Potential Effects of Oral Isotretinoin on Growth Plate and Height
Abstract
1. Introduction
2. Methods
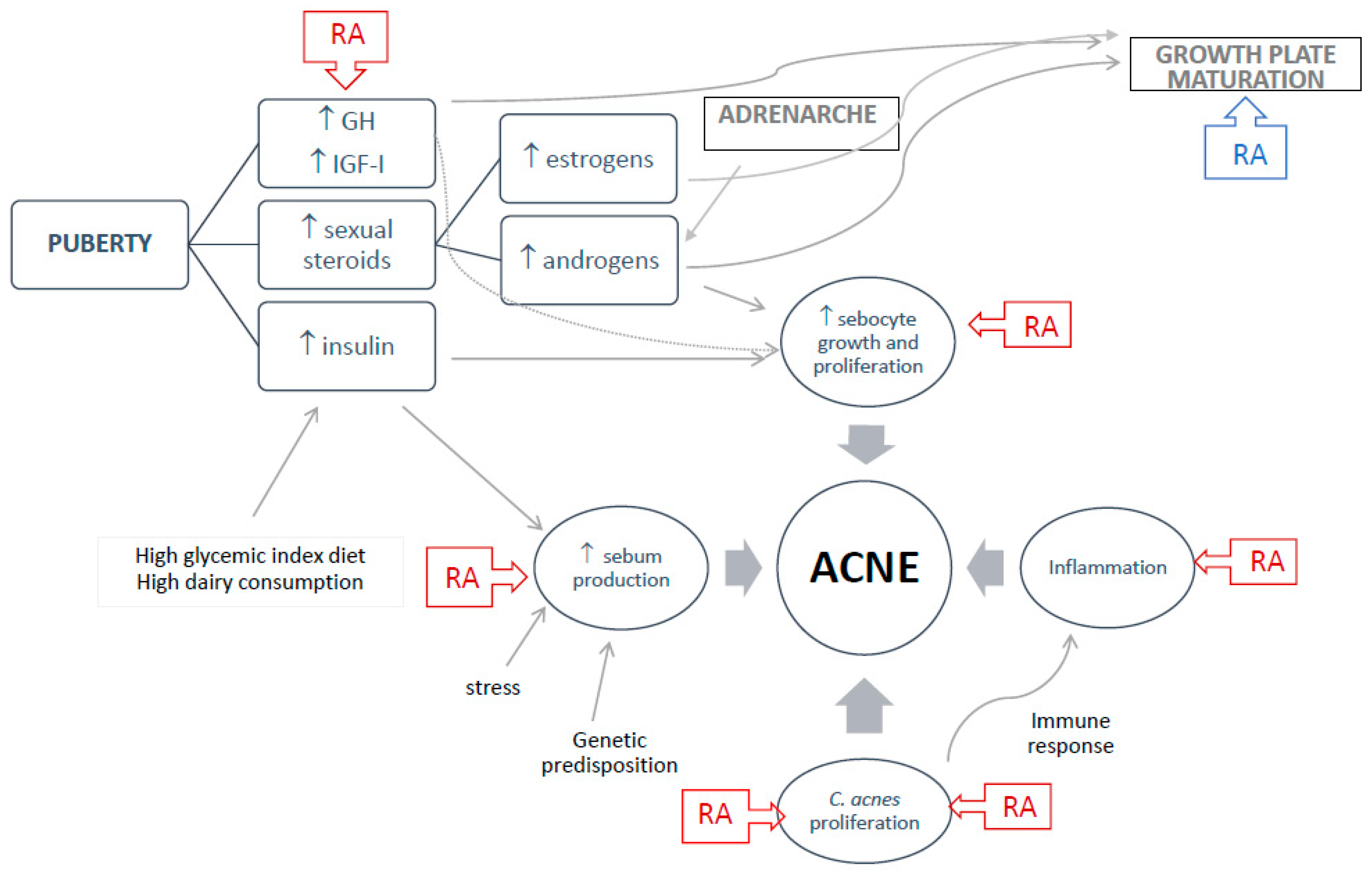
3. Normal Growth, Adrenarche, and Puberty
4. Growth Plate Regulation
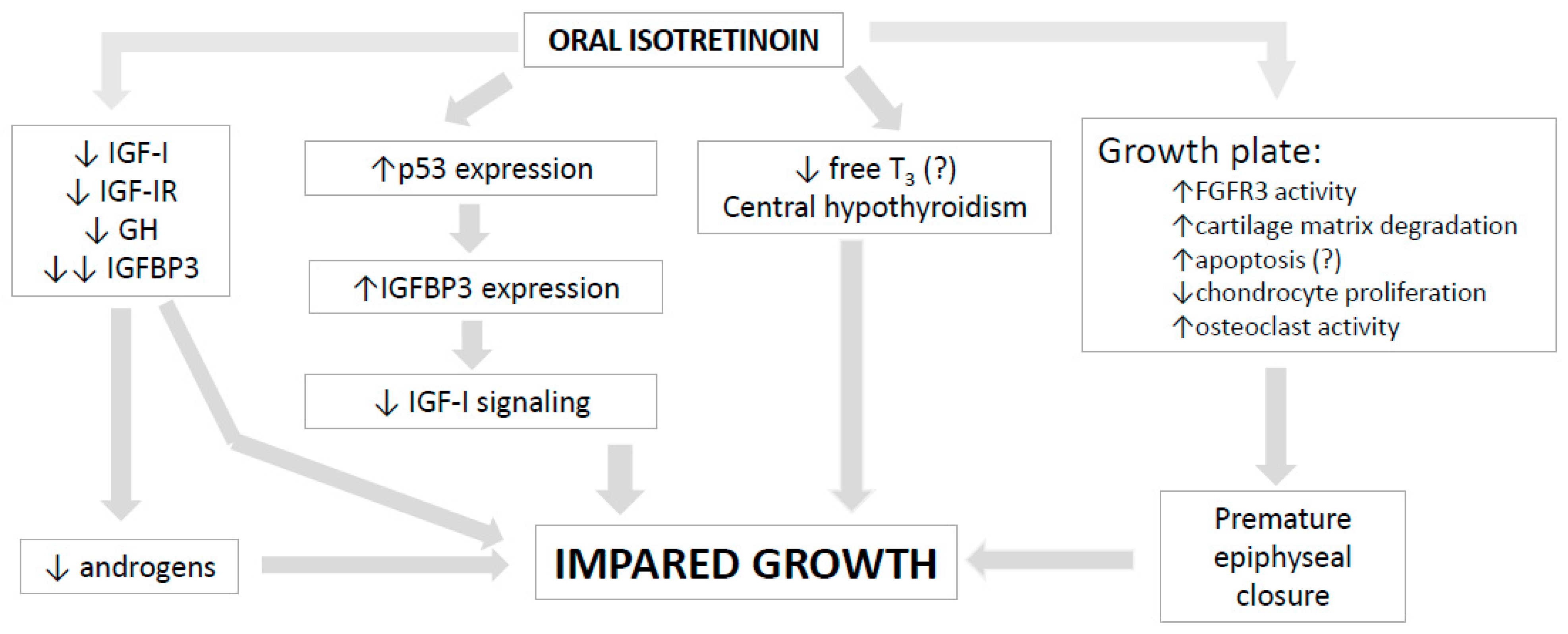
5. Isotretinoin Effects on Bone and Growth Plate
6. Isotretinoin Effects on GH-IGF-I Axis and Pituitary Hormones
7. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Bagatin, E.; Costa, C.S.; Rocha, M.; Picosse, F.R.; Kamamoto, C.S.L.; Pirmez, R.; Ianhez, M.; Miot, H.A. Consensus on the use of oral isotretinoin in dermatology-Brazilian Society of Dermatology. An. Bras. Dermatol. 2020, 95 (Suppl. 1), 19–38. [Google Scholar] [CrossRef] [PubMed]
- Habeshian, K.A.; Cohen, B.A. Current Issues in the Treatment of Acne Vulgaris. Pediatrics 2020, 145 (Suppl. 2), S225–S230. [Google Scholar] [CrossRef] [PubMed]
- Karadag, A.S.; Ertugrul, D.T.; Tutal, E.; Akin, K.O. Isotretinoin influences pituitary hormone levels in acne patients. Acta Derm. Venereol. 2011, 91, 31–34. [Google Scholar] [CrossRef] [PubMed]
- George, R.; Clarke, S.; Thiboutot, D. Hormonal therapy for acne. Semin. Cutan. Med. Surg. 2008, 27, 188–196. [Google Scholar] [CrossRef] [PubMed]
- Lolis, M.S.; Bowe, W.P.; Shalita, A.R. Acne and systemic disease. Med. Clin. N. Am. 2009, 93, 1161–1181. [Google Scholar] [CrossRef]
- Boguszewski, C.L.; Boguszewski, M. Growth Hormone’s Links to Cancer. Endocr. Rev. 2019, 40, 558–574. [Google Scholar] [CrossRef]
- Feily, A.; Namazi, M.R. Decrease of insulin growth factor-1 as a novel mechanism for anti-androgen effect of isotretinoin and its reported association with depression in some cases. J. Drugs Dermatol. 2011, 10, 793–794. [Google Scholar]
- Zouboulis, C.C.; Jourdan, E.; Picardo, M. Acne is an inflammatory disease and alterations of sebum composition initiate acne lesions. J. Eur. Acad. Dermatol. Venereol. 2014, 28, 527–532. [Google Scholar] [CrossRef]
- Kara, Y.A. Evaluation of serum insulin-like growth factor-1, insulin, glucose levels in patients with adolescent and post-adolescent acne. J. Cosmet. Dermatol. 2022, 21, 1292–1296. [Google Scholar] [CrossRef]
- Smith, T.M.; Gilliland, K.; Clawson, G.A.; Thiboutot, D. IGF-1 induces SREBP-1 expression and lipogenesis in SEB-1 sebocytes via activation of the phosphoinositide 3-kinase/Akt pathway. J. Investig. Dermatol. 2008, 128, 1286–1293. [Google Scholar] [CrossRef]
- Ben-Amitai, D.; Laron, Z. Effect of insulin-like growth factor-1 deficiency or administration on the occurrence of acne. J. Eur. Acad. Dermatol. Venereol. 2011, 25, 950–954. [Google Scholar] [CrossRef] [PubMed]
- Klinger, B.; Anin, S.; Silbergeld, A.; Eshet, R.; Laron, Z. Development of hyperandrogenism during treatment with insulin-like growth factor-I (IGF-I) in female patients with Laron syndrome. Clin. Endocrinol. 1998, 48, 81–87. [Google Scholar] [CrossRef]
- Berbis, P. Retinoids: Mechanisms of action. Ann. Dermatol. Venereol. 2010, 137 (Suppl. 3), S97–S103. [Google Scholar] [CrossRef]
- Zaenglein, A.L.; Levy, M.L.; Stefanko, N.S.; Benjamin, L.T.; Bruckner, A.L.; Choate, K.; Craiglow, B.G.; DiGiovanna, J.J.; Eichenfield, L.F.; Elias, P.; et al. Consensus recommendations for the use of retinoids in ichthyosis and other disorders of cornification in children and adolescents. Pediatr. Dermatol. 2021, 38, 164–180. [Google Scholar] [CrossRef] [PubMed]
- Rademaker, M. Isotretinoin: Dose, duration and relapse. What does 30 years of usage tell us? Australas. J. Dermatol. 2013, 54, 157–162. [Google Scholar] [CrossRef] [PubMed]
- Alazawi, S.; Hendriksz, T. Analysis of the effects of isotretinoin on the premature epiphyseal closure in pediatric populations: A literature review. J. Osteopath Med. 2022, 122, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Bagatin, E.; Costa, C.S. The use of isotretinoin for acne-an update on optimal dosing, surveillance, and adverse effects. Expert Rev. Clin. Pharmacol. 2020, 13, 885–897. [Google Scholar] [CrossRef]
- Melnik, B.C. Apoptosis May Explain the Pharmacological Mode of Action and Adverse Effects of Isotretinoin, Including Teratogenicity. Acta Derm. Venereol. 2017, 97, 173–181. [Google Scholar] [CrossRef]
- Karadag, A.S.; Ertugrul, D.T.; Tutal, E.; Akin, K.O. Short-term isotretinoin treatment decreases insulin-like growth factor-1 and insulin-like growth factor binding protein-3 levels: Does isotretinoin affect growth hormone physiology? Br. J. Dermatol. 2010, 162, 798–802. [Google Scholar] [CrossRef]
- Pease, C.N. Focal retardation and arrestment of growth of bones due to vitamin A intoxication. JAMA 1962, 182, 980–985. [Google Scholar] [CrossRef]
- Kodaka, T.; Takaki, H.; Soeta, S.; Mori, R.; Naito, Y. Local disappearance of epiphyseal growth plates in rats with hypervitaminosis A. J. Vet. Med. Sci. 1998, 60, 815–821. [Google Scholar] [CrossRef] [PubMed]
- Matsuoka, M.; Onodera, T.; Majima, T.; Iwasaki, K.; Takahashi, D.; Kondo, E.; Iwasaki, N. Correction osteotomy for bilateral varus knee deformity caused by premature epiphyseal closure induced by hypervitaminosis A: A case report. BMC Musculoskelet. Disord. 2019, 20, 287. [Google Scholar] [CrossRef]
- Carroll Woodard, J.; Donovan, A.G.; Eckhoff, C. Vitamin (A and D)-induced premature physeal closure (hyena disease) in calves. J. Comp. Pathol. 1997, 116, 353–366. [Google Scholar] [CrossRef] [PubMed]
- Woodard, J.C.; Donovan, G.A.; Fisher, L.W. Pathogenesis of vitamin (A and D)-induced premature growth-plate closure in calves. Bone 1997, 21, 171–182. [Google Scholar] [CrossRef] [PubMed]
- Karlberg, J. On the modelling of human growth. Stat. Med. 1987, 6, 185–192. [Google Scholar] [CrossRef] [PubMed]
- Aguiar-Oliveira, M.H.; Souza, A.H.O.; Oliveira, C.R.P.; Campos, V.C.; Oliveira-Neto, L.A.; Salvatori, R. Mechanisms in Endocrinology: The multiple facets of GHRH/GH/IGF-I axis: Lessons from lifetime, untreated, isolated GH deficiency due to a GHRH receptor gene mutation. Eur. J. Endocrinol. 2017, 177, R85–R97. [Google Scholar] [CrossRef]
- Laron, Z. Lessons from 50 Years of Study of Laron Syndrome. Endocr. Pract. 2015, 21, 1395–1402. [Google Scholar] [CrossRef]
- Bernardini, S.; Spadoni, G.L.; Povoa, G.; Boscherini, B.; Hall, K. Plasma levels of insulin-like growth factor binding protein-1, and growth hormone binding protein activity from birth to the third month of life. Acta Endocrinol. 1992, 127, 313–318. [Google Scholar] [CrossRef]
- Bozzola, M.; Tettoni, K.; Locatelli, F.; Radetti, G.; Belloni, C.; Autelli, M.; Zecca, M.; Valentini, R.; Severi, F.; Tato, L. Postnatal variations of growth hormone bioactivity and of growth hormone-dependent factors. Arch. Pediatr. Adolesc. Med. 1996, 150, 1068–1071. [Google Scholar] [CrossRef]
- Low, L.C.; Tam, S.Y.; Kwan, E.Y.; Tsang, A.M.; Karlberg, J. Onset of significant GH dependence of serum IGF-I and IGF-binding protein 3 concentrations in early life. Pediatr. Res. 2001, 50, 737–742. [Google Scholar] [CrossRef]
- Ong, K.; Kratzsch, J.; Kiess, W.; Dunger, D.; Team, A.S. Circulating IGF-I levels in childhood are related to both current body composition and early postnatal growth rate. J. Clin. Endocrinol. Metab. 2002, 87, 1041–1044. [Google Scholar] [CrossRef] [PubMed]
- Albertsson-Wikland, K.; Rosberg, S.; Karlberg, J.; Groth, T. Analysis of 24-hour growth hormone profiles in healthy boys and girls of normal stature: Relation to puberty. J. Clin. Endocrinol. Metab. 1994, 78, 1195–1201. [Google Scholar] [CrossRef] [PubMed]
- Coutant, R.; de Casson, F.B.; Rouleau, S.; Douay, O.; Mathieu, E.; Gatelais, F.; Bouhours-Nouet, N.; Voinot, C.; Audran, M.; Limal, J.M. Divergent effect of endogenous and exogenous sex steroids on the insulin-like growth factor I response to growth hormone in short normal adolescents. J. Clin. Endocrinol. Metab. 2004, 89, 6185–6192. [Google Scholar] [CrossRef] [PubMed]
- Juul, A.; Bang, P.; Hertel, N.T.; Main, K.; Dalgaard, P.; Jorgensen, K.; Muller, J.; Hall, K.; Skakkebaek, N.E. Serum insulin-like growth factor-I in 1030 healthy children, adolescents, and adults: Relation to age, sex, stage of puberty, testicular size, and body mass index. J. Clin. Endocrinol. Metab. 1994, 78, 744–752. [Google Scholar] [CrossRef]
- Lofqvist, C.; Andersson, E.; Gelander, L.; Rosberg, S.; Blum, W.F.; Albertsson Wikland, K. Reference values for IGF-I throughout childhood and adolescence: A model that accounts simultaneously for the effect of gender, age, and puberty. J. Clin. Endocrinol. Metab. 2001, 86, 5870–5876. [Google Scholar] [CrossRef]
- Wood, C.L.; Lane, L.C.; Cheetham, T. Puberty: Normal physiology (brief overview). Best. Pract. Res. Clin. Endocrinol. Metab. 2019, 33, 101265. [Google Scholar] [CrossRef]
- Nilsson, O.; Marino, R.; De Luca, F.; Phillip, M.; Baron, J. Endocrine regulation of the growth plate. Horm. Res. 2005, 64, 157–165. [Google Scholar] [CrossRef]
- Biro, F.M.; Pinney, S.M.; Huang, B.; Baker, E.R.; Walt Chandler, D.; Dorn, L.D. Hormone changes in peripubertal girls. J. Clin. Endocrinol. Metab. 2014, 99, 3829–3835. [Google Scholar] [CrossRef]
- Rosenfield, R.L. Normal and Premature Adrenarche. Endocr. Rev 2021, 42, 783–814. [Google Scholar] [CrossRef]
- D’Andrea, C.R.; Alfraihat, A.; Singh, A.; Anari, J.B.; Cahill, P.J.; Schaer, T.; Snyder, B.D.; Elliott, D.; Balasubramanian, S. Part 1. Review and meta-analysis of studies on modulation of longitudinal bone growth and growth plate activity: A macro-scale perspective. J. Orthop. Res. 2021, 39, 907–918. [Google Scholar] [CrossRef]
- Agirdil, Y. The growth plate: A physiologic overview. EFORT Open Rev. 2020, 5, 498–507. [Google Scholar] [CrossRef]
- Shimo, T.; Koyama, E.; Okui, T.; Masui, M.; Kunisada, Y.; Ibaragi, S.; Yoshioka, N.; Kurio, N.; Yoshida, S.; Sasaki, A.; et al. Retinoic Receptor Signaling Regulates Hypertrophic Chondrocyte-specific Gene Expression. Vivo 2019, 33, 85–91. [Google Scholar] [CrossRef] [PubMed]
- De Luca, F.; Uyeda, J.A.; Mericq, V.; Mancilla, E.E.; Yanovski, J.A.; Barnes, K.M.; Zile, M.H.; Baron, J. Retinoic acid is a potent regulator of growth plate chondrogenesis. Endocrinology 2000, 141, 346–353. [Google Scholar] [CrossRef] [PubMed]
- Rausch, S.; Barholz, M.; Foller, M.; Feger, M. Vitamin A regulates fibroblast growth factor 23 (FGF23). Nutrition 2020, 79–80, 110988. [Google Scholar] [CrossRef]
- Nilsson, O.; Isoherranen, N.; Guo, M.H.; Lui, J.C.; Jee, Y.H.; Guttmann-Bauman, I.; Acerini, C.; Lee, W.; Allikmets, R.; Yanovski, J.A.; et al. Accelerated Skeletal Maturation in Disorders of Retinoic Acid Metabolism: A Case Report and Focused Review of the Literature. Horm. Metab. Res. 2016, 48, 737–744. [Google Scholar] [CrossRef]
- DiGiovanna, J.J. Isotretinoin effects on bone. J. Am. Acad. Dermatol. 2001, 45, S176–S182. [Google Scholar] [CrossRef] [PubMed]
- DiGiovanna, J.J.; Langman, C.B.; Tschen, E.H.; Jones, T.; Menter, A.; Lowe, N.J.; Eichenfield, L.; Hebert, A.A.; Pariser, D.; Savin, R.P.; et al. Effect of a single course of isotretinoin therapy on bone mineral density in adolescent patients with severe, recalcitrant, nodular acne. J. Am. Acad. Dermatol. 2004, 51, 709–717. [Google Scholar] [CrossRef]
- Duvalyan, A.; Cha, A.; Goodarzian, F.; Arkader, A.; Villablanca, J.G.; Marachelian, A. Premature epiphyseal growth plate arrest after isotretinoin therapy for high-risk neuroblastoma: A case series and review of the literature. Pediatr. Blood Cancer 2020, 67, e28236. [Google Scholar] [CrossRef]
- Milstone, L.M.; McGuire, J.; Ablow, R.C. Premature epiphyseal closure in a child receiving oral 13-cis-retinoic acid. J. Am. Acad. Dermatol. 1982, 7, 663–666. [Google Scholar] [CrossRef]
- Prendiville, J.; Bingham, E.A.; Burrows, D. Premature epiphyseal closure–a complication of etretinate therapy in children. J. Am. Acad. Dermatol. 1986, 15, 1259–1262. [Google Scholar] [CrossRef]
- Marini, J.C.; Hill, S.; Zasloff, M.A. Dense metaphyseal bands and growth arrest associated with isotretinoin therapy. Am. J. Dis. Child. 1988, 142, 316–318. [Google Scholar] [CrossRef]
- Standeven, A.M.; Davies, P.J.; Chandraratna, R.A.; Mader, D.R.; Johnson, A.T.; Thomazy, V.A. Retinoid-induced epiphyseal plate closure in guinea pigs. Fundam. Appl. Toxicol. 1996, 34, 91–98. [Google Scholar] [CrossRef]
- Steele, R.G.; Lugg, P.; Richardson, M. Premature epiphyseal closure secondary to single-course vitamin A therapy. Aust. N. Z. J. Surg. 1999, 69, 825–827. [Google Scholar] [CrossRef] [PubMed]
- Hobbie, W.L.; Mostoufi, S.M.; Carlson, C.A.; Gruccio, D.; Ginsberg, J.P. Prevalence of advanced bone age in a cohort of patients who received cis-retinoic acid for high-risk neuroblastoma. Pediatr. Blood Cancer 2011, 56, 474–476. [Google Scholar] [CrossRef]
- Luthi, F.; Eggel, Y.; Theumann, N. Premature epiphyseal closure in an adolescent treated by retinoids for acne: An unusual cause of anterior knee pain. Joint. Bone Spine 2012, 79, 314–316. [Google Scholar] [CrossRef]
- Zhao, S.; Goodson, N.J. Diffuse idiopathic skeletal hyperostosis and isotretinoin in cystic acne. BMJ Case Rep. 2015, 2015, bcr2015209775. [Google Scholar] [CrossRef] [PubMed]
- Noyes, J.J.; Levine, M.A.; Belasco, J.B.; Mostoufi-Moab, S. Premature Epiphyseal Closure of the Lower Extremities Contributing to Short Stature after cis-Retinoic Acid Therapy in Medulloblastoma: A Case Report. Horm. Res. Paediatr. 2016, 85, 69–73. [Google Scholar] [CrossRef]
- Steineck, A.; MacKenzie, J.D.; Twist, C.J. Premature physeal closure following 13-cis-retinoic acid and prolonged fenretinide administration in neuroblastoma. Pediatr. Blood Cancer 2016, 63, 2050–2053. [Google Scholar] [CrossRef] [PubMed]
- Park, W.K.; Choi, H.S.; Chung, C.Y.; Park, M.S.; Sung, K.H. Genu varum deformity due to premature epiphyseal closure after treatment with isotretinoin for neuroblastoma: A case report. J. Orthop. Surg. 2020, 28, 2309499020924483. [Google Scholar] [CrossRef]
- Koh, K.N.; Jeon, J.Y.; Park, S.S.; Im, H.J.; Kim, H.; Kang, M.S. Physeal Abnormalities in Children With High-risk Neuroblastoma Intensively Treated With/Without 13-Cis-Retinoic Acid. J. Pediatr. Orthop. 2021, 41, e841–e848. [Google Scholar] [CrossRef]
- Kvist, O.; Luiza Dallora, A.; Nilsson, O.; Anderberg, P.; Sanmartin Berglund, J.; Flodmark, C.E.; Diaz, S. A cross-sectional magnetic resonance imaging study of factors influencing growth plate closure in adolescents and young adults. Acta Paediatr. 2021, 110, 1249–1256. [Google Scholar] [CrossRef]
- Delgado, J.; Jaramillo, D.; Chauvin, N.A.; Guo, M.; Stratton, M.S.; Sweeney, H.E.; Barrera, C.A.; Mostoufi-Moab, S. Evaluating growth failure with diffusion tensor imaging in pediatric survivors of high-risk neuroblastoma treated with high-dose cis-retinoic acid. Pediatr. Radiol. 2019, 49, 1056–1065. [Google Scholar] [CrossRef] [PubMed]
- Horton, W.E.; Yamada, Y.; Hassell, J.R. Retinoic acid rapidly reduces cartilage matrix synthesis by altering gene transcription in chondrocytes. Dev. Biol. 1987, 123, 508–516. [Google Scholar] [CrossRef] [PubMed]
- Melnik, B.C. p53: Key conductor of all anti-acne therapies. J. Transl. Med. 2017, 15, 195. [Google Scholar] [CrossRef] [PubMed]
- Shen, Q.; Wang, X.; Bai, H.; Tan, X.; Liu, X. Effects of high-dose all-trans retinoic acid on longitudinal bone growth of young rats. Growth Horm. IGF Res. 2022, 62, 101446. [Google Scholar] [CrossRef]
- Karadag, A.S.; Takci, Z.; Ertugrul, D.T.; Bilgili, S.G.; Balahoroglu, R.; Takir, M. The effect of different doses of isotretinoin on pituitary hormones. Dermatology 2015, 230, 354–359. [Google Scholar] [CrossRef]
- Rodighiero, E.; Bertolani, M.; Saleri, R.; Pedrazzi, G.; Lotti, T.; Feliciani, C.; Satolli, F. Do acne treatments affect insulin-like growth factor-1 serum levels? A clinical and laboratory study on patients with acne vulgaris. Dermatol. Ther. 2020, 33, e13439. [Google Scholar] [CrossRef] [PubMed]
- Kaymak, Y.; Adisen, E.; Ilter, N.; Bideci, A.; Gurler, D.; Celik, B. Dietary glycemic index and glucose, insulin, insulin-like growth factor-I, insulin-like growth factor binding protein 3, and leptin levels in patients with acne. J. Am. Acad. Dermatol. 2007, 57, 819–823. [Google Scholar] [CrossRef]
- Smith, T.M.; Cong, Z.; Gilliland, K.L.; Clawson, G.A.; Thiboutot, D.M. Insulin-like growth factor-1 induces lipid production in human SEB-1 sebocytes via sterol response element-binding protein-1. J. Investig. Dermatol. 2006, 126, 1226–1232. [Google Scholar] [CrossRef] [PubMed]
- Rahaman, S.M.A.; De, D.; Handa, S.; Pal, A.; Sachdeva, N.; Ghosh, T.; Kamboj, P. Association of insulin-like growth factor (IGF)-1 gene polymorphisms with plasma levels of IGF-1 and acne severity. J. Am. Acad. Dermatol. 2016, 75, 768–773. [Google Scholar] [CrossRef]
- Sherman, S.I. Etiology, diagnosis, and treatment recommendations for central hypothyroidism associated with bexarotene therapy for cutaneous T-cell lymphoma. Clin. Lymphoma 2003, 3, 249–252. [Google Scholar] [CrossRef] [PubMed]
- Masood, M.Q.; Hakeem, H. Isotretinoin associated reversible hypothyroidism. Thyroid 2011, 21, 1039–1040. [Google Scholar] [CrossRef] [PubMed]
| Authors | Study Type | Reason for Retinoid Use | Dose and Duration of Therapy | Main Findings |
|---|---|---|---|---|
| Milstone et al., 1982 [49] | Case report 10-year-old boy | Epidermolytic hyperkeratosis | Oral isotretinoin: 0.5–4.5 mg/kg/day, cycles of 6 months followed by 2- to 4-week periods off drug; total of 4 years | Right knee pain; radiographic evidence of partial closure of the proximal epiphysis of the right tibia |
| Prendiville et al., 1986 [50] | Case reports: Case 1: 8.5-year-old boy Case 2: 11-year-old girl | Case 1: nonbullous ichthyosiform erythroderma Case 2: systematized verrucous nevi | Case 1: etretinate (0.5–2.5 mg/kg/day for 6.3 years) Case 2: etretinate (1 mg/kg/day for 5.4 years) | Case 1: premature growth plate closure of the right distal tibial epiphysis; shortness of stature, thinning of long bones, and traumatic fractures Case 2: bilateral fusion of both elbow epiphyses and precocious narrowing of the upper and lower femoral epiphyses |
| Marini et al., 1988 [51] | Case report: 13-year-old boy | Fibrodysplasia ossificans progressiva | Oral isotretinoin: 4–5 mg/kg/day, for 5 months | Striking growth arrest lines on long bones, metaphysis of upper and lower extremities. 5–9 months after discontinuance: gradual decrease of metaphyseal bands and resumption of clinical growth |
| Standeven et al., 1996 [52] | Animal study: guinea pigs | Experimental study | Intraperitoneal isotretinoin: 21 mg/kg/day for 7 days via osmotic pump | Irreversible histological features of epiphyseal closure |
| Woodard et al., 1997 [24] | Animal study: calves (6 treated and 6 controls) | Experimental study | Intramuscular vitamin A (2,000,000 IU) + vitamin D (300,000 IU) on the first day after birth, and oral vitamin A (10,000 IU/kg/day per 8 weeks), and after oral vitamin A (30,000 IU/kg/day per 8 weeks) | Premature closure of growth plate (proximal and distal tibia, radius, hind, and fore limbs) by microscopical examination in treated animals. After 1 week, bone growth of the proximal tibia of a control animal was 136 μg/day; in the treated animal it was 25 μg/day |
| Kodaka et al., 1998 [21] | Animal study: 5 rats treated and 5 controls | Experimental study | Oral vitamin A: 50,000, 100,000, and 150,000 IU/100 g/day for 5 days from 4 weeks after birth | Premature growth plate disappearance; eosinophilic cartilage bands in higher dose groups |
| Steele et al., 1999 [53] | Case report: 14-year-old boy | Cystic acne | Oral isotretinoin: 75 mg/kg/day for 6 months | Bilateral knee pain, genu valgum; closure of lateral femoral physis |
| Hobbie et al., 2011 [54] | Retrospective review: 32 children (13 girls), 7.4–16.4-year-old | Neuroblastoma | Oral isotretinoin: Group 1 (24 patients): 6 cycles 160 mg/m2/day (2 weeks on, 2 weeks off) Group 2 (8 patients): did not receive isotretinoin | Group 1: advanced bone age in 7 children, 9.5 years (6–10.5) from diagnosis-younger median age at neuroblastoma diagnosis |
| Luthi et al., 2012 [55] | Case report: 16-year-old boy | Acne refractory to topical treatments | Oral isotretinoin: 0.5 mg/kg/day for 7 months | Bilateral knee pain. Knee RM: acute epiphysiodesis lesions with irregular epiphyseal cartilage, and marked metaphyseal-epiphyseal oedema |
| Zhao et al., 2015 [56] | Case report: 35-year-old man | Cystic acne | Oral isotretinoin: 4 cycles of 500 mg/kg/day for 6 months and a final long-term course gradually down-titrated to 20 mg/day from 15-year-old | Thoracic back pain, diffuse idiopathic skeletal hyperostosis |
| Noyes et al., 2016 [57] | Case report: 9-year-old girl | Medulloblastoma | Oral isotretinoin: 11 cycles of 180 mg/m2/day (14-day cycles) for 13 months | Bilateral premature closure of distal femur and proximal tibia growth plates; normal bone age in the hand and wrist |
| Steineck et al., 2016 [58] | Case series: Case A: 6-year-old girl (at diagnosis) Case B: 5-year-old boy (at diagnosis) | Neuroblastoma | Oral fenretide: Case A and B: 2475 mg/m2/day, delivered as 800 mg orally three times daily for 7 days, repeated every 21 days, 70 courses over 5 years (cumulative dose 1212,750 mg/m2) | Premature epiphyseal closure Case A: left knee pain; arm and leg length discrepancy, short adult stature (21-year-old): 154 cm (8th percentile), mid-parental height 172.7 cm (90th percentile). Case B: genu varum; short adult stature (20-year-old): 165 cm (5th percentile), mid-parental height unavailable. |
| Matsuoka et al., 2019 [22] | Case report: 10-year-old girl | Neuroblastoma | Oral isotretinoin: initial dose of 20 mg/day and maintenance dose of 40 mg/day for a total period of 9.8 years from 1 year- old. | Knee pain, bilateral varus knee deformity due to premature epiphyseal closure; polar irregularity of chondrocytes and decreased cartilage matrix without apoptosis by histopathological examination of the growth plate |
| Duvalyan et al., 2019 [48] | Case series: Case A: 9-year-old girl Case B: 11-year-old boy Case C: 10-year-old boy | Neuroblastoma | Oral isotretinoin Case A: 160 mg/m2/day, 2 weeks on, 2 weeks off (cumulative dose: 19,200 mg/m2) Case B and C: 160 mg/m2/day, 2 weeks on, 2 weeks off (cumulative dose: 13,440 mg/m2) | Bilateral knees plate closure Case A: right leg deformity and length discrepancy. Height at the 2.5 percentile (17-year-old) Case B: progressive genu valgum. Height at the 0.06 percentile (14-year-old) Case C: leg length discrepancy. Height at the 4.3 percentile (13-year-old) |
| Park et al., 2020 [59] | Case report: 10-year-old boy | Neuroblastoma | Oral isotretinoin (72.3 mg/m2/day) for 1 year | Genu varum; premature epiphyseal closure |
| Koh et al., 2021 [60] | Case-control study: 15 patients (8 girls): 4.9±1.7 years 12 controls (7 girls): 4.9 ± 1.9 years | Neuroblastoma | Oral isotretinoin (13 patients): 6 cycles of 160 mg/m2/day, 2 weeks on, 2 weeks off | 6/13: physeal abnormalities; asymmetric genu valgum deformity-higher risk of deformity if a child was above 5 years. No significant growth differences in height during follow-up |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cardoso-Demartini, A.A.; Boguszewski, C.L.; Boguszewski, M.C.S. Potential Effects of Oral Isotretinoin on Growth Plate and Height. Endocrines 2023, 4, 281-292. https://doi.org/10.3390/endocrines4020023
Cardoso-Demartini AA, Boguszewski CL, Boguszewski MCS. Potential Effects of Oral Isotretinoin on Growth Plate and Height. Endocrines. 2023; 4(2):281-292. https://doi.org/10.3390/endocrines4020023
Chicago/Turabian StyleCardoso-Demartini, Adriane A., Cesar Luiz Boguszewski, and Margaret C. S. Boguszewski. 2023. "Potential Effects of Oral Isotretinoin on Growth Plate and Height" Endocrines 4, no. 2: 281-292. https://doi.org/10.3390/endocrines4020023
APA StyleCardoso-Demartini, A. A., Boguszewski, C. L., & Boguszewski, M. C. S. (2023). Potential Effects of Oral Isotretinoin on Growth Plate and Height. Endocrines, 4(2), 281-292. https://doi.org/10.3390/endocrines4020023






