The Progression of Prediabetes to Type 2 Diabetes in Children and Adolescents in the United States: Current Challenges and Solutions
Abstract
:1. Introduction
2. Lack of Consensus on the Approaches to the Management of Prediabetes in Children and Adolescents
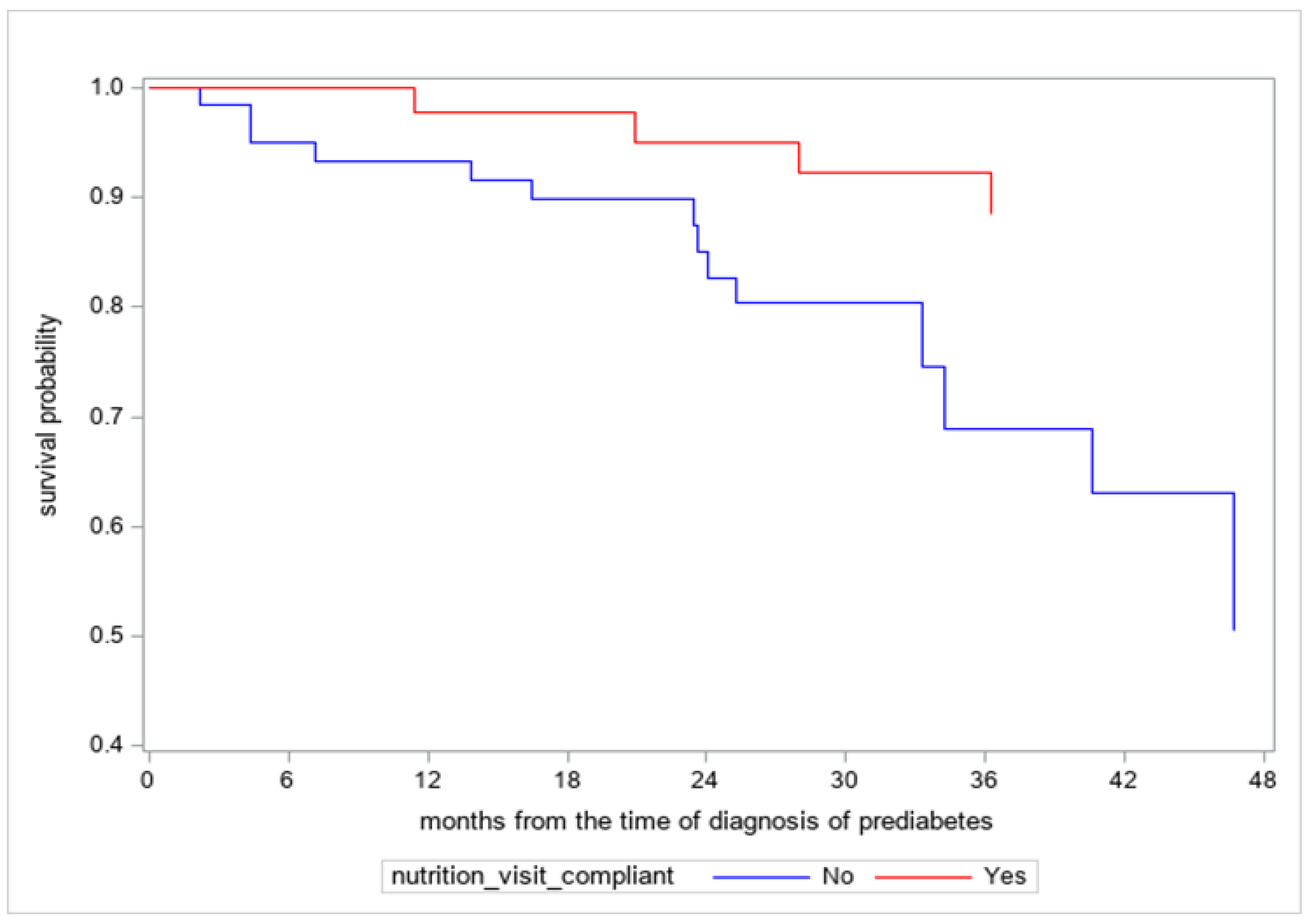
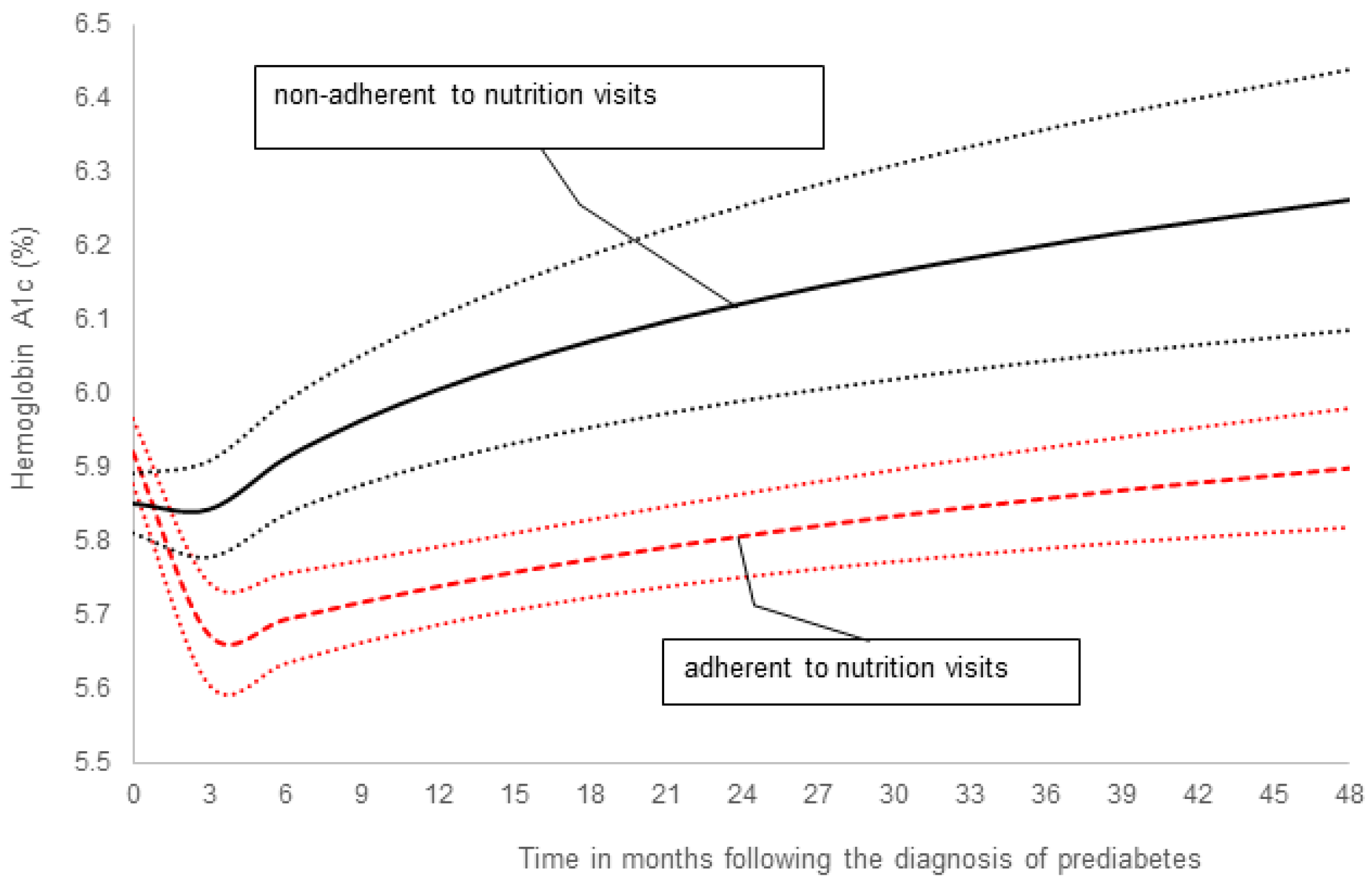
3. New Data on the Efficacy of Medical Nutrition Therapy in Children and Adolescents with Prediabetes
4. Discussion
5. Conclusions and Future Directions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wallace, A.S.; Wang, D.; Shin, J.-I.; Selvin, E. Screening and Diagnosis of Prediabetes and Diabetes in US Children and Adolescents. Pediatrics 2020, 146, e20200265. [Google Scholar] [CrossRef]
- Galderisi, A.; Giannini, C.; Weiss, R.; Kim, G.; Shabanova, V.; Santoro, N.; Pierpont, B.; Savoye, M.; Caprio, S. Trajectories of changes in glucose tolerance in a multiethnic cohort of obese youths: An observational prospective analysis. Lancet Child Adolesc. Health 2018, 2, 726–735. [Google Scholar] [CrossRef]
- Andes, L.J.; Cheng, Y.J.; Rolka, D.B.; Gregg, E.W.; Imperatore, G. Prevalence of Prediabetes Among Adolescents and Young Adults in the United States, 2005–2016. JAMA Pediatr. 2020, 174, e194498. [Google Scholar] [CrossRef]
- Cunningham, S.A.; Hardy, S.T.; Jones, R.; Ng, C.; Kramer, M.R.; Narayan, K.V. Changes in the Incidence of Childhood Obesity. Pediatrics 2022, 150, e2021053708. [Google Scholar] [CrossRef]
- Liu, J.; Li, Y.; Zhang, D.; Yi, S.S.; Liu, J. Trends in Prediabetes Among Youths in the US From 1999 Through 2018. JAMA Pediatr. 2022, 176, 608. [Google Scholar] [CrossRef]
- Tuso, P. Prediabetes and Lifestyle Modification: Time to Prevent a Preventable Disease. Perm. J. 2014, 18, 88–93. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention. Report, National Diabetes Statistics Report Website, Centers for Disease Control and Prevention; Centers for Disease Control and Prevention: Atlanta, GA, USA, 2022. [Google Scholar]
- American Diabetes Association. Children and Adolescents: Standards of Medical Care in Diabetes. Diabetes Care 2020, 43, S163–S182. [Google Scholar] [CrossRef] [PubMed]
- DiMeglio, L.A.; Evans-Molina, C.; Oram, R.A. Type 1 diabetes. Lancet 2018, 391, 2449–2462. [Google Scholar] [CrossRef]
- Broome, D.T.; Pantalone, K.M.; Kashyap, S.R.; Philipson, L.H. Approach to the Patient with MODY-Monogenic Diabetes. J. Clin. Endocrinol. Metab. 2021, 106, 237–250. [Google Scholar] [CrossRef]
- Rubio-Cabezas, O.; Hattersley, A.T.; Njølstad, P.R.; Mlynarski, W.; Ellard, S.; White, N.; Chi, D.V.; Craig, M.E. ISPAD Clinical Practice Consensus Guidelines The diagnosis and management of monogenic diabetes in children and adolescents. Pediatr. Diabetes 2014, 15 (Suppl. S20), 47–64. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, S.; Khunti, K.; Davies, M.J. Type 2 diabetes. Lancet 2017, 389, 2239–2251. [Google Scholar] [CrossRef]
- RISE Consortium Investigators. Effects of Treatment of Impaired Glucose Tolerance or Recently Diagnosed Type 2 Diabetes With Metformin Alone or in Combination With Insulin Glargine on beta-Cell Function: Comparison of Responses In Youth And Adults. Diabetes 2019, 68, 1670–1680. [Google Scholar]
- Yahyavi, S.K.; Snorgaard, O.; Knop, F.K.; Schou, M.; Lee, C.; Selmer, C.; Gislason, G.; Torp-Pedersen, C.; Jensen, M.B.; Bonde, A.N. Prediabetes Defined by First Measured HbA1c Predicts Higher Cardiovascular Risk Compared With HbA1c in the Diabetes Range: A Cohort Study of Nationwide Registries. Diabetes Care 2021, 44, 2767–2774. [Google Scholar] [CrossRef]
- Thota, G. Prediabetes is a risk factor for myocardial infarction-a national inpatient sample study. In Proceedings of the Annual Endocrine Society Meeting, Atlanta, GA, USA, 12 June 2022. [Google Scholar]
- Magge, S.; Silverstein, J.; Elder, D.; Nadeau, K.; Hannon, T.S. Evaluation and Treatment of Prediabetes in Youth. J. Pediatr. 2020, 219, 11–22. [Google Scholar] [CrossRef]
- Tabák, A.G.; Herder, C.; Rathmann, W.; Brunner, E.J.; Kivimäki, M. Prediabetes: A high-risk state for diabetes development. Lancet 2012, 379, 2279–2290. [Google Scholar] [CrossRef]
- RISE Consortium Investigators. Impact of Insulin and Metformin Versus Metformin Alone on beta-Cell Function in Youth With Impaired Glucose Tolerance or Recently Diagnosed Type 2 Diabetes. Diabetes Care 2018, 41, 1717–1725. [Google Scholar] [CrossRef]
- Coughlan, K.A.; Valentine, R.J.; Ruderman, N.B.; Saha, A.K. AMPK activation: A therapeutic target for type 2 diabetes? Diabetes Metab. Syndr. Obes. Targets Ther. 2014, 7, 241–253. [Google Scholar]
- McDuffie, J.R.; Calis, K.; Uwaifo, G.; Sebring, N.; Fallon, E.; Frazer, T.; Hubbard, V.; Yanovski, J. Efficacy of Orlistat as an Adjunct to Behavioral Treatment in Overweight African American and Caucasian Adolescents with Obesity-related Co-morbid Conditions. J. Pediatr. Endocrinol. Metab. 2004, 17, 307–320. [Google Scholar] [CrossRef]
- Gomez-Diaz, R.; Talavera, J.O.; Pool, E.C.; Ortiz-Navarrete, V.; Solórzano-Santos, F.; Mondragon, R.; Valladares-Salgado, A.; Cruz, M.; Aguilar-Salinas, C.A.; Wacher, N.H. Metformin decreases plasma resistin concentrations in pediatric patients with impaired glucose tolerance: A placebo-controlled randomized clinical trial. Metabolism 2012, 61, 1247–1255. [Google Scholar] [CrossRef]
- Arslanian, S.A.; Lewy, V.; Danadian, K.; Saad, R. Metformin therapy in obese adolescents with polycystic ovary syndrome and impaired glucose tolerance: Amelioration of exaggerated adrenal response to adrenocorticotropin with reduction of insulinemia/insulin resistance. J. Clin. Endocrinol. Metab. 2002, 87, 1555–1559. [Google Scholar] [CrossRef]
- Kendall, D.; Vail, A.; Amin, R.; Barrett, T.; Dimitri, P.; Ivison, F.; Kibirige, M.; Mathew, V.; Matyka, K.; McGovern, A.; et al. Metformin in Obese Children and Adolescents: The MOCA Trial. J. Clin. Endocrinol. Metab. 2013, 98, 322–329. [Google Scholar] [CrossRef] [PubMed]
- Cali, A.M.; Pierpont, B.M.; Taksali, S.E.; Allen, K.; Shaw, M.M.; Savoye, M.; Caprio, S. Rosiglitazone Improves Glucose Metabolism in Obese Adolescents With Impaired Glucose Tolerance: A Pilot Study. Obesity 2011, 19, 94–99. [Google Scholar] [CrossRef]
- Garnett, S.P.; Gow, M.; Ho, M.; Baur, L.A.; Noakes, M.; Woodhead, H.J.; Broderick, C.R.; Burrell, S.; Chisholm, K.; Halim, J.; et al. Optimal Macronutrient Content of the Diet for Adolescents With Prediabetes; RESIST a Randomised Control Trial. J. Clin. Endocrinol. Metab. 2013, 98, 2116–2125. [Google Scholar] [CrossRef] [PubMed]
- Savoye, M.; Caprio, S.; Dziura, J.; Camp, A.; Germain, G.; Summers, C.; Li, F.; Shaw, M.; Nowicka, P.; Kursawe, R.; et al. Reversal of Early Abnormalities in Glucose Metabolism in Obese Youth: Results of an Intensive Lifestyle Randomized Controlled Trial. Diabetes Care 2014, 37, 317–324. [Google Scholar] [CrossRef] [PubMed]
- Gerstein, H.C.; Bosch, J.; Dagenais, G.R.; Díaz, R.; Jung, H.; Maggioni, A.P.; Pogue, J.; Probstfield, J.; Ramachandran, A.; Riddle, M.C.; et al. Basal insulin and cardiovascular and other outcomes in dysglycemia. N. Engl. J. Med. 2012, 367, 319–328. [Google Scholar] [CrossRef]
- Tuomilehto, J.; Lindström, J.; Eriksson, J.G.; Valle, T.T.; Hämäläinen, H.; Ilanne-Parikka, P.; Keinänen-Kiukaanniemi, S.; Laakso, M.; Louheranta, A.; Rastas, M.; et al. Prevention of Type 2 Diabetes Mellitus by Changes in Lifestyle among Subjects with Impaired Glucose Tolerance. N. Engl. J. Med. 2001, 344, 1343–1350. [Google Scholar] [CrossRef] [PubMed]
- Pan, X.-R.; Li, G.-W.; Hu, Y.-H.; Wang, J.-X.; Yang, W.-Y.; An, Z.-X.; Hu, Z.-X.; Lin, J.; Xiao, J.-Z.; Cao, H.-B.; et al. Effects of Diet and Exercise in Preventing NIDDM in People With Impaired Glucose Tolerance: The Da Qing IGT and Diabetes Study. Diabetes Care 1997, 20, 537–544. [Google Scholar] [CrossRef] [PubMed]
- Ramachandran, A.; Snehalatha, C.; Mary, S.; Mukesh, B.; Bhaskar, A.D.; Vijay, V.; Indian Diabetes Prevention Programme (IDPP). The Indian Diabetes Prevention Programme shows that lifestyle modification and metformin prevent type 2 diabetes in Asian Indian subjects with impaired glucose tolerance (IDPP-1). Diabetologia 2006, 49, 289–297. [Google Scholar] [CrossRef]
- Torgerson, J.S.; Hauptman, J.; Boldrin, M.N.; Sjöström, L. XENical in the prevention of diabetes in obese subjects (XENDOS) study: A randomized study of orlistat as an adjunct to lifestyle changes for the prevention of type 2 diabetes in obese patients. Diabetes Care 2004, 27, 155–161. [Google Scholar] [CrossRef]
- DREAM (Diabetes REduction Assessment with Ramipril and Rosiglitazone Medication) Trial Investigators. Effect of rosiglitazone on the frequency of diabetes in patients with impaired glucose tolerance or impaired fasting glucose: A randomised controlled trial. Lancet 2006, 368, 1096–1105. [Google Scholar] [CrossRef]
- Carlsson, L.M.; Peltonen, M.; Ahlin, S.; Anveden, C.; Bouchard, C.; Carlsson, B.; Jacobson, P.; Lönroth, H.; Maglio, C.; Näslund, I.; et al. Bariatric Surgery and Prevention of Type 2 Diabetes in Swedish Obese Subjects. N. Engl. J. Med. 2012, 367, 695–704. [Google Scholar] [CrossRef] [PubMed]
- Holman, R.R.; Haffner, S.M.; McMurray, J.J.; Bethel, M.A.; Holzhauer, B.; Hua, T.A.; Belenkov, Y.; Boolell, M.; Buse, J.B.; Buckley, B.M.; et al. Effect of Nateglinide on the Incidence of Diabetes and Cardiovascular Events. N. Engl. J. Med. 2010, 362, 1463–1476. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chiasson, J.-L.; Josse, R.G.; Gomis, R.; Hanefeld, M.; Karasik, A.; Laakso, M.; STOP-NIDDM Trail Research Group. Acarbose for prevention of type 2 diabetes mellitus: The STOP-NIDDM randomised trial. Lancet 2002, 359, 2072–2077. [Google Scholar] [CrossRef]
- Kahn, S.E.; Haffner, S.M.; Heise, M.A.; Herman, W.H.; Holman, R.R.; Jones, N.P.; Kravitz, B.G.; Lachin, J.M.; O’Neill, M.C.; Zinman, B.; et al. Glycemic durability of rosiglitazone, metformin, or glyburide monotherapy. N. Engl. J. Med. 2006, 355, 2427–2443. [Google Scholar] [CrossRef]
- Nwosu, B.U.; Parajuli, S.; Jasmin, G.; Fleshman, J.; Sharma, R.B.; Alonso, L.C.; Lee, A.F.; Barton, B.A. Ergocalciferol in New-onset Type 1 Diabetes: A Randomized Controlled Trial. J. Endocr. Soc. 2022, 6, bvab179. [Google Scholar] [CrossRef]
- Pittas, A.G.; Dawson-Hughes, B.; Sheehan, P.; Ware, J.H.; Knowler, W.C.; Aroda, V.R.; Brodsky, I.; Ceglia, L.; Chadha, C.; Chatterjee, R.; et al. Vitamin D Supplementation and Prevention of Type 2 Diabetes. N. Engl. J. Med. 2019, 381, 520–530. [Google Scholar] [CrossRef]
- Jorde, R.; Sollid, S.T.; Svartberg, J.; Schirmer, H.; Joakimsen, R.M.; Njølstad, I.; Fuskevåg, O.M.; Figenschau, Y.; Hutchinson, M.Y. Vitamin D 20 000 IU per Week for Five Years Does Not Prevent Progression From Prediabetes to Diabetes. J. Clin. Endocrinol. Metab. 2016, 101, 1647–1655. [Google Scholar] [CrossRef]
- Yu, L.; Zhai, Y.; Shen, S. Association between vitamin D and prediabetes: A PRISMA-compliant meta-analysis. Medicine 2020, 99, e19034. [Google Scholar] [CrossRef]
- Zhang, Y.; Xue, Y.; Zhang, D.; Liu, Y.; Xu, Z.; Gao, J.; Li, W.; Li, X. Effect of Vitamin D Supplementation on Glycemic Control in Prediabetes: A Meta-Analysis. Nutrients 2021, 13, 4464. [Google Scholar] [CrossRef]
- Poolsup, N.; Suksomboon, N.; Plordplong, N. Effect of vitamin D supplementation on insulin resistance and glycaemic control in prediabetes: A systematic review and meta-analysis. Diabet. Med. 2016, 33, 290–299. [Google Scholar] [CrossRef]
- Parajuli, S.; Jasmin, G.; Sirak, H.; Lee, A.F.; Nwosu, B.U. Prediabetes: Adherence to Nutrition Visits Decreases HbA1c in Children and Adolescents. Front. Endocrinol. 2022, 13, 916785. [Google Scholar] [CrossRef]
- Chiang, J.-K.; Lai, N.-S.; Chang, J.-K.; Koo, M. Predicting insulin resistance using the triglyceride-to-high-density lipoprotein cholesterol ratio in Taiwanese adults. Cardiovasc. Diabetol. 2011, 10, 93. [Google Scholar] [CrossRef] [Green Version]
- Rajappa, M.; Sridhar, M.G.; Balachander, J.; Sethuraman, K.R.; Rajendiran, K.S. Lipoprotein Ratios as Surrogate Markers for Insulin Resistance in South Indians with Normoglycemic Nondiabetic Acute Coronary Syndrome. ISRN Endocrinol. 2014, 2014, 981524. [Google Scholar] [CrossRef] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nwosu, B.U. The Progression of Prediabetes to Type 2 Diabetes in Children and Adolescents in the United States: Current Challenges and Solutions. Endocrines 2022, 3, 545-551. https://doi.org/10.3390/endocrines3030045
Nwosu BU. The Progression of Prediabetes to Type 2 Diabetes in Children and Adolescents in the United States: Current Challenges and Solutions. Endocrines. 2022; 3(3):545-551. https://doi.org/10.3390/endocrines3030045
Chicago/Turabian StyleNwosu, Benjamin Udoka. 2022. "The Progression of Prediabetes to Type 2 Diabetes in Children and Adolescents in the United States: Current Challenges and Solutions" Endocrines 3, no. 3: 545-551. https://doi.org/10.3390/endocrines3030045




