Neuroendocrine Changes during Menopausal Transition
Abstract
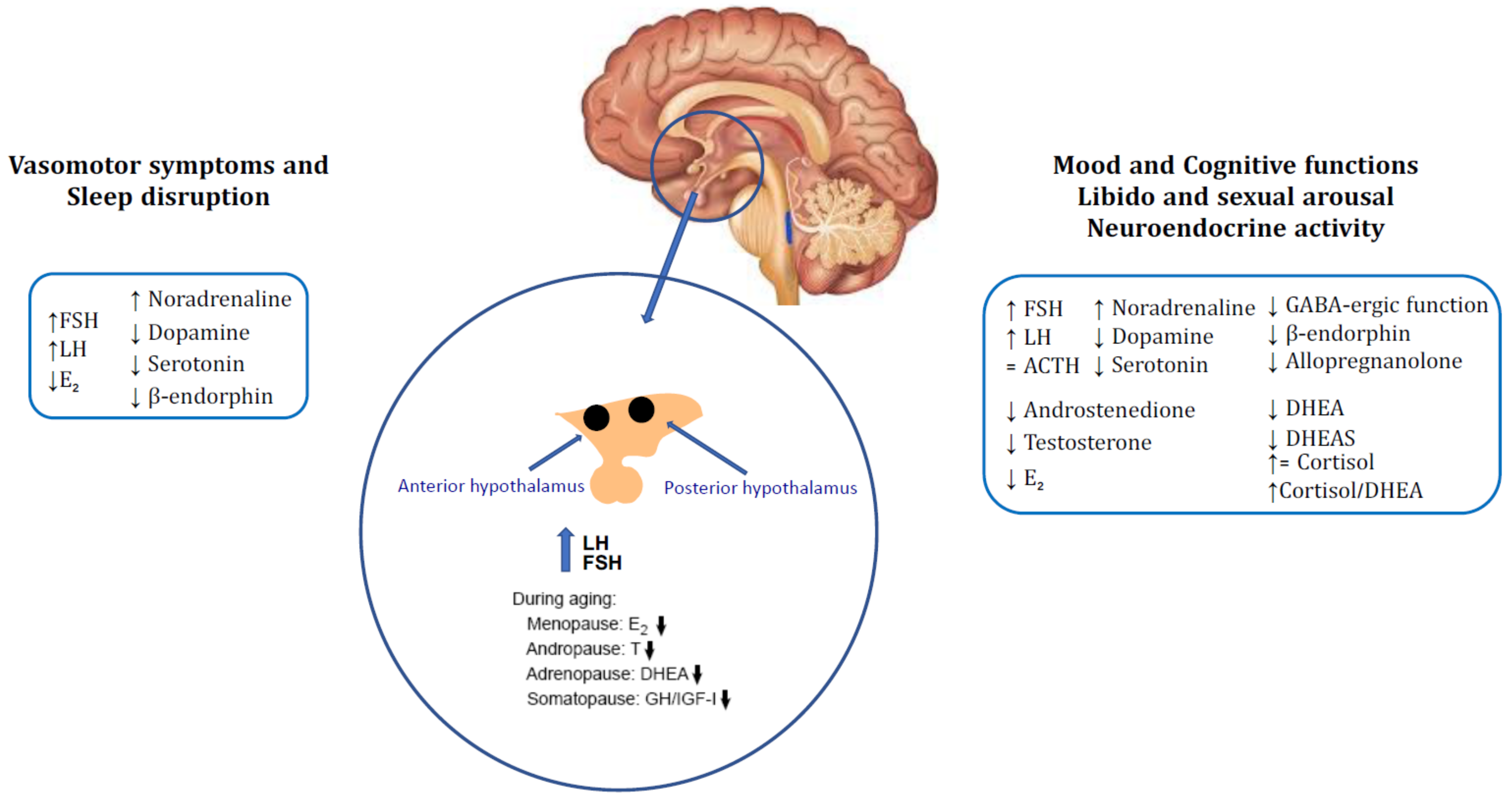
:1. Introduction
2. Neuroendocrine Changes in Menopause
2.1. Vasomotor Symptoms and Sleep Disruption
2.2. Mood Disorders
2.3. Neurological Disorders, Alzheimer’s Disease and Parkinson’s Disease
2.4. Migraine
2.5. Sexual Dysfunctions
3. New Evidence in Neuroendocrine Ageing and Menopause
3.1. Relevance of Perimenopausal Neuroendocrine Changes in the Quality of Life
3.2. Rationale and Role of Menopausal Hormonal Treatment on Neuroendocrine Ageing
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Genazzani, A.R.; Gambacciani, M. Hormone replacement therapy: The prospectives for the 21st century. Maturitas 1999, 32, 11–17. [Google Scholar] [CrossRef]
- Genazzani, A.R.; Spinetti, A.; Gallo, R.; Bernardi, F. Menopause and the central nervous system: Intervention options. Maturitas 1999, 3, 103–110. [Google Scholar] [CrossRef]
- Davis, S.R. Menopause. Nat. Rev. Dis. Primers 2015, 1, 15004. [Google Scholar] [CrossRef] [PubMed]
- Harlow, S.D.; Gass, M.; Hall, J.E.; Lobo, R.; Maki, P.; Rebar, R.W.; Sherman, S.; Sluss, P.M.; de Villiers, T.J. Executive summary of the stages of reproductive aging workshop. Addressing the unfinished agenda of staging reproductive aging. J. Clin. Endocrinol. Metab. 2012, 97, 1159–1168. [Google Scholar] [CrossRef]
- Farrag, A.F.; Khedr, E.M.; Abdel-Aleem, H.; Rageh, T.A. Effects of Surgical Menopause on Cognitive Functions. Dement. Geriatr. Cogn. Disord. 2002, 13, 193–198. [Google Scholar] [CrossRef]
- Orentreich, N.; Brind, J.L.; Vogelman, J.H.; Andres, R.; Baldwin, H. Long-term longitudinal measurements of plasma dehydroepiandrosterone sulfate in normal men. J. Clin. Endocrinol. Metab. 1992, 75, 1002–1004. [Google Scholar] [PubMed]
- Yamaji, T.; Ibayashi, H. Serum deydroepiandrosterone sulphate in normal and pathological canditions. J. Clin. Endocrinol. Metab. 1969, 29, 273–278. [Google Scholar] [CrossRef] [PubMed]
- Corpechot, C.; Robert, P.; Axelson, M.; Sjovall, J.; Baulieu, E.E. Characterization and measurement of deydriepiandrosterone sulfate in the rat brain. Proc. Natl. Acad. Sci. USA 1981, 78, 4704–4707. [Google Scholar] [CrossRef] [Green Version]
- Majewska, M.D.; Demirgoren, S.; Spivak, C.E.; London, E.D. The neurosteroid DHEA is an allosteric antagonist of the GABA A receptor. Brain Res. 1990, 526, 143–146. [Google Scholar] [CrossRef]
- Baulieu, E.E. Dehydroepiandrosterone: A fountain of youth? J. Clin. Endocrinol. Metab. 1996, 81, 3147–3151. [Google Scholar] [CrossRef]
- Davis, S.; Shah, S.M.; McKenzie, D.P.; Kulkarni, J.; Davison, S.; Bel, R.J. Dehydroepiandrosterone Sulfate Levels Are Associated with More Favorable Cognitive Function in Women. J. Clin. Endocrinol. Metab. 2008, 93, 3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baulieu, E.E. Neurosteroids: A new function in the brain. Biol. Cell 1991, 71, 3–10. [Google Scholar] [CrossRef]
- Robel, P.; Baulieu, E.E. Neurosteroids. Biosynthesis and function. Trends Endocrinol. Metab. 1994, 5, 1–8. [Google Scholar] [CrossRef]
- Stoffel-Wagner, B. Neurosteroid metabolism in the human brain. Eur. J. Endocrinol. 2001, 145, 669–679. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stoffel-Wagner, B.; Watzka, M.; Steckelbroeck, S.; Ludwig, M.; Clusmann, H.; Bidlingmaier, F.; Casarosa, E.; Luisi, S.; Elger, C.E.; Beyenburg, S. Allopregnanolone serum levels and expression of 5-alpha-reductase and 3-alpha hydroxysteroid dehydrogenase isoforms in hippocampal and temporal cortex of patients with epilepsy. Epilepsy Res. 2003, 54, 11–19. [Google Scholar] [CrossRef]
- Sundstrom, I.; Andersson, A.; Nyberg, S.; Purdy, R.H.; Bäckström, T. Patients with premenstrual syndrome have a different sensitivity to a neuroactive steroid during the menstrual cycle compared to control subjects. Neuroendocrinology 1998, 67, 126–138. [Google Scholar] [CrossRef] [PubMed]
- Khisti, R.T.; Mandhane, S.N.; Chopde, C.T. The neurosteroid 3-alpha-hydroxy-5-alpha-pregnan-20-one induces catalepsy in mice. Neurosci. Lett. 1998, 251, 85–88. [Google Scholar] [CrossRef]
- Johansson, I.M.; Birzniece, V.; Lindblad, C.; Olsson, T.; Bäckström, T. Allopregnanolone inhibits learning in the Morris water maze. Brain Res. 2002, 934, 125–131. [Google Scholar] [CrossRef]
- Genazzani, A.R.; Bernardi, F.; Pluchino, N.; Begliuomini, S.; Lenzi, E.; Casarosa, E.; Luisi, M. Endocrinology of menopausal transition and its brain implications. CNS Spectr. 2005, 10, 449–457. [Google Scholar] [CrossRef]
- Słopien, R.; Junik, R.; Meczekalski, B.; Halerz-Nowakowska, B.; Maciejewska, M.; Warenik-Szymankiewicz, A.; Sowińskib, J. Influence of hormonal replacement therapy on the regional cerebral blood flow in postmenopausal women. Maturitas 2003, 46, 255–262. [Google Scholar] [CrossRef]
- Słopien, R.; Jasniewicz, J.; Meczekalski, B.; Warenik-Szymankiewicza, A.; Lianeri, M.; Jagodzińskib, P.P. Polymorphic variants of genes encoding MTHFR, MTR and MTHFD1 and the risk of depression in postmenopausal women in Poland. Maturitas 2008, 8, 252–255. [Google Scholar] [CrossRef]
- Downs, J.L.; Wise, P.M. The role of the brain in female reproductive aging. Mol. Cell Endocrinol. 2009, 299, 32–38. [Google Scholar] [CrossRef] [Green Version]
- Santoro, N. Symptoms of menopause: Hot flushes. Clin. Obstet. Gynecol. 2008, 51, 539–548. [Google Scholar] [CrossRef]
- Freedman, R.R. Menopausal hot flashes: Mechanisms, endocrinology, treatment. J. Steroid Biochem. Mol. Biol. 2014, 142, 115–120. [Google Scholar] [CrossRef] [Green Version]
- Richard-Davis, G.; Wellons, M. Racial and ethnic differences in the physiology and clinical symptoms of menopause. Semin. Reprod. Med. 2013, 31, 380–386. [Google Scholar]
- Duffy, O.K.; Iversen, L.; Aucott, L.; Hannaford, P.C. Factors associated with resilience or vulnerability to hot flushes and night sweats during the menopausal transition. Menopause 2013, 20, 383–392. [Google Scholar] [CrossRef] [PubMed]
- Thurston, R.C.; Chang, Y.; Mancuso, P.; Matthews, K.A. Adipokines, adiposity, and vasomotor symptoms during the menopause transition: Findings from the Study of Women’s Health across the Nation. Fertil. Steril. 2013, 100, 793–800. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thurston, R.C.; Khoudary, S.R.E.; Sutton-Tyrrell, K.; Crandall, C.J.; Sternfeld, B.; Joffe, H.; Gold, E.B.; Selzer, F.; Matthews, K.A. Vasomotor symptoms and insulin resistance in the study of women’s health across the nation. J. Clin. Endocrinol. Metab. 2021, 97, 3487–3494. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kravitz, H.M.; Joffe, H. Sleep during the perimenopause: A Swan story. Obstet. Gynecol. Clin. N. Am. 2011, 38, 567–586. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yaffe, K.; Falvey, C.M.; Hoang, T. Connections between sleep and cognition in older adults. Lancet Neurol. 2014, 13, 1017–1028. [Google Scholar] [CrossRef]
- Rasgon, N.L.; Silverman, D.; Siddarth, P.; Miller, K.; Ercoli, L.M.; Elman, S.; Lavretsky, H.; Huang, S.-C.; Phelpsc, M.E.; Small, G.W. Estrogen use and brain metabolic change in postmenopausal women. Neurobiol. Aging 2005, 26, 229–235. [Google Scholar] [CrossRef]
- Maki, P.M.; Resnick, S.M. Longitudinal effects of estrogen replacement therapy on PET cerebral blood flow and cognition. Neurobiol. Aging 2000, 21, 373–383. [Google Scholar] [CrossRef]
- Majewska, M.D. Neurosteroids: Endogenous bimodal modulators of the GABA A receptors. Mechanism of action and physiological significance. Prog. Neurobiol. 1992, 38, 379–395. [Google Scholar] [CrossRef]
- Mellon, S.H. Neurosteroids: Action and clinical relevance. J. Clin. Endocrinol. Metab. 1994, 78, 1003–1008. [Google Scholar] [PubMed]
- Bernardi, F.; Pieri, M.; Stomati, M.; Luisi, S.; Palumbo, M.; Pluchino, N.; Ceccarelli, C.; Genazzani, A.R. Effect of different hormonal replacement therapies on circulating allopregnanolone and dehydroepiandrosterone levels in postmenopausal women. Gynecol. Endocrinol. 2003, 17, 65–77. [Google Scholar] [CrossRef] [PubMed]
- Davis, S.R.; Burger, H.G. Androgens and the postmenopausal woman, Clinical Review. J. Clin. Endocrinol. Metab. 1996, 81, 2759–2763. [Google Scholar] [PubMed]
- Paganini-Hill, A.; Henderson, V. Estrogen deficiency and risk of Alzheimer’s Disease in women. Am. J. Epidemiol. 1994, 140, 256–261. [Google Scholar] [CrossRef] [PubMed]
- Yao, J.; Irwin, R.W.; Zhao, L.; Nilsen, J.; Hamilton, R.T.; Brinton, R.D. Mitochondrial bioenergetic deficit precedes Alzheimer’s pathology in female mouse model of Alzheimer’s disease. Proc. Natl. Acad. Sci. USA 2009, 106, 14670–14675. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sherwin, B.B. Estrogen effects on cognition in menopausal women. Neurology 1997, 48, S21–S26. [Google Scholar] [CrossRef] [PubMed]
- Zandi, P.P.; Carlson, M.C.; Plassman, B.L.; Welshbohmer, K.A.; Mayer, L.S.; Steffens, D.C.; Breitner, J.C. Hormone replacement therapy and incidence of Alzheimer disease in older women: The Cache County Study. JAMA 2002, 288, 2123–2129. [Google Scholar] [CrossRef] [Green Version]
- Brinton, R.D. Investigative models for determining hormone therapy-induced outcomes in brain: Evidence in support of a healthy cell bias of estrogen action. Ann. N. Y. Acad. Sci. 2005, 1052, 57–74. [Google Scholar] [CrossRef]
- Rocca, W.A.; Rocca, L.G.; Smith, C.Y.; Grossardt, B.R.; Faubion, S.; Shuster, L.T.; Kirkland, J.L.; LeBrasseur, N.K.; Schafer, M.J.; Mielke, M.; et al. Loss of ovarian hormones and accelerated somatic and mental aging. Physiology 2018, 33, 374–383. [Google Scholar] [CrossRef]
- Zeydan, B.; Tosakulwong, N.; Schwarz, C.G.; Senjem, M.L.; Gunter, J.L.; Reid, R.I.; Rocca, G.L.; Lesnick, T.G.; Smith, C.Y.; Bailey, K.R.; et al. Association of bilateral salpingo-oophorectomy before menopause onset with medial temporal lobe neurodegeneration. JAMA Neurol. 2019, 76, 1. [Google Scholar] [CrossRef] [Green Version]
- Sawada, H.; Ibi, M.; Kihara, T.; Urushitani, M.; Akaika, A.; Shimohama, S. Estradiol protects mesencephalic dopaminergic neurons from oxidative stress-induced neuronal death. J. Neurosci. Res. 1998, 54, 707–719. [Google Scholar] [CrossRef]
- Dluzen, D.E.; MCDermott, J.L. Gender differences in neurotoxicity of the nigrostriatal dopaminergic system: Implications for Parkinson’s disease. J. Gend. Specif. Med. 2000, 3, 36–42. [Google Scholar]
- Saunders-Pullman, R.; Gordon-Elliott, J.; Parides, M.; Fahn, S.; Saunders, H.R.; Bressman, S. The effect of estrogen replacement on early Parkinson’s disease. Neurology 1999, 52, 1417–1421. [Google Scholar] [CrossRef]
- Short, R.A.; Bowen, R.L.; O’Brien, P.C.; Graff-Radford, N.R. Elevated gonadotropin levels in patients with Alzheimer disease. Mayo Clin. Proc. 2001, 76, 906–909. [Google Scholar] [CrossRef]
- Hagemann, G.; Ugur, T.; Schleussner, E.; Mentzel, H.J.; Fitzek, C.; Witte, O.W.; Gaser, C. Changes in Brain Size during the Menstrual Cycle. PLoS ONE 2011, 6, e14655. [Google Scholar] [CrossRef] [PubMed]
- Laumann, E.; Paik, A.; Rosen, R.C. Sexual dysfunction in the United States: Prevalence and predictors. JAMA 1999, 281, 537–544. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bacon, E.R.; Brinton, R.D. Epigenetics of the developing and aging brain: Mechanisms that regulate onset and outcomes of brain reorganization. Neurosci. Biobehav. Rev. 2021, 125, 503–516. [Google Scholar] [CrossRef]
- Rahkola-Soisalo, P.; Savolainen-Peltonen, H.; Gissler, M.; Hoti, F.; Vattulainen, P.; Ylikorkala, O.; Mikkola, T.S. Increased risk for stress urinary incontinence in women with postmenopausal hormone therapy. Int. Urogynecol. J. 2019, 30, 251–256. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nappi, P.R.; Cucinella, L.; Martella, S.; Rossi, M.; Tiranini, L.; Martini, E. Female sexual dysfunction (FSD): Prevalence and impact on quality of life (QoL). Maturitas 2016, 94, 87–91. [Google Scholar] [CrossRef] [PubMed]
- Gass, M.L.; Cochrane, B.B.; Larson, J.C.; Manson, J.E.; Barnabei, V.M.; Brzyski, R.G.; Lane, D.S.; LaValleur, J.; Ockene, J.K.; Mouton, C.P.; et al. Patterns and predictors of sexual activity among women in the Hormone Therapy trials of the Women’s Health Initiative. Menopause 2011, 18, 1160–1171. [Google Scholar] [CrossRef] [PubMed]
- Scheffers, C.S.; Armstrong, S.; Cantineau, A.E.; Farquhar, C.; Jordan, V. Dehydroepiandrosterone for women in the peri- or postmenopausal phase. Cochrane Database Syst. Rev. 2015, 1, CD011066. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peixoto, C.; Carrilho, C.G.; Barros, J.A.; Ribeiro, T.T.; Silva, L.M.; Nardi, A.E.; Cardoso, A.; Veras, A.B. The effects of dehydroepiandrosterone on sexual function: A systematic review. Climacteric 2017, 20, 129–137. [Google Scholar] [CrossRef]
- Dören, M.; Ruebig, A.; Holzgreve, W. Differential effects on the androgen status of postmenopausal women treated with tibolone and continuous combined estradiol and norethindrone acetate replacement therapy. Fertil. Steril. 2001, 75, 554–558. [Google Scholar] [CrossRef]
- Gartoulla, P.; Worsley, R.; Bell, R.J.; Davis, S.R. Moderate to severe vasomotor and sexual symptoms remain problematic for women aged 60 to 65 years. Menopause 2018, 25, 1331–1338. [Google Scholar] [CrossRef]
- Ockene, J.K.; Barad, D.H.; Cochrane, B.B. Symptom experience after discontinuing use of estrogen plus progestin. JAMA 2005, 294, 183–193. [Google Scholar] [CrossRef]
- Schmidt, P.J.; Dor, R.B.; Martinez, P.E.; Guerrieri, G.M.; Harsh, V.L.; Thompson, K.; Koziol, D.E.; Nieman, L.K.; Rubinow, D.R. Effects of Estradiol Withdrawal on Mood in Women With Past Perimenopausal Depression: A Randomized Clinical Trial. JAMA Psychiatry 2015, 72, 714–726. [Google Scholar] [CrossRef] [Green Version]
- Mikkola, T.S.; Tuomikoski, P.; Lyytinen, H.; Korhonen, P.; Hoti, F.; Vattulainen, P.; Gissler, M.; Ylikorkala, O. Increased cardiovascular mortality risk in women discontinuing postmenopausal hormone therapy. J. Clin. Endocrinol. Metab. 2015, 100, 4588–4594. [Google Scholar] [CrossRef] [Green Version]
- Monteleone, P.; Mascagni, G.; Giannini, A.; Genazzani, A.R.; Simoncini, T. Symptoms of menopause—global prevalence, physiology and implications. Nat. Rev. Endocrinol. 2018, 14, 199–215. [Google Scholar] [CrossRef] [PubMed]
- Genazzani, A.R.; Monteleone, P.; Giannini, A.; Simoncini, T. Hormone therapy in the postmenopausal years: Considering benefits and risks in clinical practice. Hum. Reprod. Update 2021. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giannini, A.; Caretto, M.; Genazzani, A.R.; Simoncini, T. Neuroendocrine Changes during Menopausal Transition. Endocrines 2021, 2, 405-416. https://doi.org/10.3390/endocrines2040036
Giannini A, Caretto M, Genazzani AR, Simoncini T. Neuroendocrine Changes during Menopausal Transition. Endocrines. 2021; 2(4):405-416. https://doi.org/10.3390/endocrines2040036
Chicago/Turabian StyleGiannini, Andrea, Marta Caretto, Andrea R. Genazzani, and Tommaso Simoncini. 2021. "Neuroendocrine Changes during Menopausal Transition" Endocrines 2, no. 4: 405-416. https://doi.org/10.3390/endocrines2040036
APA StyleGiannini, A., Caretto, M., Genazzani, A. R., & Simoncini, T. (2021). Neuroendocrine Changes during Menopausal Transition. Endocrines, 2(4), 405-416. https://doi.org/10.3390/endocrines2040036






