRef]
- Wellen, K.E.; Hotamisligil, G.S. Inflammation, stress, and diabetes. J. Clin. Investig. 2005, 115, 1111–1119. [Google Scholar] [CrossRef]
- Milutinović, D.V.; MacUt, D.; Božić, I.; Nestorov, J.; Damjanović, S.; Matić, G. Hypothalamic-pituitary-adrenocortical axis hypersensitivity and glucocorticoid receptor expression and function in women with polycystic ovary syndrome. Exp. Clin. Endocrinol. Diabetes 2011, 119, 636–643. [Google Scholar] [CrossRef]
- Kumar, S.; Kelly, A.S. Review of Childhood Obesity: From Epidemiology, Etiology, and Comorbidities to Clinical Assessment and Treatment. In Mayo Clinic Proceedings; Elsevier Ltd.: Amsterdam, The Netherlands, 2017; Volume 92, pp. 251–265. [Google Scholar]
- Marx, W.; Moseley, G.; Berk, M.; Jacka, F. Nutritional psychiatry: The present state of the evidence. Proc. Nutr. Soc. 2017, 76, 427–436. [Google Scholar] [CrossRef]
- Marinac, C.R.; Quante, M.; Mariani, S.; Weng, J.; Redline, S.; Cespedes Feliciano, E.M.; Aaron Hipp, J.; Wang, D.; Kaplan, E.R.; James, P.; et al. Associations between timing of meals, physical activity, light exposure, and sleep with body mass index in free-living adults. J. Phys. Act. Health 2019, 16, 214–221. [Google Scholar] [CrossRef]
- Bae, S.A.; Fang, M.Z.; Rustgi, V.; Zarbl, H.; Androulakis, I.P. At the interface of lifestyle, behavior, and circadian rhythms: Metabolic implications. In Frontiers in Nutrition; Frontiers Media S.A.: Lausanne, Switzerland, 2019; Volume 6. [Google Scholar]
- Cespedes Feliciano, E.M.; Rifas-Shiman, S.L.; Quante, M.; Redline, S.; Oken, E.; Taveras, E.M. Chronotype, Social Jet Lag, and Cardiometabolic Risk Factors in Early Adolescence. JAMA Pediatr. 2019, 173, 1049–1057. [Google Scholar] [CrossRef]
- Shimabukuro, M. Leptin resistance and lipolysis of white adipose tissue: An implication to ectopic fat disposition and its consequences. In Journal of Atherosclerosis and Thrombosis; Japan Atherosclerosis Society: Tokyo, Japan, 2017; Volume 24, pp. 1088–1089. [Google Scholar]
- Fang, H.; Judd, R.L. Adiponectin regulation and function. Compr. Physiol. 2018, 8, 1031–1063. [Google Scholar] [CrossRef]
- Wu, H.; Ballantyne, C.M. Skeletal muscle inflammation and insulin resistance in obesity. J. Clin. Investig. 2017, 127, 43–54. [Google Scholar] [CrossRef]
- Chait, A.; den Hartigh, L.J. Adipose Tissue Distribution, Inflammation and Its Metabolic Consequences, Including Diabetes and Cardiovascular Disease. Front. Cardiovasc. Med. 2020, 7, 22. [Google Scholar] [CrossRef]
- Frontera, W.R.; Rodriguez Zayas, A.; Rodriguez, N. Aging of Human Muscle: Understanding Sarcopenia at the Single Muscle Cell Level. Phys. Med. Rehabil. Clin. N. Am. 2012, 23, 201–207. [Google Scholar] [CrossRef]
- Colaianni, G.; Cinti, S.; Colucci, S.; Grano, M. Irisin and musculoskeletal health. In Annals of the New York Academy of Sciences; Blackwell Publishing Inc.: Hoboken, NJ, USA, 2017; Volume 1402, pp. 5–9. [Google Scholar]
- Colaianni, G.; Cuscito, C.; Mongelli, T.; Pignataro, P.; Buccoliero, C.; Liu, P.; Lu, P.; Sartini, L.; Comite, M.D.; Mori, G.; et al. The myokine irisin increases cortical bone mass. Proc. Natl. Acad. Sci. USA 2015, 112, 12157–12162. [Google Scholar] [CrossRef]
- D’Antona, G.; Pellegrino, M.A.; Adami, R.; Rossi, R.; Naccari Carlizzi, C.; Canepari, M.; Saltin, B.; Bottinelli, R. The effect of ageing and immobilization on structure and function of human skeletal muscle fibres. J. Physiol. 2003, 552, 499–511. [Google Scholar] [CrossRef] [PubMed]
- Coskun, G.; Sencar, L.; Tuli, A.; Saker, D.; Alparslan, M.M.; Polat, S.; Silvestrini, A. Effects of osteocalcin on synthesis of testosterone and INSL3 during adult leydig cell differentiation. Int. J. Endocrinol. 2019, 2019, 1041760. [Google Scholar] [CrossRef] [PubMed]
- Zaidi, S.K.; Pande, S.; Pratap, J.; Gaur, T.; Grigoriu, S.; Ali, S.A.; Stein, J.L.; Lian, J.B.; Van Wijnen, A.J.; Stein, G.S. Runx2 deficiency and defective subnuclear targeting bypass senescence to promote immortalization and tumorigenic potential. Proc. Natl. Acad. Sci. USA 2007, 104, 19861–19866. [Google Scholar] [CrossRef] [PubMed]
- Burnstock, G.; Dale, N. Purinergic signalling during development and ageing. In Purinergic Signalling; Kluwer Academic Publishers: Amsterdam, The Netherlands, 2015; Volume 11, pp. 277–305. [Google Scholar]
- Zimmermann, H.; Zebisch, M.; Sträter, N. Cellular function and molecular structure of ecto-nucleotidases. Purinergic Signal. 2012, 8, 437–502. [Google Scholar] [CrossRef]
- Orriss, I.R.; Key, M.L.; Hajjawi, M.O.R.; Arnett, T.R. Extracellular ATP Released by Osteoblasts Is A Key Local Inhibitor of Bone Mineralisation. PLoS ONE 2013, 8, e69057. [Google Scholar] [CrossRef]
- Orriss, I.R. The role of purinergic signalling in the musculoskeletal system. In Autonomic Neuroscience: Basic and Clinical; Elsevier: Amsterdam, The Netherlands, 2015; Volume 191, pp. 124–134. [Google Scholar]
- Buckley, K.A.; Hipskind, R.A.; Gartland, A.; Bowler, W.B.; Gallagher, J.A. Adenosine triphosphate stimulates human osteoclast activity via upregulation of osteoblast-expressed receptor activator of nuclear factor-κB ligand. Bone 2002, 31, 582–590. [Google Scholar] [CrossRef]
- Wesselius, A.; Bours, M.J.L.; Agrawal, A.; Gartland, A.; Dagnelie, P.C.; Schwarz, P.; Jorgensen, N.R. Role of purinergic receptor polymorphisms in human bone. Front. Biosci. 2011, 16, 2572–2585. [Google Scholar] [CrossRef]
- Jørgensen, N.R.; Husted, L.B.; Skarratt, K.K.; Stokes, L.; Tofteng, C.L.; Kvist, T.; Jensen, J.E.B.; Eiken, P.; Brixen, K.; Fuller, S.; et al. Single-nucleotide polymorphisms in the P2X7 receptor gene are associated with post-menopausal bone loss and vertebral fractures. Eur. J. Hum. Genet. 2012, 20, 675–681. [Google Scholar] [CrossRef]
- Coccurello, R.; Volonté, C. P2X7 Receptor in the Management of Energy Homeostasis: Implications for Obesity, Dyslipidemia, and Insulin Resistance. Front. Endocrinol. 2020, 11, 199. [Google Scholar] [CrossRef]
- Costa, M.A.; Barbosa, A.; Neto, E.; Sá-E-Sousa, A.; Freitas, R.; Neves, J.M.; Magalhães-Cardoso, T.; Ferreirinha, F.; Correia-De-Sá, P. On the role of subtype selective adenosine receptor agonists during proliferation and osteogenic differentiation of human primary bone marrow stromal cells. J. Cell Physiol. 2011, 226, 1353–1366. [Google Scholar] [CrossRef]
- Gnad, T.; Scheibler, S.; Kugelgen, I.V.; Scheele, C.; Kilic, A.; Glode, A.; Hoffmann, L.S.; Reverte-Salisa, L.; Horn, P.; Mutlu, S.; et al. Adenosine activates brown adipose tissue and recruits beige adipocytes via A2A receptors. Nature 2014, 516, 395–399. [Google Scholar] [CrossRef]
- Tozzi, M.; Novak, I. Purinergic receptors in adipose tissue as potential targets in metabolic disorders. In Frontiers in Pharmacology; Frontiers Media S.A.: Lausanne, Switzerland, 2017; Volume 8. [Google Scholar]
- Noronha-Matos, J.B.; Coimbra, J.; Sá-e-Sousa, A.; Rocha, R.; Marinhas, J.; Freitas, R.; Guerra-Gomes, S.; Ferreirinha, F.; Costa, M.A.; Correia-de-Sá, P. P2X7-induced zeiosis promotes osteogenic differentiation and mineralization of postmenopausal bone marrow-derived mesenchymal stem cells. FASEB J. 2014, 28, 5208–5222. [Google Scholar] [CrossRef]
- Pandolfi, J.; Ferraro, A.; Lerner, M.; Serrano, J.R.; Dueck, A.; Fainboim, L.; Arruvito, L. Purinergic signaling modulates human visceral adipose inflammatory responses: Implications in metabolically unhealthy obesity. J. Leukoc. Biol. 2015, 97, 941–949. [Google Scholar] [CrossRef]
- Haussler, M.R.; Norman, A.W. Chromosomal receptor for a vitamin D metabolite. Proc. Natl. Acad. Sci. USA 1969, 62, 155–162. [Google Scholar] [CrossRef]
- Kuchuk, N.O.; van Schoor, N.M.; Pluijm, S.M.; Chines, A.; Lips, P. Vitamin D status, parathyroid function, bone turnover, and BMD in postmenopausal women with osteoporosis: Global perspective. J. Bone Miner. Res. Off. J. Am. Soc. Bone Miner. Res. 2009, 24, 693–701. [Google Scholar] [CrossRef]
- McGill, A.T.; Stewart, J.M.; Lithander, F.E.; Strik, C.M.; Poppitt, S.D. Relationships of low serum vitamin D3 with anthropometry and markers of the metabolic syndrome and diabetes in overweight and obesity. Nutr. J. 2008, 7, 1–5. [Google Scholar] [CrossRef]
- Bischoff, H.A.; Borchers, M.; Gudat, F.; Duermueller, U.; Theiler, R.; Stahelin, H.B.; Dick, W. In situ detection of 1,25-dihydroxyvitamin D3 receptor in human skeletal muscle tissue. Histochem. J. 2001, 33, 19–24. [Google Scholar] [CrossRef]
- Lips, P.; Duong, T.U.; Oleksik, A.; Black, D.; Cummings, S.; Cox, D.; Nickelsen, T. A global study of vitamin D status and parathyroid function in postmenopausal women with osteoporosis: Baseline data from the multiple outcomes of raloxifene evaluation clinical trial. J. Clin. Endocrinol. Metab. 2001, 86, 1212–1221. [Google Scholar] [CrossRef]
- Bischoff, H.A.; Stähelin, H.B.; Dick, W.; Akos, R.; Knecht, M.; Salis, C.; Nebiker, M.; Theiler, R.; Pfeifer, M.; Conzelmann, M.; et al. Effects of vitamin D and calcium supplementation on falls: A randomized controlled trial. J. Bone Miner. Res. Off. J. Am. Soc. Bone Miner. Res. 2003, 18, 343–351. [Google Scholar] [CrossRef]
- Zhang, L.; Quan, M.; Cao, Z.B. Effect of vitamin D supplementation on upper and lower limb muscle strength and muscle power in athletes: A meta-analysis. PLoS ONE 2019, 14, e0215826. [Google Scholar] [CrossRef] [PubMed]
- Thanapluetiwong, S.; Chewcharat, A.; Takkavatakarn, K.; Praditpornsilpa, K.; Eiam-Ong, S.; Susantitaphong, P. Vitamin D supplement on prevention of fall and fracture: A Meta-analysis of Randomized Controlled Trials. Medicine 2020, 99, e21506. [Google Scholar] [CrossRef] [PubMed]
- Bouillon, R.; Marcocci, C.; Carmeliet, G.; Bikle, D.; White, J.H.; Dawson-Hughes, B.; Lips, P.; Munns, C.F.; Lazaretti-Castro, M.; Giustina, A.; et al. Skeletal and Extraskeletal Actions of Vitamin D: Current Evidence and Outstanding Questions. Endocr. Rev. 2019, 40, 1109. [Google Scholar] [CrossRef] [PubMed]
- Soares, M.J.; Murhadi, L.L.; Kurpad, A.V.; Chan She Ping-Delfos, W.L.; Piers, L.S. Mechanistic roles for calcium and vitamin D in the regulation of body weight. Obes. Rev. 2012, 13, 592–605. [Google Scholar] [CrossRef]
- Coskun, G.; Sencar, L.; Tuli, A.; Saker, D.; Alparslan, M.M.; Polat, S. Serum 25-hydroxyvitamin D level in relation to weight change and the risk of weight gain in adults of normal weight at baseline: The Norwegian HUNT cohort study. BMJ Open 2020, 10, e039192. [Google Scholar] [CrossRef]
- Letavernier, E.; Daudon, M. Vitamin D, Hypercalciuria and Kidney Stones. Nutrients 2018, 10, 366. [Google Scholar] [CrossRef]
- Locke, A.E.; Kahali, B.; Berndt, S.I.; Justice, A.E.; Pers, T.H.; Day, F.R.; Powell, C.; Vedantam, S.; Buchkovich, M.L.; Yang, J.; et al. Genetic studies of body mass index yield new insights for obesity biology. Nature 2015, 518, 197–206. [Google Scholar] [CrossRef]
- Pigeyre, M.; Yazdi, F.T.; Kaur, Y.; Meyre, D. Recent progress in genetics, epigenetics and metagenomics unveils the pathophysiology of human obesity. In Clinical Science; Portland Press Ltd.: London, UK, 2016; Volume 130, pp. 943–986. [Google Scholar]
- Kirk, B.; Zanker, J.; Duque, G. Osteosarcopenia: Epidemiology, diagnosis, and treatment—Facts and numbers. J. Cachexia Sarcopenia Muscle 2020, 11, 609–618. [Google Scholar] [CrossRef]
- Li, S.; Chen, B.; Chen, H.; Hua, Z.; Shao, Y.; Yin, H.; Wang, J. Analysis of potential genetic biomarkers and molecular mechanism of smoking-related postmenopausal osteoporosis using weighted gene co-expression network analysis and machine learning. PLoS ONE 2021, 16, e0257343. [Google Scholar] [CrossRef]
- Zhu, X.; Bai, W.; Zheng, H. Twelve years of GWAS discoveries for osteoporosis and related traits: Advances, challenges and applications. Bone Res. 2021, 9, 23. [Google Scholar] [CrossRef]
- Urzi, F.; Pokorny, B.; Buzan, E. Pilot Study on Genetic Associations with Age-Related Sarcopenia. Front. Genet. 2020, 11, 615238. [Google Scholar] [CrossRef]
- Jin, Y.; Kim, D.; Choi, Y.J.; Song, I.; Chung, Y.S. Gene Network Analysis for Osteoporosis, Sarcopenia, Diabetes, and Obesity in Human Mesenchymal Stromal Cells. Genes 2022, 13, 459. [Google Scholar] [CrossRef]
- Khan, M.J.; Gerasimidis, K.; Edwards, C.A.; Shaikh, M.G. Role of Gut Microbiota in the Aetiology of Obesity: Proposed Mechanisms and Review of the Literature. J. Obes. 2016, 2016, 7353642. [Google Scholar]
- Bocheva, G.; Slominski, R.M.; Slominski, A.T. The Impact of Vitamin D on Skin Aging. Int. J. Mol. Sci. 2021, 22, 9097. [Google Scholar] [CrossRef]
- Bischoff-Ferrari, H.A.; Borchers, M.; Gudat, F.; Dürmüller, U.; Stähelin, H.B.; Dick, W. Vitamin D Receptor Expression in Human Muscle Tissue Decreases with Age. J. Bone Miner. Res. 2004, 19, 265–269. [Google Scholar] [CrossRef]
- Alalwan, T.A. Phenotypes of sarcopenic obesity: Exploring the effects on peri-muscular fat, the obesity paradox, hormone-related responses and the clinical implications. Geriatrics 2020, 5, 8. [Google Scholar] [CrossRef]
- Noronha-Matos, J.B.; Correia-de-Sá, P. Mesenchymal Stem Cells Ageing: Targeting the "Purinome" to Promote Osteogenic Differentiation and Bone Repair. J. Cell Physiol. 2016, 231, 1852–1861. [Google Scholar] [CrossRef]
- Snijders, T.; Parise, G. Role of muscle stem cells in sarcopenia. Curr. Opin. Clin. Nutr. Metab. Care 2017, 20, 186–190. [Google Scholar] [CrossRef]
- Patel, M.S.; Lee, J.; Baz, M.; Wells, C.E.; Bloch, S.; Lewis, A.; Donaldson, A.V.; Garfield, B.E.; Hopkinson, N.S.; Natanek, A.; et al. Growth differentiation factor-15 is associated with muscle mass in chronic obstructive pulmonary disease and promotes muscle wasting in vivo. J. Cachexia Sarcopenia Muscle 2016, 7, 436–448. [Google Scholar] [CrossRef]
- Dawson-Hughes, B.; Looker, A.C.; Tosteson, A.N.A.; Johansson, H.; Kanis, J.A.; Melton, L.J. The potential impact of new National Osteoporosis Foundation guidance on treatment patterns. Osteoporos. Int. 2010, 21, 41–52. [Google Scholar] [CrossRef]
- Bernfeld, E.; Menon, D.; Vaghela, V.; Zerin, I.; Faruque, P.; Frias, M.A.; Foster, D.A. Phospholipase D-dependent mTOR complex 1 (mTORC1) activation by glutamine. J. Biol. Chem. 2018, 293, 16390–16401. [Google Scholar] [CrossRef]
- Rizzoli, R.; Stevenson, J.C.; Bauer, J.M.; Van Loon, L.J.C.; Walrand, S.; Kanis, J.A.; Cooper, C.; Brandi, M.L.; Diez-Perez, A.; Reginster, J.Y. The role of dietary protein and vitamin D in maintaining musculoskeletal health in postmenopausal women: A consensus statement from the European Society for Clinical and Economic Aspects of Osteoporosis and Osteoarthritis (ESCEO). Maturitas 2014, 79, 122–132. [Google Scholar] [CrossRef] [PubMed]
- Macfarlane, S.; Macfarlane, G.T.; Cummings, J.H. Review article: Prebiotics in the gastrointestinal tract. Aliment. Pharmacol. Ther. 2006, 24, 701–714. [Google Scholar] [CrossRef] [PubMed]
- Gomarasca, M.; Banfi, G.; Lombardi, G. Myokines: The endocrine coupling of skeletal muscle and bone. In Advances in Clinical Chemistry; Academic Press Inc.: Cambridge, MA, USA, 2020; Volume 94, pp. 155–218. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).