Ovarian Rejuvenation Using Autologous Platelet-Rich Plasma
Abstract
1. Introduction
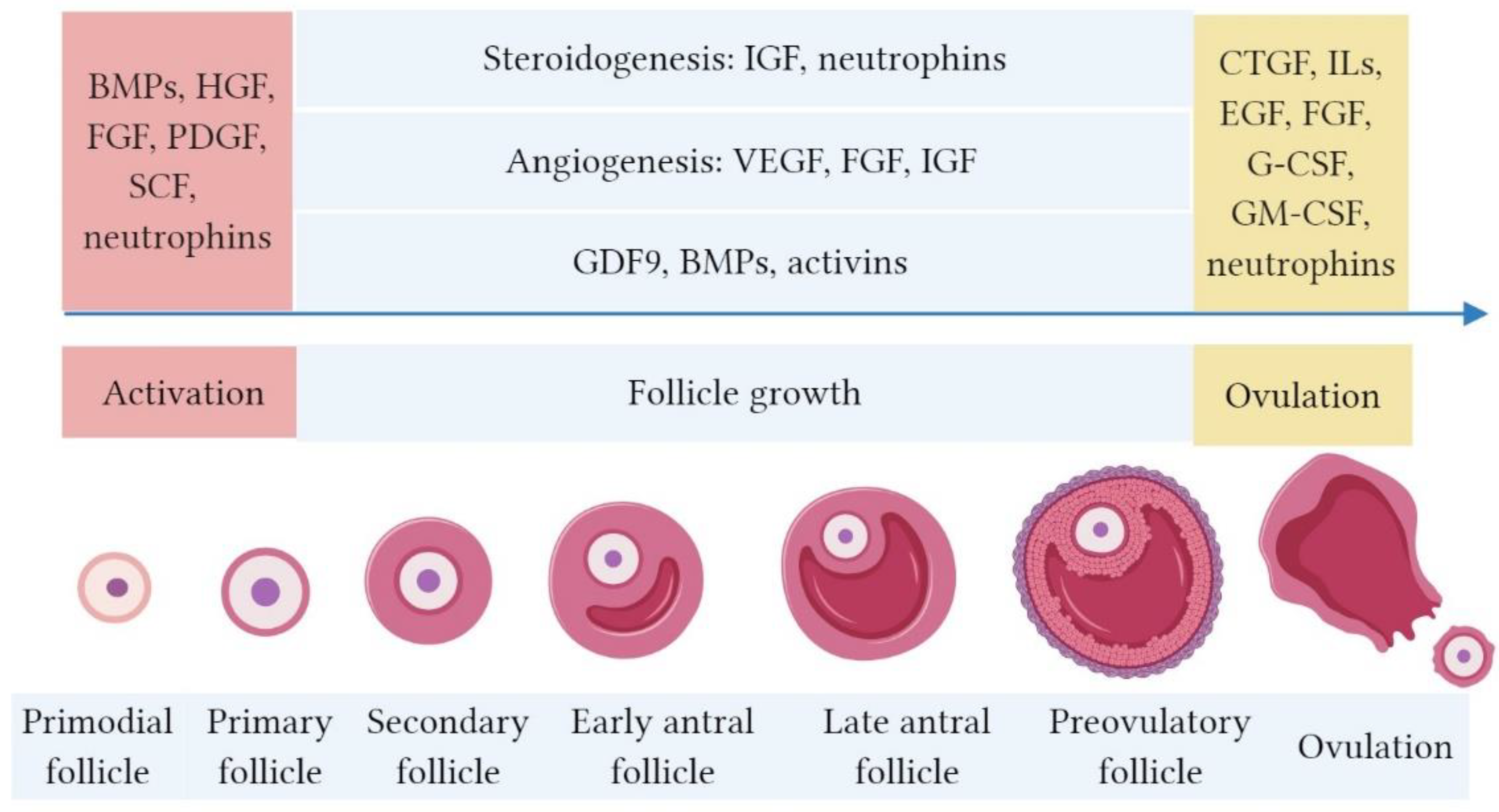
2. Bioactive Factors in PRP and their Role in Folliculogenesis
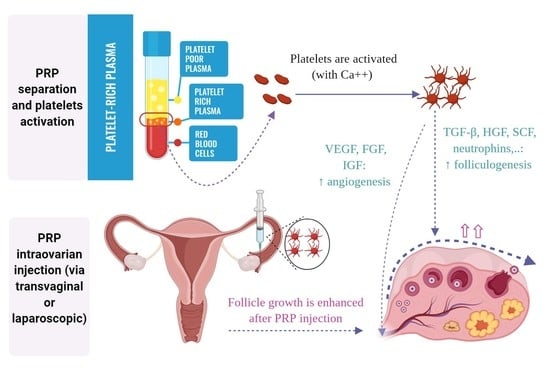
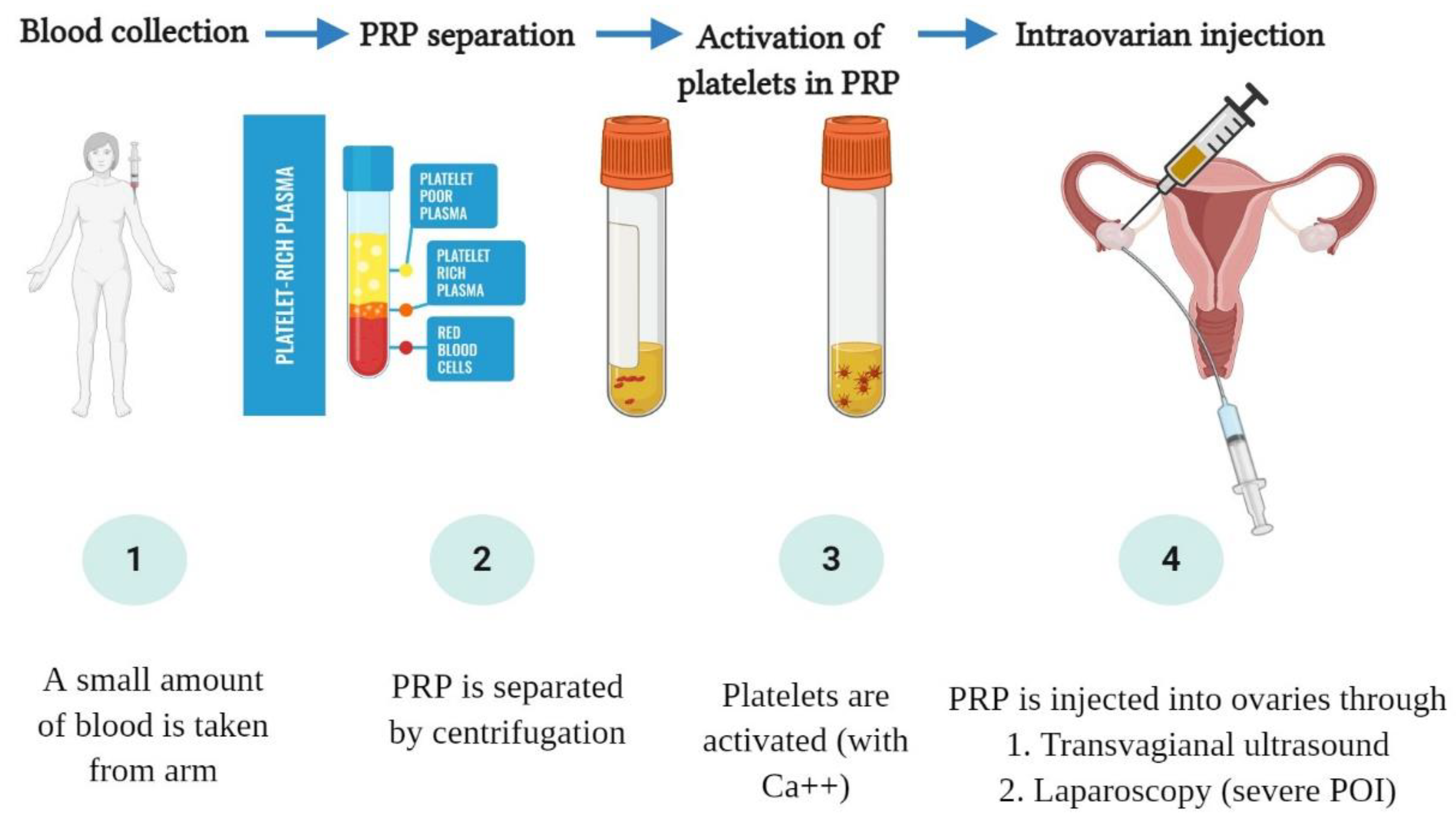
3. The Hypothesized Mechanism of PRP in Ovarian “Rejuvenation”
4. Clinical Outcomes of Intraovarian PRP Injection
4.1. Case Report and Case Series Studies
4.2. Comparative Studies
4.3. Clinical Practice and Future Perspective of Intraovarian PRP Injection
5. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Martin, J.A.; Hamilton, B.E.; Osterman, M.J.K.; Driscoll, A.K.; Drake, P. Births: Final Data for 2016. Natl. Vital Stat. Rep. 2018, 67, 1–55. [Google Scholar]
- Mathews, T.J.; Hamilton, B.E. Mean Age of Mothers is on the Rise: United States, 2000–2014. NCHS Data Brief. 2016, 232, 1–8. [Google Scholar]
- Coulam, C.B.; Adamson, S.C.; Annegers, J.F. Incidence of premature ovarian failure. Obstet. Gynecol. 1986, 67, 604–606. [Google Scholar] [CrossRef]
- Lagergren, K.; Hammar, M.; Nedstrand, E.; Bladh, M.; Sydsjö, G. The prevalence of primary ovarian insufficiency in Sweden: A national register study. BMC Womens Health 2018, 18, 175. [Google Scholar] [CrossRef]
- Sundman, E.A.; Cole, B.J.; Fortier, L.A. Growth factor and catabolic cytokine concentrations are influenced by the cellular composition of platelet-rich plasma. Am. J. Sports Med. 2011, 39, 2135–2140. [Google Scholar] [CrossRef] [PubMed]
- Marx, R.E. Platelet-rich plasma (PRP): What is PRP and what is not PRP? Implant. Dent. 2001, 10, 225–228. [Google Scholar] [CrossRef] [PubMed]
- Nikolidakis, D.; Jansen, J.A. The biology of platelet-rich plasma and its application in oral surgery: Literature review. Tissue Eng. Part B Rev. 2008, 14, 249–258. [Google Scholar] [CrossRef] [PubMed]
- Alsousou, J.; Ali, A.; Willett, K.; Harrison, P. The role of platelet-rich plasma in tissue regeneration. Platelets 2013, 24, 173–182. [Google Scholar] [CrossRef] [PubMed]
- Liao, H.T.; James, I.B.; Marra, K.G.; Rubin, J.P. The Effects of Platelet-Rich Plasma on Cell Proliferation and Adipogenic Potential of Adipose-Derived Stem Cells. Tissue Eng. Part A 2015, 21, 2714–2722. [Google Scholar] [CrossRef]
- Reurink, G.; Goudswaard, G.J.; Moen, M.H.; Weir, A.; Verhaar, J.A.; Bierma-Zeinstra, S.M.; Maas, M.; Tol, J.L. Platelet-rich plasma injections in acute muscle injury. N. Engl. J. Med. 2014, 370, 2546–2547. [Google Scholar] [CrossRef]
- Leo, M.S.; Kumar, A.S.; Kirit, R.; Konathan, R.; Sivamani, R.K. Systematic review of the use of platelet-rich plasma in aesthetic dermatology. J. Cosmet. Dermatol. 2015, 14, 315–323. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.; Li, J.; Chen, Y.; Wei, L.; Yang, X.; Shi, Y.; Liang, X. Autologous platelet-rich plasma promotes endometrial growth and improves pregnancy outcome during in vitro fertilization. Int. J. Clin. Exp. Med. 2015, 8, 1286–1290. [Google Scholar] [PubMed]
- Noh, K.C.; Liu, X.N.; Zhuan, Z.; Yang, C.J.; Kim, Y.T.; Lee, G.W.; Choi, K.H.; Kim, K.O. Leukocyte-Poor Platelet-Rich Plasma-Derived Growth Factors Enhance Human Fibroblast Proliferation In Vitro. Clin. Orthop. Surg. 2018, 10, 240–247. [Google Scholar] [CrossRef] [PubMed]
- Lubkowska, A.; Dolegowska, B.; Banfi, G. Growth factor content in PRP and their applicability in medicine. J. Biol. Regul. Homeost. Agents 2012, 26, 3s–22s. [Google Scholar]
- Mussano, F.; Genova, T.; Munaron, L.; Petrillo, S.; Erovigni, F.; Carossa, S. Cytokine, chemokine, and growth factor profile of platelet-rich plasma. Platelets 2016, 27, 467–471. [Google Scholar] [CrossRef]
- Krüger, J.P.; Freymannx, U.; Vetterlein, S.; Neumann, K.; Endres, M.; Kaps, C. Bioactive factors in platelet-rich plasma obtained by apheresis. Transfus. Med. Hemother. 2013, 40, 432–440. [Google Scholar] [CrossRef]
- Schär, M.O.; Diaz-Romero, J.; Kohl, S.; Zumstein, M.A.; Nesic, D. Platelet-rich concentrates differentially release growth factors and induce cell migration in vitro. Clin. Orthop. Relat. Res. 2015, 473, 1635–1643. [Google Scholar] [CrossRef]
- Chu, Y.L.; Xu, Y.R.; Yang, W.X.; Sun, Y. The role of FSH and TGF-β superfamily in follicle atresia. Aging 2018, 10, 305–321. [Google Scholar] [CrossRef]
- Coutts, S.M.; Childs, A.J.; Fulton, N.; Collins, C.; Bayne, R.A.; McNeilly, A.S.; Anderson, R.A. Activin signals via SMAD2/3 between germ and somatic cells in the human fetal ovary and regulates kit ligand expression. Dev. Biol. 2008, 314, 189–199. [Google Scholar] [CrossRef][Green Version]
- Durlinger, A.L.; Visser, J.A.; Themmen, A.P. Regulation of ovarian function: The role of anti-Müllerian hormone. Reproduction 2002, 124, 601–609. [Google Scholar] [CrossRef]
- Nilsson, E.E.; Skinner, M.K. Bone morphogenetic protein-4 acts as an ovarian follicle survival factor and promotes primordial follicle development. Biol. Reprod. 2003, 69, 1265–1272. [Google Scholar] [CrossRef] [PubMed]
- Vitt, U.A.; Hsueh, A.J. Stage-dependent role of growth differentiation factor-9 in ovarian follicle development. Mol. Cell. Endocrinol. 2001, 183, 171–177. [Google Scholar] [CrossRef]
- Vitt, U.A.; Hayashi, M.; Klein, C.; Hsueh, A.J. Growth differentiation factor-9 stimulates proliferation but suppresses the follicle-stimulating hormone-induced differentiation of cultured granulosa cells from small antral and preovulatory rat follicles. Biol. Reprod. 2000, 62, 370–377. [Google Scholar] [CrossRef] [PubMed]
- Dong, J.; Albertini, D.F.; Nishimori, K.; Kumar, T.R.; Lu, N.; Matzuk, M.M. Growth differentiation factor-9 is required during early ovarian folliculogenesis. Nature 1996, 383, 531–535. [Google Scholar] [CrossRef]
- Otsuka, F.; McTavish, K.J.; Shimasaki, S. Integral role of GDF-9 and BMP-15 in ovarian function. Mol. Reprod. Dev. 2011, 78, 9–21. [Google Scholar] [CrossRef] [PubMed]
- Orisaka, M.; Orisaka, S.; Jiang, J.Y.; Craig, J.; Wang, Y.; Kotsuji, F.; Tsang, B.K. Growth differentiation factor 9 is antiapoptotic during follicular development from preantral to early antral stage. Mol. Endocrinol. 2006, 20, 2456–2468. [Google Scholar] [CrossRef]
- Sanfins, A.; Rodrigues, P.; Albertini, D.F. GDF-9 and BMP-15 direct the follicle symphony. J. Assist. Reprod. Genet. 2018, 35, 1741–1750. [Google Scholar] [CrossRef]
- Gode, F.; Gulekli, B.; Dogan, E.; Korhan, P.; Dogan, S.; Bige, O.; Cimrin, D.; Atabey, N. Influence of follicular fluid GDF9 and BMP15 on embryo quality. Fertil. Steril. 2011, 95, 2274–2278. [Google Scholar] [CrossRef]
- Knight, P.G.; Glister, C. TGF-beta superfamily members and ovarian follicle development. Reproduction 2006, 132, 191–206. [Google Scholar] [CrossRef]
- Findlay, J.K.; Drummond, A.E.; Dyson, M.; Baillie, A.J.; Robertson, D.M.; Ethier, J.F. Production and actions of inhibin and activin during folliculogenesis in the rat. Mol. Cell. Endocrinol. 2001, 180, 139–144. [Google Scholar] [CrossRef]
- Namwanje, M.; Brown, C.W. Activins and Inhibins: Roles in Development, Physiology, and Disease. Cold Spring Harb. Perspect. Biol. 2016, 8. [Google Scholar] [CrossRef] [PubMed]
- Quezada, M.; Wang, J.; Hoang, V.; McGee, E.A. Smad7 is a transforming growth factor-beta-inducible mediator of apoptosis in granulosa cells. Fertil. Steril. 2012, 97, 1452–1459.e6. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.; Zhang, Y.L.; Fan, H.Y. Selective Smad4 knockout in ovarian preovulatory follicles results in multiple defects in ovulation. Mol. Endocrinol. 2013, 27, 966–978. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, E.; Parrott, J.A.; Skinner, M.K. Basic fibroblast growth factor induces primordial follicle development and initiates folliculogenesis. Mol. Cell. Endocrinol. 2001, 175, 123–130. [Google Scholar] [CrossRef]
- Guglielmo, M.C.; Ricci, G.; Catizone, A.; Barberi, M.; Galdieri, M.; Stefanini, M.; Canipari, R. The effect of hepatocyte growth factor on the initial stages of mouse follicle development. J. Cell. Physiol. 2011, 226, 520–529. [Google Scholar] [CrossRef] [PubMed]
- Thuwanut, P.; Comizzoli, P.; Wildt, D.E.; Keefer, C.L.; Songsasen, N. Stem cell factor promotes in vitro ovarian follicle development in the domestic cat by upregulating c-kit mRNA expression and stimulating the phosphatidylinositol 3-kinase/AKT pathway. Reprod. Fertil. Dev. 2017, 29, 1356–1368. [Google Scholar] [CrossRef]
- Liu, K.; Rajareddy, S.; Liu, L.; Jagarlamudi, K.; Boman, K.; Selstam, G.; Reddy, P. Control of mammalian oocyte growth and early follicular development by the oocyte PI3 kinase pathway: New roles for an old timer. Dev. Biol. 2006, 299, 1–11. [Google Scholar] [CrossRef]
- Passos, J.R.; Costa, J.J.; da Cunha, E.V.; Silva, A.W.; Ribeiro, R.P.; de Souza, G.B.; Barroso, P.A.; Dau, A.M.; Saraiva, M.V.; Gonçalves, P.B.; et al. Protein and messenger RNA expression of interleukin 1 system members in bovine ovarian follicles and effects of interleukin 1β on primordial follicle activation and survival in vitro. Domest. Anim. Endocrinol. 2016, 54, 48–59. [Google Scholar] [CrossRef]
- Nagashima, T.; Kim, J.; Li, Q.; Lydon, J.P.; DeMayo, F.J.; Lyons, K.M.; Matzuk, M.M. Connective tissue growth factor is required for normal follicle development and ovulation. Mol. Endocrinol. 2011, 25, 1740–1759. [Google Scholar] [CrossRef]
- Schindler, R.; Nilsson, E.; Skinner, M.K. Induction of ovarian primordial follicle assembly by connective tissue growth factor CTGF. PLoS ONE 2010, 5, e12979. [Google Scholar] [CrossRef][Green Version]
- Harlow, C.R.; Hillier, S.G. Connective tissue growth factor in the ovarian paracrine system. Mol. Cell. Endocrinol. 2002, 187, 23–27. [Google Scholar] [CrossRef]
- Hsueh, A.J.; Kawamura, K.; Cheng, Y.; Fauser, B.C. Intraovarian control of early folliculogenesis. Endocr. Rev. 2015, 36, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Kawamura, K.; Kawamura, N.; Mulders, S.M.; Sollewijn Gelpke, M.D.; Hsueh, A.J. Ovarian brain-derived neurotrophic factor (BDNF) promotes the development of oocytes into preimplantation embryos. Proc. Natl. Acad. Sci. USA 2005, 102, 9206–9211. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.M.; Wu, H.C.; Sun, Z.G.; Lian, F.; Leung, P.C.K. Neurotrophins and glial cell line-derived neurotrophic factor in the ovary: Physiological and pathophysiological implications. Hum. Reprod. Update 2019, 25, 224–242. [Google Scholar] [CrossRef]
- Cui, L.; Fang, L.; Mao, X.; Chang, H.M.; Leung, P.C.K.; Ye, Y. GDNF-Induced Downregulation of miR-145-5p Enhances Human Oocyte Maturation and Cumulus Cell Viability. J. Clin. Endocrinol. Metab. 2018, 103, 2510–2521. [Google Scholar] [CrossRef]
- Pinkas, H.; Fisch, B.; Rozansky, G.; Felz, C.; Kessler-Icekson, G.; Krissi, H.; Nitke, S.; Ao, A.; Abir, R. Platelet-derived growth factors (PDGF-A and -B) and their receptors in human fetal and adult ovaries. Mol. Hum. Reprod. 2008, 14, 199–206. [Google Scholar] [CrossRef]
- Kim, S.O.; Trau, H.A.; Duffy, D.M. Vascular endothelial growth factors C and D may promote angiogenesis in the primate ovulatory follicle. Biol. Reprod. 2017, 96, 389–400. [Google Scholar] [CrossRef]
- Li, S.H.; Hwu, Y.M.; Lu, C.H.; Chang, H.H.; Hsieh, C.E.; Lee, R.K. VEGF and FGF2 Improve Revascularization, Survival, and Oocyte Quality of Cryopreserved, Subcutaneously-Transplanted Mouse Ovarian Tissues. Int. J. Mol. Sci. 2016, 17, 1237. [Google Scholar] [CrossRef]
- Mattioli, M.; Barboni, B.; Turriani, M.; Galeati, G.; Zannoni, A.; Castellani, G.; Berardinelli, P.; Scapolo, P.A. Follicle activation involves vascular endothelial growth factor production and increased blood vessel extension. Biol. Reprod. 2001, 65, 1014–1019. [Google Scholar] [CrossRef]
- Yamamoto, S.; Konishi, I.; Tsuruta, Y.; Nanbu, K.; Mandai, M.; Kuroda, H.; Matsushita, K.; Hamid, A.A.; Yura, Y.; Mori, T. Expression of vascular endothelial growth factor (VEGF) during folliculogenesis and corpus luteum formation in the human ovary. Gynecol. Endocrinol. 1997, 11, 371–381. [Google Scholar] [CrossRef]
- Fraser, H.M. Regulation of the ovarian follicular vasculature. Reprod. Biol. Endocrinol. 2006, 4, 18. [Google Scholar] [CrossRef] [PubMed]
- Plendl, J. Angiogenesis and vascular regression in the ovary. Anat. Histol. Embryol. 2000, 29, 257–266. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Kumar, T.R.; Matzuk, M.M.; Bondy, C. Insulin-like growth factor I regulates gonadotropin responsiveness in the murine ovary. Mol. Endocrinol. 1997, 11, 1924–1933. [Google Scholar] [CrossRef] [PubMed]
- Zhou, P.; Baumgarten, S.C.; Wu, Y.; Bennett, J.; Winston, N.; Hirshfeld-Cytron, J.; Stocco, C. IGF-I signaling is essential for FSH stimulation of AKT and steroidogenic genes in granulosa cells. Mol. Endocrinol. 2013, 27, 511–523. [Google Scholar] [CrossRef] [PubMed]
- Baumgarten, S.C.; Armouti, M.; Ko, C.; Stocco, C. IGF1R Expression in Ovarian Granulosa Cells Is Essential for Steroidogenesis, Follicle Survival, and Fertility in Female Mice. Endocrinology 2017, 158, 2309–2318. [Google Scholar] [CrossRef]
- Serafim, M.K.; Duarte, A.B.; Silva, G.M.; Souza, C.E.; Magalhães-Padilha, D.M.; Moura, A.A.; Silva, L.D.; Campello, C.C.; Figueiredo, J.R. Impact of growth hormone (GH) and follicle stimulating hormone (FSH) on in vitro canine preantral follicle development and estradiol production. Growth Horm. IGF Res. 2015, 25, 85–89. [Google Scholar] [CrossRef]
- Wang, H.; Wen, Y.; Polan, M.L.; Boostanfar, R.; Feinman, M.; Behr, B. Exogenous granulocyte-macrophage colony-stimulating factor promotes follicular development in the newborn rat in vivo. Hum. Reprod. 2005, 20, 2749–2756. [Google Scholar] [CrossRef][Green Version]
- Ito, M.; Harada, T.; Tanikawa, M.; Fujii, A.; Shiota, G.; Terakawa, N. Hepatocyte growth factor and stem cell factor involvement in paracrine interplays of theca and granulosa cells in the human ovary. Fertil. Steril. 2001, 75, 973–979. [Google Scholar] [CrossRef]
- Baskind, N.E.; Orsi, N.M.; Sharma, V. Follicular-phase ovarian follicular fluid and plasma cytokine profiling of natural cycle in vitro fertilization patients. Fertil. Steril. 2014, 102, 410–418. [Google Scholar] [CrossRef]
- Richani, D.; Gilchrist, R.B. The epidermal growth factor network: Role in oocyte growth, maturation and developmental competence. Hum. Reprod. Update 2018, 24, 1–14. [Google Scholar] [CrossRef]
- Peralta, O.A.; Bucher, D.; Fernandez, A.; Berland, M.; Strobel, P.; Ramirez, A.; Ratto, M.H.; Concha, I. Granulocyte-macrophage colony stimulating factor (GM-CSF) enhances cumulus cell expansion in bovine oocytes. Reprod. Biol. Endocrinol. 2013, 11, 55. [Google Scholar] [CrossRef] [PubMed]
- Salmassi, A.; Schmutzler, A.G.; Huang, L.; Hedderich, J.; Jonat, W.; Mettler, L. Detection of granulocyte colony-stimulating factor and its receptor in human follicular luteinized granulosa cells. Fertil. Steril. 2004, 81 (Suppl. 1), 786–791. [Google Scholar] [CrossRef] [PubMed]
- Yanagi, K.; Makinoda, S.; Fujii, R.; Miyazaki, S.; Fujita, S.; Tomizawa, H.; Yoshida, K.; Iura, T.; Takegami, T.; Nojima, T. Cyclic changes of granulocyte colony-stimulating factor (G-CSF) mRNA in the human follicle during the normal menstrual cycle and immunolocalization of G-CSF protein. Hum. Reprod. 2002, 17, 3046–3052. [Google Scholar] [CrossRef] [PubMed]
- Cai, L.; Jeon, Y.; Yoon, J.D.; Hwang, S.U.; Kim, E.; Park, K.M.; Kim, K.J.; Jin, M.H.; Lee, E.; Kim, H.; et al. The effects of human recombinant granulocyte-colony stimulating factor treatment during in vitro maturation of porcine oocyte on subsequent embryonic development. Theriogenology 2015, 84, 1075–1087. [Google Scholar] [CrossRef]
- Check, J.H.; Vaniver, J.; Senft, D.; DiAntonio, G.; Summers, D. The use of granulocyte colony stimulating factor to enhance oocyte release in women with the luteinized unruptured follicle syndrome. Clin. Exp. Obstet. Gynecol. 2016, 43, 178–180. [Google Scholar] [CrossRef]
- Hou, H.Y.; Wang, X.; Yu, Q.; Li, H.Y.; Li, S.J.; Tang, R.Y.; Guo, Z.X.; Chen, Y.Q.; Hu, C.X.; Yang, Z.J.; et al. Evidence that growth hormone can improve mitochondrial function in oocytes from aged mice. Reproduction 2018, 157, 345–358. [Google Scholar] [CrossRef]
- Dehghani, F.; Aboutalebi, H.; Esmaeilpour, T.; Panjehshahin, M.R.; Bordbar, H. Effect of platelet-rich plasma (PRP) on ovarian structures in cyclophosphamide-induced ovarian failure in female rats: A stereological study. Toxicol. Mech. Methods 2018, 28, 653–659. [Google Scholar] [CrossRef]
- Ahmadian, S.; Sheshpari, S.; Pazhang, M.; Bedate, A.M.; Beheshti, R.; Abbasi, M.M.; Nouri, M.; Rahbarghazi, R.; Mahdipour, M. Intra-ovarian injection of platelet-rich plasma into ovarian tissue promoted rejuvenation in the rat model of premature ovarian insufficiency and restored ovulation rate via angiogenesis modulation. Reprod. Biol. Endocrinol. 2020, 18, 78. [Google Scholar] [CrossRef]
- Cremonesi, F.; Bonfanti, S.; Idda, A.; Lange-Consiglio, A. Platelet Rich Plasma for Regenerative Medicine Treatment of Bovine Ovarian Hypofunction. Front. Vet. Sci. 2020, 7, 517. [Google Scholar] [CrossRef]
- Cremonesi, F.; Bonfanti, S.; Idda, A.; Anna, L.C. Improvement of Embryo Recovery in Holstein Cows Treated by Intra-Ovarian Platelet Rich Plasma before Superovulation. Vet. Sci. 2020, 7, 16. [Google Scholar] [CrossRef]
- Hosseini, L.; Shirazi, A.; Naderi, M.M.; Shams-Esfandabadi, N.; Borjian Boroujeni, S.; Sarvari, A.; Sadeghnia, S.; Behzadi, B.; Akhondi, M.M. Platelet-rich plasma promotes the development of isolated human primordial and primary follicles to the preantral stage. Reprod. Biomed. Online 2017, 35, 343–350. [Google Scholar] [CrossRef] [PubMed]
- Younis, J.S.; Haddad, S.; Matilsky, M.; Radin, O.; Ben-Ami, M. Undetectable basal ovarian stromal blood flow in infertile women is related to low ovarian reserve. Gynecol. Endocrinol. 2007, 23, 284–289. [Google Scholar] [CrossRef] [PubMed]
- Fenton, A.J. Premature ovarian insufficiency: Pathogenesis and management. J. Midlife Health 2015, 6, 147–153. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.H.; Oh, D.S.; Jeong, J.H.; Shin, B.S.; Joo, B.S.; Lee, K.S. Follicular blood flow is a better predictor of the outcome of in vitro fertilization-embryo transfer than follicular fluid vascular endothelial growth factor and nitric oxide concentrations. Fertil. Steril. 2004, 82, 586–592. [Google Scholar] [CrossRef]
- Wood, S.H.; Sills, E.S. Intraovarian vascular enhancement via stromal injection of platelet-derived growth factors: Exploring subsequent oocyte chromosomal status and in vitro fertilization outcomes. Clin. Exp. Reprod. Med. 2020, 47, 94–100. [Google Scholar] [CrossRef]
- Etulain, J.; Mena, H.A.; Meiss, R.P.; Frechtel, G.; Gutt, S.; Negrotto, S.; Schattner, M. An optimised protocol for platelet-rich plasma preparation to improve its angiogenic and regenerative properties. Sci. Rep. 2018, 8, 1513. [Google Scholar] [CrossRef]
- Samadi, P.; Sheykhhasan, M.; Khoshinani, H.M. The Use of Platelet-Rich Plasma in Aesthetic and Regenerative Medicine: A Comprehensive Review. Aesthetic Plast. Surg. 2019, 43, 803–814. [Google Scholar] [CrossRef]
- Mohamadi, S.; Norooznezhad, A.H.; Mostafaei, S.; Nikbakht, M.; Nassiri, S.; Safar, H.; Moghaddam, K.A.; Ghavamzadeh, A.; Kazemnejad, A. A randomized controlled trial of effectiveness of platelet-rich plasma gel and regular dressing on wound healing time in pilonidal sinus surgery: Role of different affecting factors. Biomed. J. 2019, 42, 403–410. [Google Scholar] [CrossRef]
- Bakacak, M.; Bostanci, M.S.; İnanc, F.; Yaylali, A.; Serin, S.; Attar, R.; Yildirim, G.; Yildirim, O.K. Protective Effect of Platelet Rich Plasma on Experimental Ischemia/Reperfusion Injury in Rat Ovary. Gynecol. Obstet. Investig. 2016, 81, 225–231. [Google Scholar] [CrossRef]
- Callejo, J.; Salvador, C.; González-Nuñez, S.; Almeida, L.; Rodriguez, L.; Marqués, L.; Valls, A.; Lailla, J.M. Live birth in a woman without ovaries after autograft of frozen-thawed ovarian tissue combined with growth factors. J. Ovarian Res. 2013, 6, 33. [Google Scholar] [CrossRef]
- Sfakianoudis, K.; Simopoulou, M.; Nitsos, N.; Rapani, A.; Pappas, A.; Pantou, A.; Chronopoulou, M.; Deligeoroglou, E.; Koutsilieris, M.; Pantos, K. Autologous Platelet-Rich Plasma Treatment Enables Pregnancy for a Woman in Premature Menopause. J. Clin. Med. 2018, 8, 1. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.C.; Hsu, L.; Hsu, I.; Chiu, Y.J.; Dorjee, S. Live Birth in Woman With Premature Ovarian Insufficiency Receiving Ovarian Administration of Platelet-Rich Plasma (PRP) in Combination With Gonadotropin: A Case Report. Front. Endocrinol. (Lausanne) 2020, 11, 50. [Google Scholar] [CrossRef] [PubMed]
- Pantos, K.; Nitsos, N.; Kokkali, G.; Vaxevanoglou, T.; Markomichali, C.; Pantou, A.; Grammatis, M.; Lazaros, L.; Sfakianoudis, K. Ovarian rejuvenation and folliculogenesis reactivation in peri-menopausal women after autologous platelet-rich plasma treatment. In Proceedings of the ESHRE 32nd Annual Meeting, Helsinki, Finland, 3–6 July 2016. [Google Scholar]
- Sills, E.S.; Rickers, N.S.; Li, X.; Palermo, G.D. First data on in vitro fertilization and blastocyst formation after intraovarian injection of calcium gluconate-activated autologous platelet rich plasma. Gynecol. Endocrinol. 2018, 34, 756–760. [Google Scholar] [CrossRef] [PubMed]
- Pantos, K.; Simopoulou, M.; Pantou, A.; Rapani, A.; Tsioulou, P.; Nitsos, N.; Syrkos, S.; Pappas, A.; Koutsilieris, M.; Sfakianoudis, K. A Case Series on Natural Conceptions Resulting in Ongoing Pregnancies in Menopausal and Prematurely Menopausal Women Following Platelet-Rich Plasma Treatment. Cell Transplant. 2019, 28, 1333–1340. [Google Scholar] [CrossRef] [PubMed]
- Sfakianoudis, K.; Simopoulou, M.; Nitsos, N.; Rapani, A.; Pantou, A.; Vaxevanoglou, T.; Kokkali, G.; Koutsilieris, M.; Pantos, K. A Case Series on Platelet-Rich Plasma Revolutionary Management of Poor Responder Patients. Gynecol. Obstet. Investig. 2019, 84, 99–106. [Google Scholar] [CrossRef]
- Farimani, M.; Heshmati, S.; Poorolajal, J.; Bahmanzadeh, M. A report on three live births in women with poor ovarian response following intra-ovarian injection of platelet-rich plasma (PRP). Mol. Biol. Rep. 2019, 46, 1611–1616. [Google Scholar] [CrossRef]
- Cakiroglu, Y.; Saltik, A.; Yuceturk, A.; Karaosmanoglu, O.; Kopuk, S.Y.; Scott, R.T.; Tiras, B.; Seli, E. Effects of intraovarian injection of autologous platelet rich plasma on ovarian reserve and IVF outcome parameters in women with primary ovarian insufficiency. Aging 2020, 12, 10211–10222. [Google Scholar] [CrossRef]
- Ferraretti, A.P.; La Marca, A.; Fauser, B.C.; Tarlatzis, B.; Nargund, G.; Gianaroli, L. ESHRE consensus on the definition of ‘poor response’ to ovarian stimulation for in vitro fertilization: The Bologna criteria. Hum. Reprod. 2011, 26, 1616–1624. [Google Scholar] [CrossRef]
- Sfakianoudis, K.; Simopoulou, M.; Grigoriadis, S.; Pantou, A.; Tsioulou, P.; Maziotis, E.; Rapani, A.; Giannelou, P.; Nitsos, N.; Kokkali, G.; et al. Reactivating Ovarian Function through Autologous Platelet-Rich Plasma Intraovarian Infusion: Pilot Data on Premature Ovarian Insufficiency, Perimenopausal, Menopausal, and Poor Responder Women. J. Clin. Med. 2020, 9, 1809. [Google Scholar] [CrossRef]
- Petryk, N.; Petryk, M. Ovarian Rejuvenation Through Platelet-Rich Autologous Plasma (PRP)-a Chance to Have a Baby Without Donor Eggs, Improving the Life Quality of Women Suffering from Early Menopause Without Synthetic Hormonal Treatment. Reprod. Sci. 2020, 27, 1975–1982. [Google Scholar] [CrossRef]
- Stojkovska, S.; Dimitrov, G.; Stamenkovska, N.; Hadzi-Lega, M.; Petanovski, Z. Live Birth Rates in Poor Responders’ Group after Previous Treatment with Autologous Platelet-Rich Plasma and Low Dose Ovarian Stimulation Compared with Poor Responders Used Only Low Dose Ovarian Stimulation Before in Vitro Fertilization. J. Med. Sci. 2019, 7, 3184–3188. [Google Scholar] [CrossRef]
- Melo, P.; Navarro, C.; Jones, C.; Coward, K.; Coleman, L. The use of autologous platelet-rich plasma (PRP) versus no intervention in women with low ovarian reserve undergoing fertility treatment: A non-randomized interventional study. J. Assist. Reprod. Genet. 2020, 37, 855–863. [Google Scholar] [CrossRef]
- Fraison, E.; Crawford, G.; Casper, G.; Harris, V.; Ledger, W. Pregnancy following diagnosis of premature ovarian insufficiency: A systematic review. Reprod. Biomed. Online 2019, 39, 467–476. [Google Scholar] [CrossRef]
- Fadadu, P.P.; Mazzola, A.J.; Hunter, C.W.; Davis, T.T. Review of concentration yields in commercially available platelet-rich plasma (PRP) systems: A call for PRP standardization. Reg. Anesth. Pain Med. 2019. [Google Scholar] [CrossRef]
| Growth Factors | Roles in Folliculogenesis | References |
|---|---|---|
| Activins | Primordial follicle formation | [19] |
| BDNF | Primordial follicle activation, follicular growth, ovulation | [43,44,45] |
| BMPs | Follicular growth, preantral follicle maintenance, COC expansion | [18,20,21,27] |
| CTGF | Primordial follicle assembly, ovulation | [39,40,41] |
| EGF | COC expansion | [60] |
| FGF | Primordial follicle activation | [34] |
| G-CSF | Ovulation | [62,63,64,65] |
| GDF | Follicles growth after the primary stage, FSH expression | [18,22,23,24,25,26,27] |
| GH | Steroidogenesis in granulosa cells, mitochondrial function | [56,66] |
| GM-CSF | Follicles growth, COC expansion | [57,61] |
| GDNF | Primordial follicle activation, follicular growth, ovulation | [44] |
| HGF | Primordial follicle activation | [35] |
| IGF | Steroidogenesis in granulosa cells | [53,54,55] |
| IL-1 β | Primordial follicle development, ovulation | [38,59] |
| NT-3 | Primordial follicle activation, follicular growth, ovulation | [44] |
| NT-4 | Primordial follicle activation, follicular growth, ovulation | [44] |
| PDGF | Primordial follicle activation | [46] |
| SCF | Primordial follicle activation | [36,37] |
| VEGF | Angiogenesis | [51] |
| Authors | Enrolled Case Number | Age | PRP (ml) /Blood (ml)/Ovary * | Follow Up Duration (Months) | AMH (mIU/L) | FSH (mIU/L) | AFC (n) | Number of Oocytes ** | Pregnancy Outcome | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Prior PRP | Post PRP | Prior PRP | Post PRP | Prior PRP | Post PRP | Prior PRP | Post PRP | Mode of Conception | ||||||
| Natural | IVF | |||||||||||||
| Sills E.S (2018) [84] | 4 | 42 ± 4 | 5/N/A | 5 | 0.38 | 0.61 | 13.6 | 7.7 | N/A *** | N/A | N/A | 4–7 | 0 | N/A |
| Sfakianoudis K. (2018) [81] | 1 | 40 | 4/30 | 2 | 0.02 | 0.08 | 149 | 27 | N/A | N/A | N/A | N/A | 0 | 1 |
| Pantos K. (2019) [85] | 3 | 46 40 27 (POI) | 4/30 4/30 4/30 | 1 3 5 | 0.16 0.06 0.17 | 0.22 0.2 0.3 | 119 65 46.5 | 27 10 15.1 | 0 0 0 | 4 2 1 | N/A N/A N/A | N/A N/A N/A | 1 1 1 | N/A N/A N/A |
| Sfakianoudis K. (2019) [86] | 3 | 40 37 37 | 5/35 5/35 5/35 | N/A N/A N/A | 0.65 0.54 0.44 | 1.1 0.93 0.81 | 27.8 18.3 24.1 | 11.1 4.1 8.6 | N/A N/A N/A | N/A N/A N/A | 0/4 10/12 2/4 | 5/6 2/2 2/4 | 000 | 111 |
| Farimani M. (2019) [87] | 19 | 35.57± 3.80 | 2/N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | 0.64 ± 0.92 | 2.1 ± 2.5 | 2 | 1 |
| Hsu C.C (2020) [82] | 1 | 37 | 3/20 | N/A | 0.02 | 63.65 | 17.84 | N/A | N/A | 0 | 6/2 | 0 | 1 | |
| Cakiroglu Y. (2020) [88] | 311 | 34.8 ± 4.3 | 2-4/20 | N/A | 0.13 ± 0.16 | 0.18 ± 0.18 | 41.9 ± 24.7 | 41.6 ± 24.7 | 0.5 ± 0.5 | 1.7 ± 1.4 | N/A | N/A | 23 | 13 |
| Sfakianoudis K. (2020) **** (POR) [90] | 30 | 38.40 ± 2.01 | 4/30 | 3 | 0.66 ± 0.20 | 1.14 ± 0.26 | 10.71 ± 1.62 | 8.95 ± 1.40 | 2.63 ± 0.93 | 5.20 ± 1.35 | 1.20 ± 0.76 | N/A | 0 | 14 |
| Petryk N. (2020) [91] | 38 | 31–45 | 0.7/8.5 | 12 | p < 0.05 | p < 0.05 | N/A | N/A | N/A | N/A | 4 | 6 | ||
| Author | Characteristics | PRP Group | Without PRP Group | p Value |
|---|---|---|---|---|
| Stojkovska S. (2019) [92] | N = 20 | N = 20 | ||
| Age | 37.47 ± 3.87 | 37.64 ± 3.20 | 0.99 | |
| Baseline FSH (mIU/mL) | 19.27 ± 2.29 | 19.22 ± 4.05 | 0.97 | |
| Baseline AMH (ng/mL) | 0.35 ± 0.19 | 0.72 ± 0.42 | 0.03 | |
| Number of oocytes | 1.87 ± 1.13 | 3.71 ± 2.40 | 0.20 | |
| Fertilization rate (%) | 80.67 ± 25.42 | 65.60 ± 25.35 | 0.44 | |
| Implantation rate (%) | 33.33 ± 44.99 | 10.71 ± 28.95 | 0.70 | |
| Clinical pregnancy rate (%) | 33.33 ± 44.99 | 10.71 ± 28.95 | 0.69 | |
| Live birth rate (%) | 40.00 ± 50.71 | 14.29 ± 36.31 | 0.71 | |
| Melo P. (2020) [93] | N = 46 | N = 37 | ||
| Age | 41 (39–44) | 41 (39–44) | 0.78 | |
| Baseline FSH (mIU/mL) | 13.6 (12.9–17.5) | 14.9 (13.1–17.8) | 0.26 | |
| Post PRP FSH (mIU/mL) | 9.07 (8.3–10.5) | 15.0 (13.4–17.9) | N/A * | |
| Baseline AMH (ng/mL) | 0.62 (0.47–0.76) | 0.68 (0.41–0.78) | 0.65 | |
| Post PRP AMH (ng/mL) | 1.01 (0.9–1.3) | 0.58 (0.39–0.76) | N/A | |
| Oocyte number | 5.0 (2.0-9.0) | 3.0 (0.0-6.0) | <0.001 | |
| Biochemical pregnancy rate | 12/46 (26.1) | 2/37 (5.4) | 0.02 | |
| Clinical pregnancy rate | 11/46 (23.9) | 2/37 (5.4) | 0.03 | |
| 1st trimester miscarriages | 6/46 (13.0) ** | 1/37 (2.7) | 0.13 | |
| Live births | 4/46 (8.7) | 1/37 (2.7) | 0.38 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vo, T.K.C.; Tanaka, Y.; Kawamura, K. Ovarian Rejuvenation Using Autologous Platelet-Rich Plasma. Endocrines 2021, 2, 15-27. https://doi.org/10.3390/endocrines2010002
Vo TKC, Tanaka Y, Kawamura K. Ovarian Rejuvenation Using Autologous Platelet-Rich Plasma. Endocrines. 2021; 2(1):15-27. https://doi.org/10.3390/endocrines2010002
Chicago/Turabian StyleVo, Tuyen Kim Cat, Yuka Tanaka, and Kazuhiro Kawamura. 2021. "Ovarian Rejuvenation Using Autologous Platelet-Rich Plasma" Endocrines 2, no. 1: 15-27. https://doi.org/10.3390/endocrines2010002
APA StyleVo, T. K. C., Tanaka, Y., & Kawamura, K. (2021). Ovarian Rejuvenation Using Autologous Platelet-Rich Plasma. Endocrines, 2(1), 15-27. https://doi.org/10.3390/endocrines2010002