Grain Size- and Temperature-Dependent Phonon-Mediated Heat Transport in the Solid Electrolyte Interphase: A First-Principles Study
Abstract
1. Introduction
2. Materials and Methods
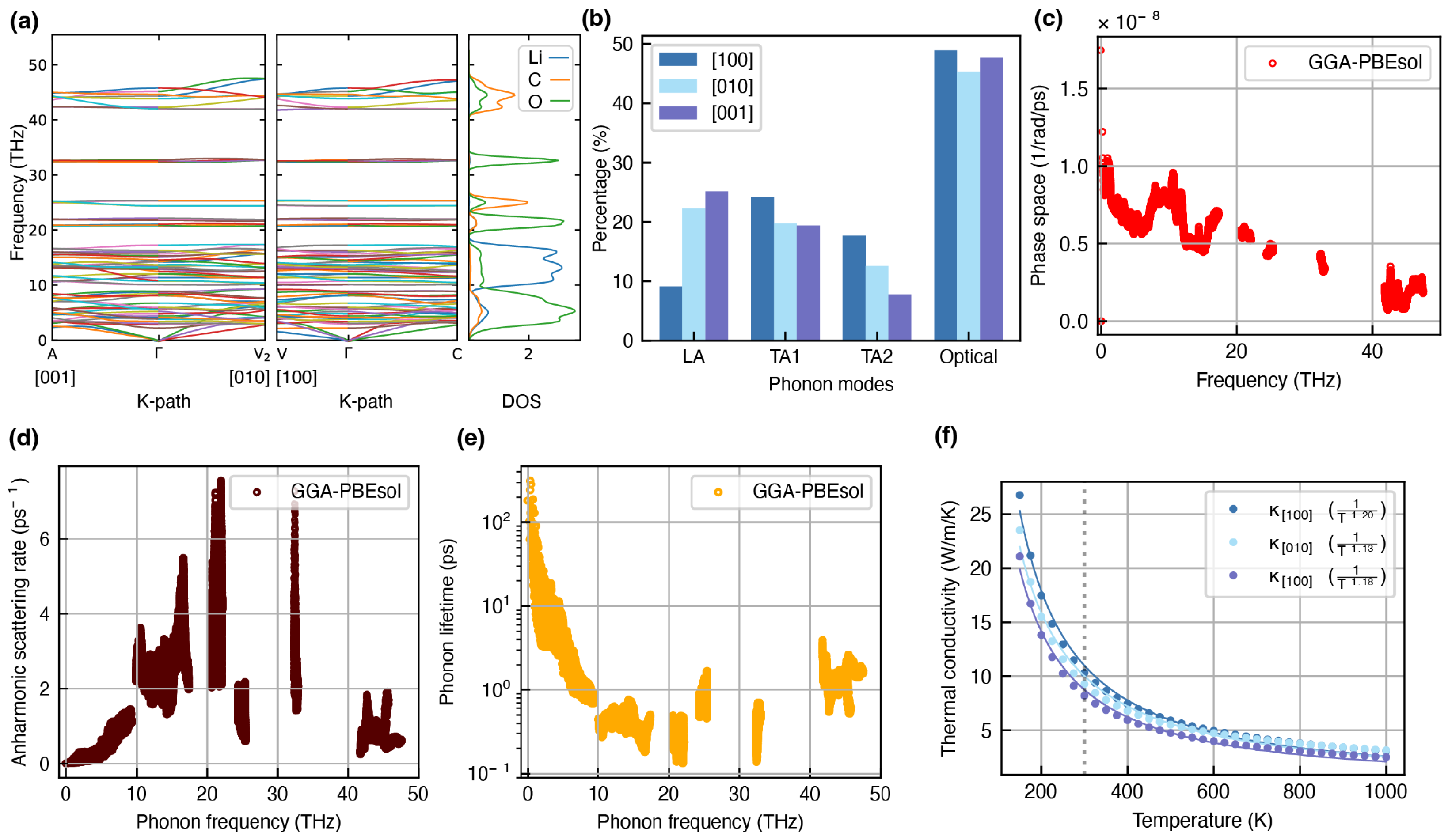
Phonon Dispersion and Thermal Conductivity
3. Results and Discussion
- LiF: LiF is a stable component of the SEI that forms from the decomposition of salts such as LiPF6 and LiTFSI (lithium bis(trifluoromethanesulfonyl)imide), additives such as fluoroethylene carbonate, or reactions involving trace HF. The exceptional stability of LiF has been widely utilized to develop durable artificial interphases due to its ability to form highly stable interfaces with electrolytes. LiF is also well known for its tolerance to temperature. For that reason, its presence in the SEI improves the high-temperature cycling performance of the battery [50,51].
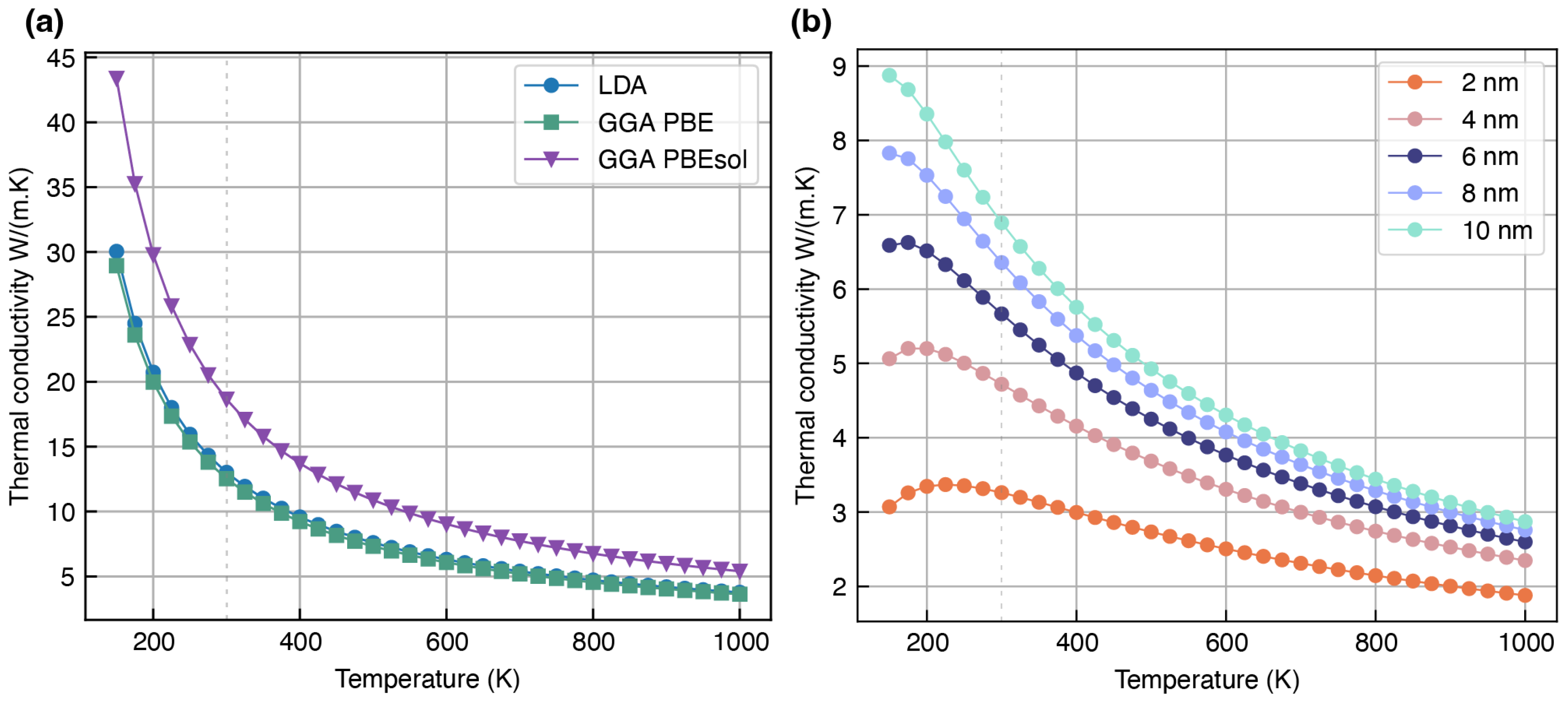
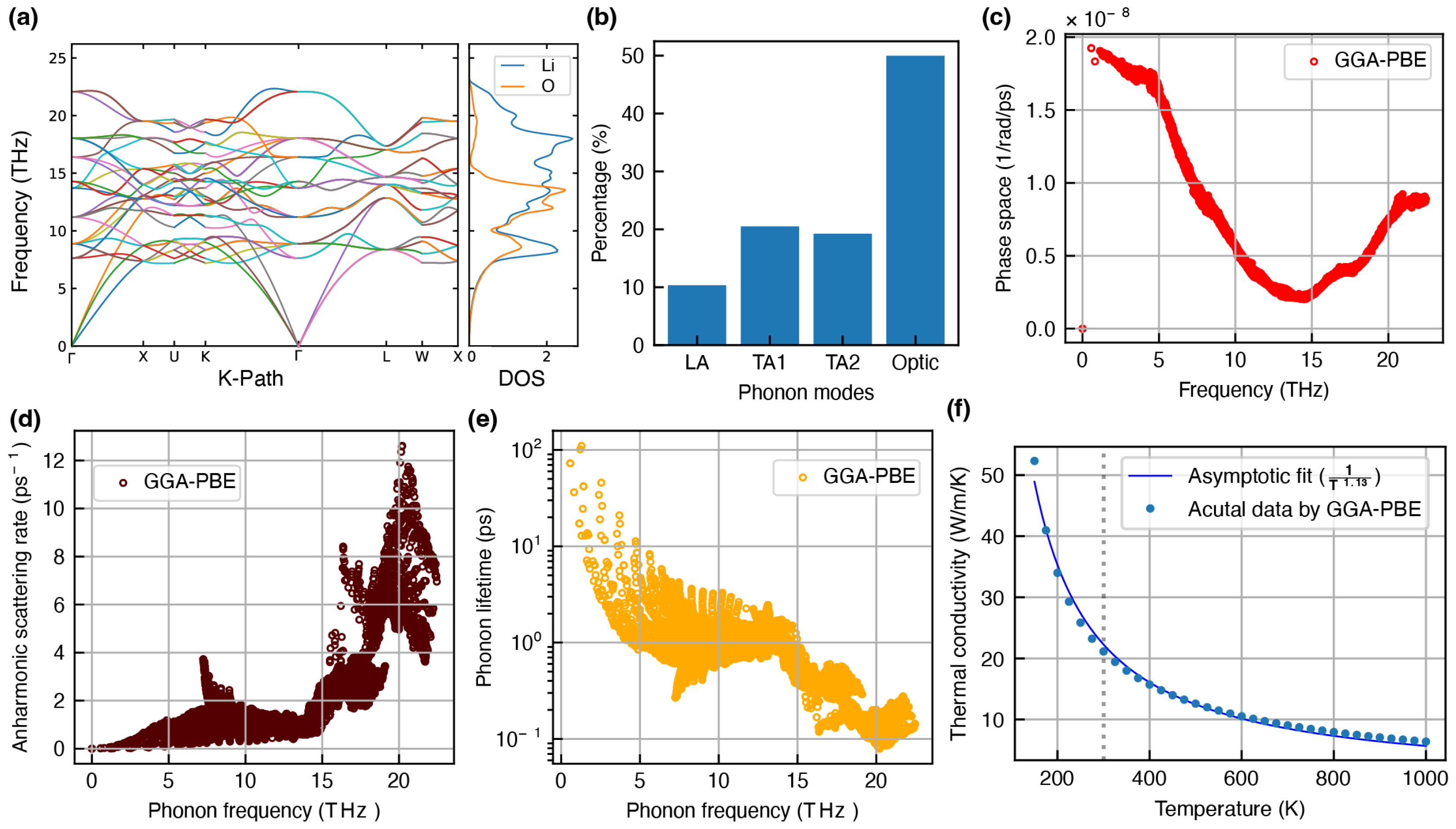
- Li2O: This study considers the face-centered cubic structure of Li2O (mp-1960), as illustrated in Figure 1b, which is reportedly stable under ambient pressure [53]. In this configuration, each Li atom is surrounded by a tetrahedral arrangement of O atoms. Li2O is a commonly reported SEI component that forms when low-voltage anodes come into contact with solid or liquid electrolytes containing oxygen [54]. With a high bandgap of 7–8 eV, Li2O exhibits strong insulating characteristics, effectively preventing any significant electron–electrolyte interaction [55]. This also suggests that heat transport in this material is predominantly governed by phonon scattering, with very minimal contribution from electron-mediated processes due to the limited availability of free charge carriers.
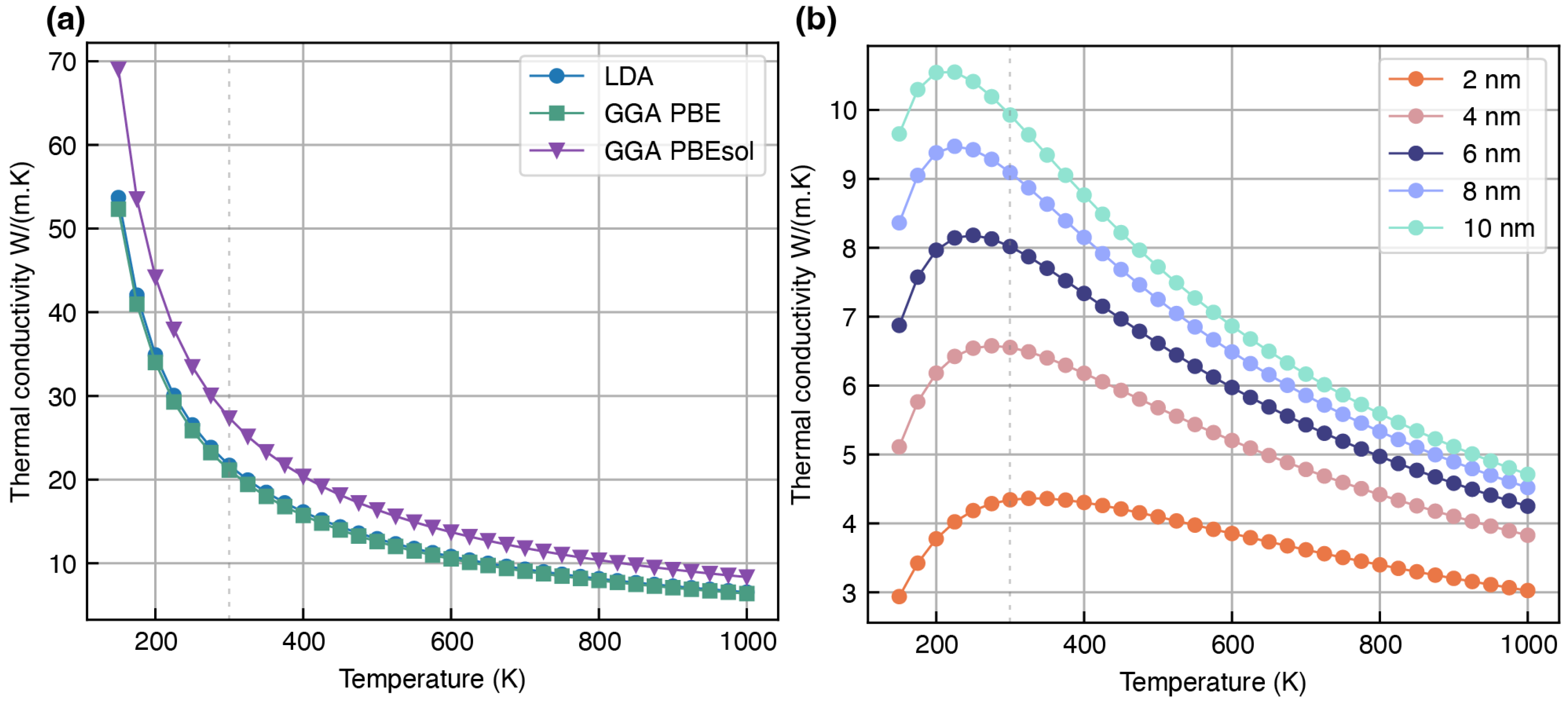
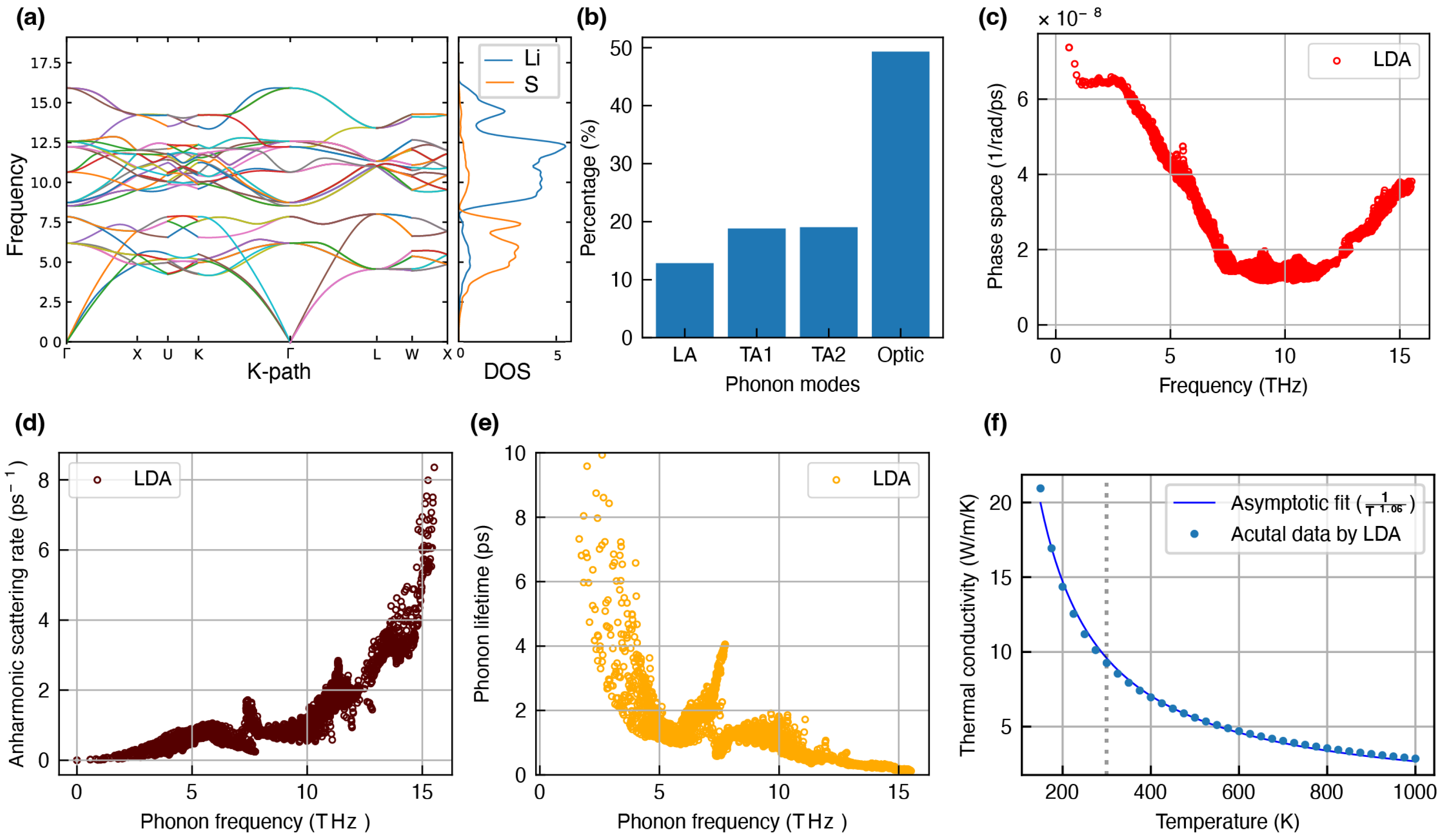
- Li2S: The presence of Li2S is confirmed in the SEI mainly when the electrolyte has polysulfide compounds of the argyrodite family [56]. Recent studies suggest that Li2S is also a promising candidate in the SEI for stable dendrite-free cycling [57,58]. This study employs the FCC structure of Li2S (Figure 1c) for phonon simulations, which shares a similar crystal structure to Li2O.
- Li2CO3: The presence of Li2CO3 reported in the SEI formed from the reduction of organic carbonate solvents, such as ethylene carbonate and propylene carbonates [1]. Fast Li-ion transport kinetics is one of the notable phenomena reported in SEI layers rich with Li2CO3 [60]. Although it is a good ionic conductor, Li2CO3 is known for its poor stability, leading to a dynamically evolving and porous SEI, especially with Li-metal electrodes [61]. Since the temperature dependence of the ionic conductivity of Li2CO3 is well known, the following discussion of its thermal transport properties provides valuable insights into the temperature-dependent Li-ion transport profile [62,63].
- LiOH: Earlier studies attributed the presence of LiOH in the SEI to water contamination [69,70]. However, more recent research suggests that LiOH forms naturally during SEI development [71,72]. Some studies reported that LiOH reduces battery efficiency by compositional transformation in the SEI from lithium ethylene dicarbonate to lithium ethylene monocarbonate, ultimately leading to capacity depletion [73]. Furthermore, when LiOH is part of the SEI, it is generally less conductive than other inorganic species, such as LiF, ultimately reducing the efficiency of the battery pack [74]. Despite its poisoning nature, LiOH can contribute to the stability of the SEI layer and can also undergo further reactions, especially in the presence of other components, such as hydrofluoric acid from the electrolyte, leading to the formation of more stable compounds such as LiF [75].
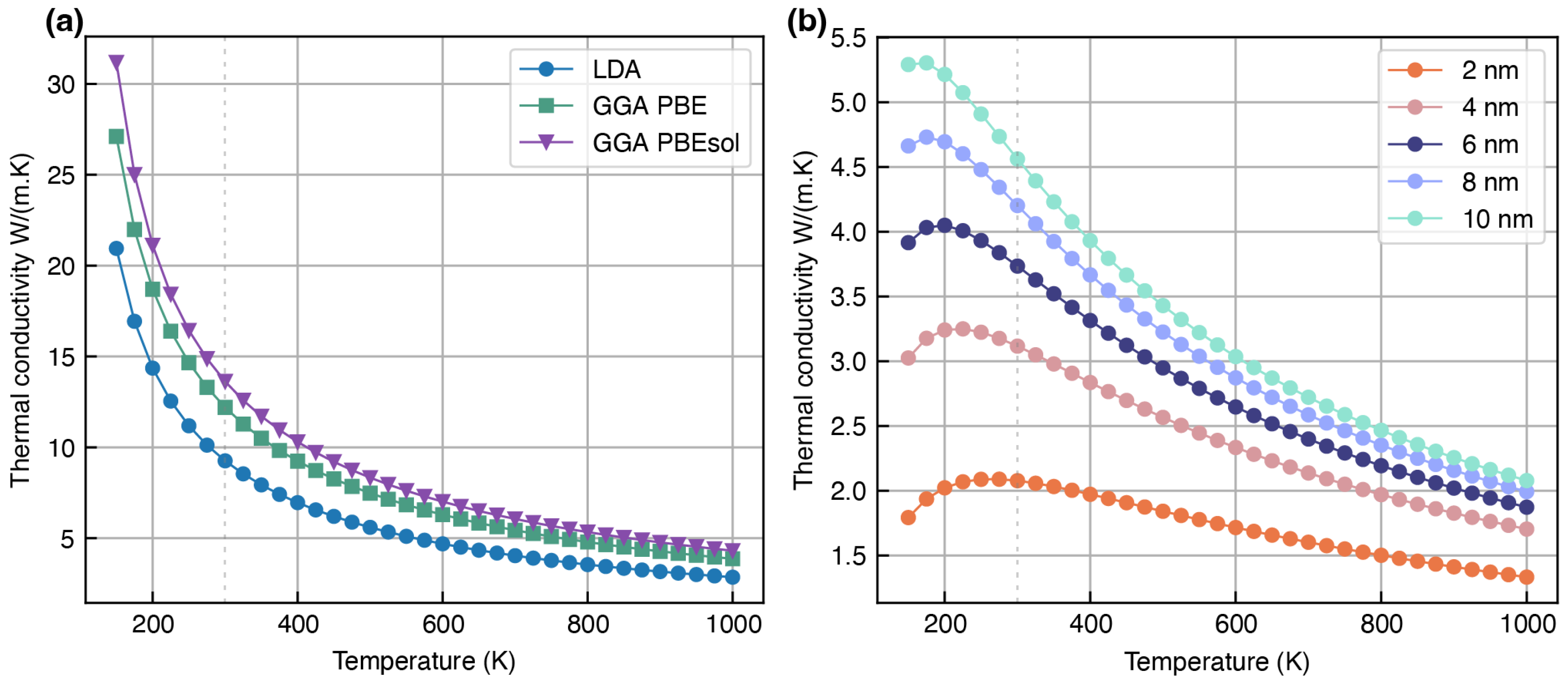
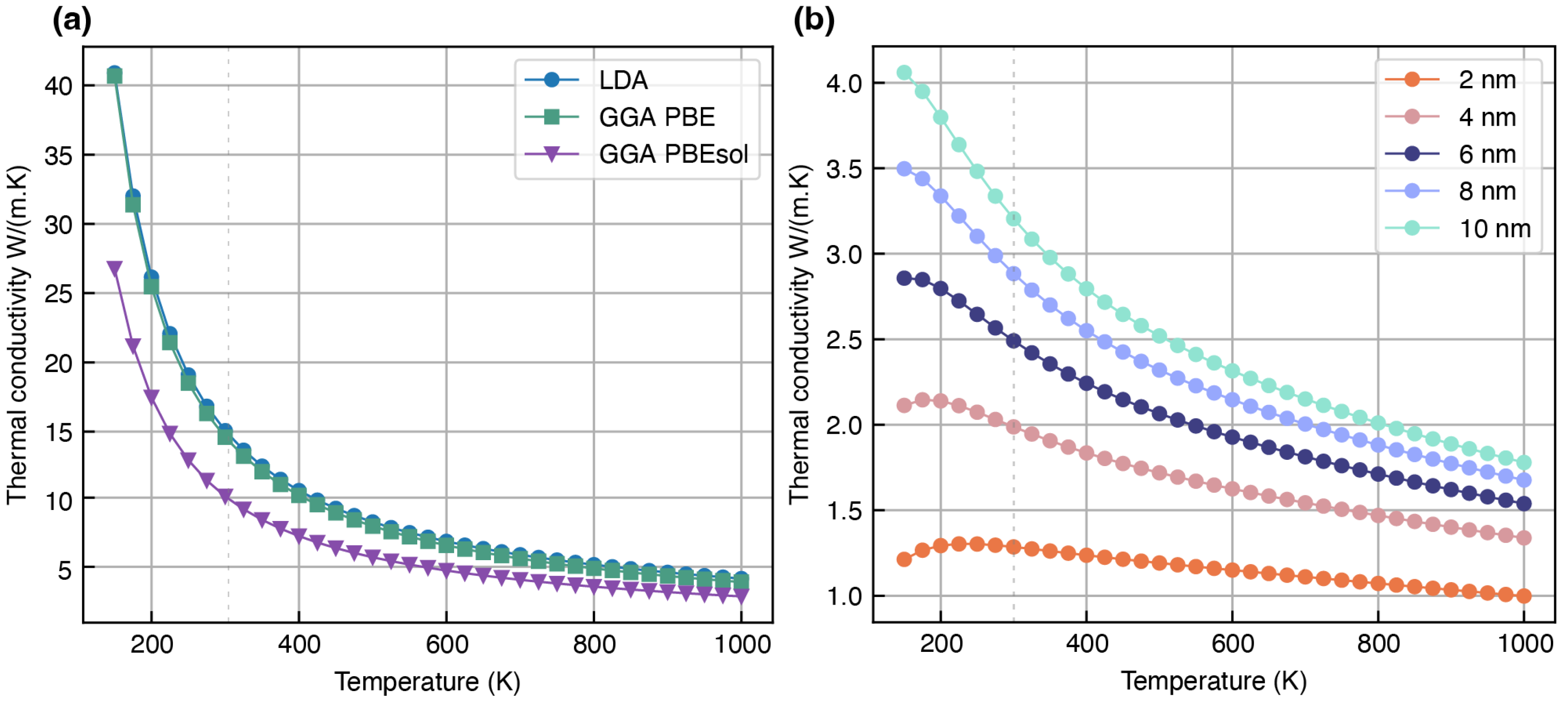
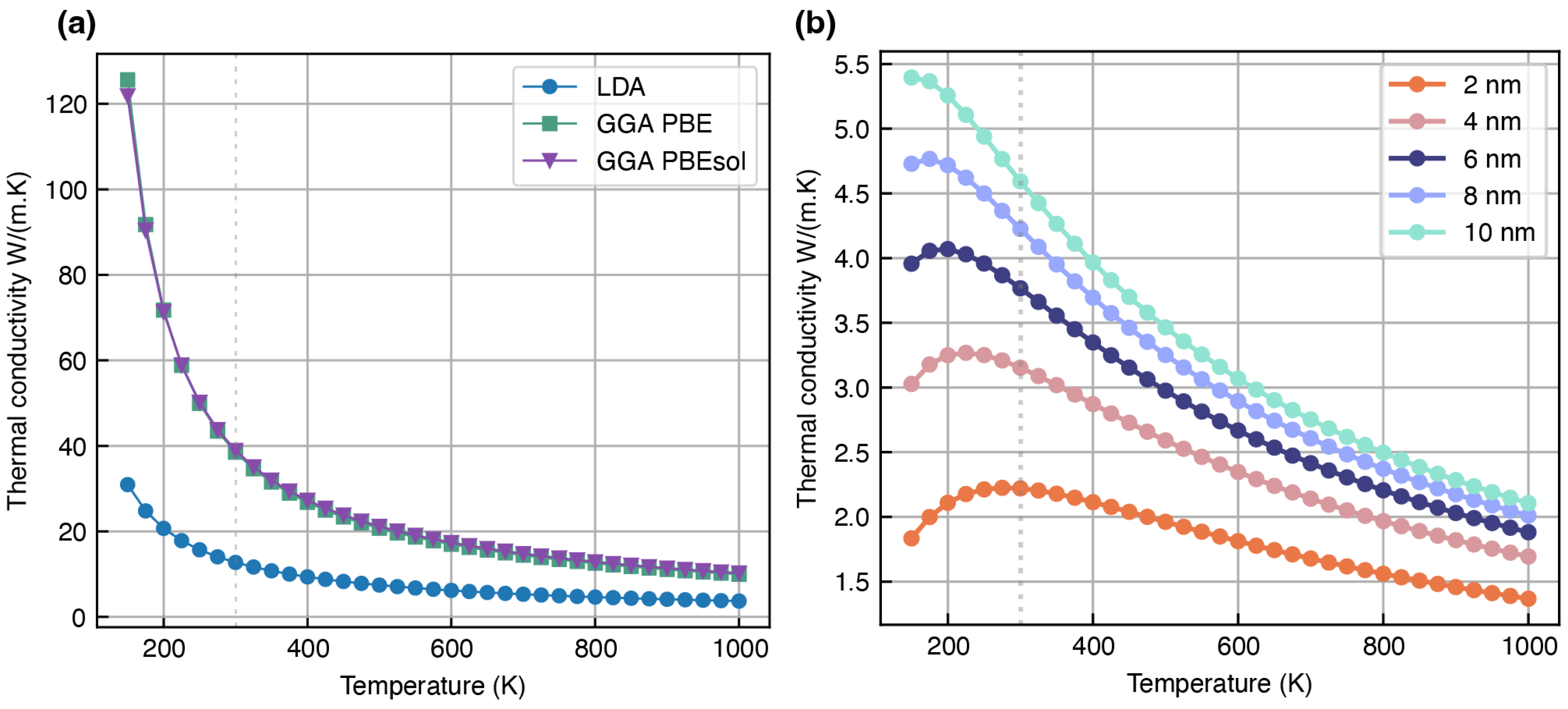
Overestimation of Lattice Thermal Conductivity
| Components | Present Work | Theoretical | Experimental | |||
|---|---|---|---|---|---|---|
| (mp-id) | LDA | GGA-PBE | GGA-PBEsol | |||
| LiF (mp-1138) | 13.01 | 12.54 | 18.65 | 13.48 a [24], 13.6 b [28], 14.8 c [29], 14.59 d [25] | 14.09 [30], 16.5 [31], 13.2 [79], 15.5 [79] | |
| Li2O (mp-1960) | 21.70 | 21.14 | 20.89 | 20 e [80], 22.38 f [81] | 15.52 [82] | |
| Li2S (mp-1153) | 9.42 | 12.90 | 13.02 | 11.96 i [81], 6 j [83] | N.A | |
| Li2CO3 * (mp-3054) | 12.03 | 11.44 | 9.26 | N.A. | 2.60 [65], 2.75 [66] | |
| 15.07 | 14.60 | 10.32 | N.A. | |||
| 9.84 | 11.42 | 9.27 | N.A. | |||
| 11.21 | 8.31 | 8.21 | N.A. | |||
| LiOH * (mp-23856) | 10.65 | 32.72 | 35.20 | N.A. | 0.69 [77], 1.69 [78,84] | |
| 12.78 | 38.53 | 38.94 | N.A. | |||
| 15.39 | 40.74 | 48.00 | N.A. | |||
| 3.78 | 18.91 | 18.66 | N.A. | |||
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Correction Statement
References
- Adenusi, H.; Chass, G.A.; Passerini, S.; Tian, K.V.; Chen, G. Lithium Batteries and the Solid Electrolyte Interphase (SEI)—Progress and Outlook. Adv. Energy Mater. 2023, 13, 2203307. [Google Scholar] [CrossRef]
- Yan, H.H.; Bie, Y.H.; Cui, X.Y.; Xiong, G.P.; Chen, L. A computational investigation of thermal effect on lithium dendrite growth. Energy Convers. Manag. 2018, 161, 193–204. [Google Scholar] [CrossRef]
- Peled, E. The Electrochemical Behavior of Alkali and Alkaline Earth Metals in Nonaqueous Battery Systems—The Solid Electrolyte Interphase Model. J. Electrochem. Soc. 1979, 126, 2047. [Google Scholar] [CrossRef]
- Aurbach, D.; Markovsky, B.; Levi, M.D.; Levi, E.; Schechter, A.; Moshkovich, M.; Cohen, Y. New insights into the interactions between electrode materials and electrolyte solutions for advanced nonaqueous batteries. J. Power Sources 1999, 81–82, 95–111. [Google Scholar] [CrossRef]
- Peled, E.; Golodnitsky, D.; Ardel, G. Advanced Model for Solid Electrolyte Interphase Electrodes in Liquid and Polymer Electrolytes. J. Electrochem. Soc. 1997, 144, L208. [Google Scholar] [CrossRef]
- Cheng, X.B.; Zhang, R.; Zhao, C.Z.; Wei, F.; Zhang, J.G.; Zhang, Q. A Review of Solid Electrolyte Interphases on Lithium Metal Anode. Adv. Sci. 2016, 3, 1500213. [Google Scholar] [CrossRef]
- Patil, A.; Patil, V.; Wook Shin, D.; Choi, J.W.; Paik, D.S.; Yoon, S.J. Issue and challenges facing rechargeable thin film lithium batteries. Mater. Res. Bull. 2008, 43, 1913–1942. [Google Scholar] [CrossRef]
- Wang, A.; Kadam, S.; Li, H.; Shi, S.; Qi, Y. Review on modeling of the anode solid electrolyte interphase (SEI) for lithium-ion batteries. npj Comput. Mater. 2018, 4, 15. [Google Scholar] [CrossRef]
- Kulathuvayal, A.S.; Su, Y. Ionic Transport through the Solid Electrolyte Interphase in Lithium-Ion Batteries: A Review from First-Principles Perspectives. ACS Appl. Energy Mater. 2023, 6, 5628–5645. [Google Scholar] [CrossRef]
- Cresce, A.v.; Russell, S.M.; Baker, D.R.; Gaskell, K.J.; Xu, K. In Situ and Quantitative Characterization of Solid Electrolyte Interphases. Nano Lett. 2014, 14, 1405–1412. [Google Scholar] [CrossRef] [PubMed]
- Shi, S.; Lu, P.; Liu, Z.; Qi, Y.; Hector, L.G., Jr.; Li, H.; Harris, S.J. Direct Calculation of Li-Ion Transport in the Solid Electrolyte Interphase. J. Am. Chem. Soc. 2012, 134, 15476–15487. [Google Scholar] [CrossRef] [PubMed]
- Peled, E.; Menkin, S. SEI: Past, present and future. J. Electrochem. Soc. 2017, 164, A1703. [Google Scholar] [CrossRef]
- Petibon, R.; Sinha, N.N.; Burns, J.C.; Aiken, C.P.; Ye, H.; VanElzen, C.M.; Jain, G.; Trussler, S.; Dahn, J.R. Comparative study of electrolyte additives using electrochemical impedance spectroscopy on symmetric cells. J. Power Sources 2014, 251, 187–194. [Google Scholar] [CrossRef]
- Ishikawa, M.; Tasaka, Y.; Yoshimoto, N.; Morita, M. Optimization of physicochemical characteristics of a lithium anode interface for high-efficiency cycling: An effect of electrolyte temperature. J. Power Sources 2001, 97–98, 262–264. [Google Scholar] [CrossRef]
- Edström, K.; Andersson, A.M.; Bishop, A.; Fransson, L.; Lindgren, J.; Hussénius, A. Carbon electrode morphology and thermal stability of the passivation layer. J. Power Sources 2001, 97–98, 87–91. [Google Scholar] [CrossRef]
- Kim, H.; Lee, J.T.; Yushin, G. High temperature stabilization of lithium–sulfur cells with carbon nanotube current collector. J. Power Sources 2013, 226, 256–265. [Google Scholar] [CrossRef]
- Ota, H.; Wang, X.; Yasukawa, E. Characterization of Lithium Electrode in Lithium Imides/Ethylene Carbonate, and Cyclic Ether Electrolytes: I. Surface Morphology and Lithium Cycling Efficiency. J. Electrochem. Soc. 2004, 151, A427. [Google Scholar] [CrossRef]
- Mogi, R.; Inaba, M.; Jeong, S.K.; Iriyama, Y.; Abe, T.; Ogumi, Z. Effects of Some Organic Additives on Lithium Deposition in Propylene Carbonate. J. Electrochem. Soc. 2002, 149, A1578. [Google Scholar] [CrossRef]
- Zhu, Y.; Xie, J.; Pei, A.; Liu, B.; Wu, Y.; Lin, D.; Li, J.; Wang, H.; Chen, H.; Xu, J.; et al. Fast lithium growth and short circuit induced by localized-temperature hotspots in lithium batteries. Nat. Commun. 2019, 10, 2067. [Google Scholar] [CrossRef]
- Liu, W.; Liu, P.; Mitlin, D. Review of Emerging Concepts in SEI Analysis and Artificial SEI Membranes for Lithium, Sodium, and Potassium Metal Battery Anodes. Adv. Energy Mater. 2020, 10, 2002297. [Google Scholar] [CrossRef]
- Wu, H.; Jia, H.; Wang, C.; Zhang, J.G.; Xu, W. Recent Progress in Understanding Solid Electrolyte Interphase on Lithium Metal Anodes. Adv. Energy Mater. 2021, 11, 2003092. [Google Scholar] [CrossRef]
- Liu, J.; Fan, L.W. Atomistic insights into the thermal transport properties of inorganic components of solid electrolyte interphase (SEI) in lithium-ion batteries. Int. J. Heat Mass Transf. 2024, 221, 125069. [Google Scholar] [CrossRef]
- Ma, S.; Jiang, M.; Tao, P.; Song, C.; Wu, J.; Wang, J.; Deng, T.; Shang, W. Temperature effect and thermal impact in lithium-ion batteries: A review. Prog. Nat. Sci. Mater. Int. 2018, 28, 653–666. [Google Scholar] [CrossRef]
- Liang, T.; Chen, W.Q.; Hu, C.E.; Chen, X.R.; Chen, Q.F. Lattice dynamics and thermal conductivity of lithium fluoride via first-principles calculations. Solid State Commun. 2018, 272, 28–32. [Google Scholar] [CrossRef]
- Lindsay, L. Isotope scattering and phonon thermal conductivity in light atom compounds: LiH and LiF. Phys. Rev. B 2016, 94, 174304. [Google Scholar] [CrossRef]
- Jones, R.E.; Ward, D.K. Estimates of crystalline LiF thermal conductivity at high temperature and pressure by a Green-Kubo method. Phys. Rev. B 2016, 94, 014309. [Google Scholar] [CrossRef]
- Wang, J.; Deng, M.; Chen, Y.; Liu, X.; Ke, W.; Li, D.; Dai, W.; He, K. Structural, elastic, electronic and optical properties of lithium halides (LiF, LiCl, LiBr, and LiI): First-principle calculations. Mater. Chem. Phys. 2020, 244, 122733. [Google Scholar] [CrossRef]
- Singh, B.K.; Roy, M.K.; Menon, V.J.; Sood, K.C. Effects of dispersion, correction term, and isotopes on the thermal conductivity of LiF crystal. Phys. Rev. B 2003, 67, 014302. [Google Scholar] [CrossRef]
- Gheribi, A.E.; Chartrand, P. Application of the CALPHAD method to predict the thermal conductivity in dielectric and semiconductor crystals. Calphad 2012, 39, 70–79. [Google Scholar] [CrossRef]
- A. V Petrov, N.S.T.; Logachev, Y.A. Temperature dependence of the thermal conductivity of alkali metal halides at elevated temperatures. Fiz. Tverd. Tela 1974, 65–70. [Google Scholar] [CrossRef]
- Basiev, T.T.; Konyushkin, V.A.; Kuznetsov, S.V.; Osiko, V.V.; Popov, P.A.; Fedorov, P.P. Thermal conductivity of γ-irradiated LiF single crystals. Tech. Phys. Lett. 2008, 34, 702–704. [Google Scholar] [CrossRef]
- Zemann, J. Crystal Structures, 2nd ed.; Wyckoff, R.W.G., Ed.; Interseience Publishers: New York, NY, USA; London, UK; Sydney, Australia, 1963. [Google Scholar] [CrossRef]
- Nakamura, H.; Machida, M. First-principles calculation study on phonon thermal conductivity of thorium and plutonium dioxides: Intrinsic anharmonic phonon-phonon and extrinsic grain-boundary–phonon scattering effects. J. Nucl. Mater. 2019, 519, 45–51. [Google Scholar] [CrossRef]
- Lin, Y.X.; Liu, Z.; Leung, K.; Chen, L.Q.; Lu, P.; Qi, Y. Connecting the irreversible capacity loss in Li-ion batteries with the electronic insulating properties of solid electrolyte interphase (SEI) components. J. Power Sources 2016, 309, 221–230. [Google Scholar] [CrossRef]
- Mikajlo, E.A.; Nixon, K.L.; Coleman, V.A.; Ford, M.J. The electronic band structure of Li2O:testing theoretical predictions using electron momentumspectroscopy. J. Phys. Condens. Matter 2002, 14, 3587. [Google Scholar] [CrossRef]
- Feng, M.; Pan, J.; Qi, Y. Impact of Electronic Properties of Grain Boundaries on the Solid Electrolyte Interphases (SEIs) in Li-ion Batteries. J. Phys. Chem. C 2021, 125, 15821–15829. [Google Scholar] [CrossRef]
- Duan, Y.; Sorescu, D.C. Density functional theory studies of the structural, electronic, and phonon properties of Li2O and Li2CO3: Application to CO2 capture reaction. Phys. Rev. B 2009, 79, 014301. [Google Scholar] [CrossRef]
- Stoneham, A. Theory of Defects in Solids: Electronic Structure of Defects in Insulators and Semiconductors; Oxford classic texts in the physical sciences; Clarendon Press: Oxford, UK, 2001. [Google Scholar]
- Baer, Y.; Schoenes, J. Electronic structure and optical properties of lithium compounds. J. Phys. C Solid State Phys. 1979, 12, 4481–4491. [Google Scholar] [CrossRef]
- Bersuker, I.B. The Jahn–Teller Effect; Cambridge University Press: Cambridge, UK, 2006. [Google Scholar] [CrossRef]
- Li, W.; Carrete, J.; A. Katcho, N.; Mingo, N. ShengBTE: A solver of the Boltzmann transport equation for phonons. Comput. Phys. Commun. 2014, 185, 1747–1758. [Google Scholar] [CrossRef]
- Togo, A.; Chaput, L.; Tadano, T.; Tanaka, I. Implementation strategies in phonopy and phono3py. J. Phys. Condens. Matter 2023, 35, 353001. [Google Scholar] [CrossRef]
- Togo, A. First-principles Phonon Calculations with Phonopy and Phono3py. J. Phys. Soc. Jpn. 2023, 92, 012001. [Google Scholar] [CrossRef]
- Chaput, L. Direct Solution to the Linearized Phonon Boltzmann Equation. Phys. Rev. Lett. 2013, 110, 265506. [Google Scholar] [CrossRef]
- Srivastava, G.P. The Physics of Phonons; Taylor & Francis: Oxford, UK, 2022. [Google Scholar] [CrossRef]
- Ziman, J. Electrons and Phonons: The Theory of Transport Phenomena in Solids; International series of monographs on physics; OUP Oxford: Oxford, UK, 2001. [Google Scholar]
- Tong, Z.; Li, S.; Ruan, X.; Bao, H. Comprehensive first-principles analysis of phonon thermal conductivity and electron-phonon coupling in different metals. Phys. Rev. B 2019, 100, 144306. [Google Scholar] [CrossRef]
- Togo, A.; Chaput, L.; Tadano, T.; Tanaka, I. Distributions of phonon lifetimes in Brillouin zones. Phys. Rev. B 2015, 91, 094306. [Google Scholar] [CrossRef]
- Tritt, T. Thermal Conductivity: Theory, Properties, and Applications; Physics of Solids and Liquids; Springer: New York, NY, USA, 2004; ISBN 978-0-306-48327-1. [Google Scholar] [CrossRef]
- Tan, J.; Matz, J.; Dong, P.; Shen, J.; Ye, M. A Growing Appreciation for the Role of LiF in the Solid Electrolyte Interphase. Adv. Energy Mater. 2021, 11, 2100046. [Google Scholar] [CrossRef]
- Ko, J.; Yoon, Y.S. Recent progress in LiF materials for safe lithium metal anode of rechargeable batteries: Is LiF the key to commercializing Li metal batteries? Ceram. Int. 2019, 45, 30–49. [Google Scholar] [CrossRef]
- Cahill, D.G.; Watson, S.K.; Pohl, R.O. Lower limit to the thermal conductivity of disordered crystals. Phys. Rev. B 1992, 46, 6131–6140. [Google Scholar] [CrossRef]
- Goel, P.; Choudhury, N.; Chaplot, S.L. Superionic behavior of lithium oxide Li2O: A lattice dynamics and molecular dynamics study. Phys. Rev. B 2004, 70, 174307. [Google Scholar] [CrossRef]
- Richards, W.D.; Miara, L.J.; Wang, Y.; Kim, J.C.; Ceder, G. Interface Stability in Solid-State Batteries. Chem. Mater. 2016, 28, 266–273. [Google Scholar] [CrossRef]
- Lorger, S.; Usiskin, R.; Maier, J. Transport and Charge Carrier Chemistry in Lithium Oxide. J. Electrochem. Soc. 2019, 166, A2215. [Google Scholar] [CrossRef]
- Aktekin, B.; Riegger, L.M.; Otto, S.K.; Fuchs, T.; Henss, A.; Janek, J. SEI growth on Lithium metal anodes in solid-state batteries quantified with coulometric titration time analysis. Nat. Commun. 2023, 14, 6946. [Google Scholar] [CrossRef]
- Liu, F.; Wang, L.; Zhang, Z.; Shi, P.; Feng, Y.; Yao, Y.; Ye, S.; Wang, H.; Wu, X.; Yu, Y. A Mixed Lithium-Ion Conductive Li2S/Li2Se Protection Layer for Stable Lithium Metal Anode. Adv. Funct. Mater. 2020, 30, 2001607. [Google Scholar] [CrossRef]
- Li, G.; Gao, Y.; He, X.; Huang, Q.; Chen, S.; Kim, S.H.; Wang, D. Organosulfide-plasticized solid-electrolyte interphase layer enables stable lithium metal anodes for long-cycle lithium-sulfur batteries. Nat. Commun. 2017, 8, 850. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.S.D.H.; Lee, M.S.B.; Park, K.Y.; Kang, K. First-principles Study on the Charge Transport Mechanism of Lithium Sulfide (Li2S) in Lithium-Sulfur Batteries. Chem. Asian J. 2016, 11, 1288–1292. [Google Scholar] [CrossRef]
- Wu, D.; He, J.; Liu, J.; Wu, M.; Qi, S.; Wang, H.; Huang, J.; Li, F.; Tang, D.; Ma, J. Li2CO3/LiF-Rich Heterostructured Solid Electrolyte Interphase with Superior Lithiophilic and Li+-Transferred Characteristics via Adjusting Electrolyte Additives. Adv. Energy Mater. 2022, 12, 2200337. [Google Scholar] [CrossRef]
- Han, B.; Zhang, Z.; Zou, Y.; Xu, K.; Xu, G.; Wang, H.; Meng, H.; Deng, Y.; Li, J.; Gu, M. Poor Stability of Li2CO3 in the Solid Electrolyte Interphase of a Lithium-Metal Anode Revealed by Cryo-Electron Microscopy. Adv. Mater. 2021, 33, 2100404. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.C.; Tagawa, H.; Asakura, S.; Mizusaki, J.; Narita, H. Solid-state electrochemical CO2 sensor by coupling lithium ion conductor (Li2CO3-Li3PO4-Asl2O3) with oxide ion-electron mixed conductor (La0.9Sr0.1MnO3). Solid State Ionics 1997, 100, 275–281. [Google Scholar] [CrossRef]
- Weng, S.; Zhang, X.; Yang, G.; Zhang, S.; Ma, B.; Liu, Q.; Liu, Y.; Peng, C.; Chen, H.; Yu, H.; et al. Temperature-dependent interphase formation and Li+ transport in lithium metal batteries. Nat. Commun. 2023, 14, 4474. [Google Scholar] [CrossRef]
- Shang, S.-L.; Hector, L.G.; Shi, S.; Qi, Y.; Wang, Y.; Liu, Z.-K. Lattice dynamics, thermodynamics and elastic properties of monoclinic Li2CO3 from density functional theory. Acta Mater. 2012, 60, 5204–5216. [Google Scholar] [CrossRef]
- Verdier, D.; Ferrière, A.; Falcoz, Q.; Siros, F.; Couturier, R. Experimentation of a High Temperature Thermal Energy Storage Prototype Using Phase Change Materials for the Thermal Protection of a Pressurized Air Solar Receiver. Energy Procedia 2014, 49, 1044–1053. [Google Scholar] [CrossRef]
- Wang, Y.; Li, S.; Wu, N.; Jia, Q.; Hoke, T.; Shi, L.; Li, Y.; Chen, X. Thermal properties and lattice anharmonicity of Li-ion conducting garnet solid electrolyte Li6.5La3Zr1.5Ta0.5O12. J. Mater. Chem. A 2024, 12, 18248–18257. [Google Scholar] [CrossRef]
- Spiteri, D.; Anaya, J.; Kuball, M. The effects of grain size and grain boundary characteristics on the thermal conductivity of nanocrystalline diamond. J. Appl. Phys. 2016, 119, 085102. [Google Scholar] [CrossRef]
- Wang, X.; Yang, Y.; Zhu, L. Effect of grain sizes and shapes on phonon thermal conductivity of bulk thermoelectric materials. J. Appl. Phys. 2011, 110, 024312. [Google Scholar] [CrossRef]
- Fujieda, T.; Xia, Y.; Koike, S.; Shikano, M.; Sakai, T. Effect of acid on passivation of a copper electrode in LiCF3SO3/propylene carbonate in underpotential region. J. Power Sources 1999, 83, 186–192. [Google Scholar] [CrossRef]
- Kanamura, K.; Tamura, H.; Takehara, Z.i. XPS analysis of a lithium surface immersed in propylene carbonate solution containing various salts. J. Electroanal. Chem. 1992, 333, 127–142. [Google Scholar] [CrossRef]
- Li, Z.; Ganapathy, S.; Xu, Y.; Heringa, J.R.; Zhu, Q.; Chen, W.; Wagemaker, M. Understanding the electrochemical formation and decomposition of Li2O2 and LiOH with operando X-ray diffraction. Chem. Mater. 2017, 29, 1577–1586. [Google Scholar] [CrossRef] [PubMed]
- Morigaki, K.i.; Ohta, A. Analysis of the surface of lithium in organic electrolyte by atomic force microscopy, Fourier transform infrared spectroscopy and scanning auger electron microscopy. J. Power Sources 1998, 76, 159–166. [Google Scholar] [CrossRef]
- Zhao, Z.; Ye, W.; Zhang, F.; Pan, Y.; Zhuo, Z.; Zou, F.; Xu, X.; Sang, X.; Song, W.; Zhao, Y.; et al. Revealing the effect of LiOH on forming a SEI using a Co magnetic “probe”. Chem. Sci. 2023, 14, 12219–12230. [Google Scholar] [CrossRef]
- Ma, X.X.; Shen, X.; Chen, X.; Fu, Z.H.; Yao, N.; Zhang, R.; Zhang, Q. The Origin of Fast Lithium-Ion Transport in the Inorganic Solid Electrolyte Interphase on Lithium Metal Anodes. Small Struct. 2022, 3, 2200071. [Google Scholar] [CrossRef]
- Lu, M.; Cheng, H.; Yang, Y. A comparison of solid electrolyte interphase (SEI) on the artificial graphite anode of the aged and cycled commercial lithium ion cells. Electrochim. Acta 2008, 53, 3539–3546. [Google Scholar] [CrossRef]
- Achchaq, F.; Moon, S.C.; Legros, P. Unlocking the power of LiOH: Key to next-generation ultra-compact thermal energy storage systems. Heliyon 2024, 10, e33992. [Google Scholar] [CrossRef]
- Yang, X.; Li, S.; Zhao, J.; Wang, X.; Huang, H.; Wang, Y.; Deng, L. Development of lithium hydroxide-metal organic framework-derived porous carbon composite materials for efficient low temperature thermal energy storage. Microporous Mesoporous Mater. 2021, 328, 111455. [Google Scholar] [CrossRef]
- Wang, Y.; Gu, J.; Li, X.; Zhu, Y.; Hu, H.; Liu, H.; Luo, G.; Yao, H. Boosted Thermal Storage Performance of LiOH·H2O by Carbon Nanotubes Isolated Multilayered Graphene Oxide Frames. Adv. Mater. Sci. Eng. 2022, 2022, 3109178. [Google Scholar] [CrossRef]
- Elhadj, S.; Matthews, M.J.; Yang, S.T.; Cooke, D.J.; Stolken, J.S.; Vignes, R.M.; Draggoo, V.G.; Bisson, S.E. Determination of the intrinsic temperature dependent thermal conductivity from analysis of surface temperature of laser irradiated materials. Appl. Phys. Lett. 2010, 96, 071110. [Google Scholar] [CrossRef]
- Lu, H.; Murphy, S.T.; Rushton, M.J.D.; Parfitt, D.C.; Grimes, R.W. Thermal conductivity and the isotope effect in Li2O. Fusion Eng. Des. 2012, 87, 1834–1838. [Google Scholar] [CrossRef]
- Yuan, X.; Zhao, Y.; Sun, Y.; Ni, J.; Dai, Z. Influence of quartic anharmonicity on lattice dynamics and thermal transport properties of 16 antifluorite structures. Phys. Rev. B 2024, 110, 014304. [Google Scholar] [CrossRef]
- Dodd, J.; Baker, L.; Kennedy, P.; Challender, R.; Coast, G. The Use of Lithium Oxide as the Breeder in Fusion Reactors. 1989. Available online: http://inis.iaea.org/search/search.aspx?orig_q=RN:21038114 (accessed on 1 March 2025).
- Zhou, Y.; Xiong, S.; Zhang, X.; Volz, S.; Hu, M. Thermal transport crossover from crystalline to partial-crystalline partial-liquid state. Nat. Commun. 2018, 9, 4712. [Google Scholar] [CrossRef]
- Li, S.; Huang, H.; Li, J.; Kobayashi, N.; Osaka, Y.; He, Z.; Yuan, H. The effect of 3D carbon nanoadditives on lithium hydroxide monohydrate based composite materials for highly efficient low temperature thermochemical heat storage. RSC Adv. 2018, 8, 8199–8208. [Google Scholar] [CrossRef]
- Wu, B.; Yi, R.; Xu, Y.; Gao, P.; Bi, Y.; Novák, L.; Liu, Z.; Hu, E.; Wang, N.; Rijssenbeek, J.; et al. Unusual Li2O sublimation promotes single-crystal growth and sintering. Nat. Energy 2025, 10, 605–615. [Google Scholar] [CrossRef]
- Han, B.; Anwar U., I.; Haq, R.; Louhi-Kultanen, M. Lithium carbonate precipitation by homogeneous and heterogeneous reactive crystallization. Hydrometallurgy 2020, 195, 105386. [Google Scholar] [CrossRef]
- Chen, C.; Liu, P.; Lu, C. Synthesis and characterization of nano-sized ZnO powders by direct precipitation method. Chem. Eng. J. 2008, 144, 509–513. [Google Scholar] [CrossRef]
- Tomboc, G.M.; Wang, Y.; Wang, H.; Li, J.; Lee, K. Sn-based metal oxides and sulfides anode materials for Na ion battery. Energy Storage Mater. 2021, 39, 21–44. [Google Scholar] [CrossRef]
- Zheng, L.; Li, G.; Zhang, J. Grain boundaries boost the prelithiation capability of the Li2CO3 cathode additives for high-energy-density lithium-ion batteries. Chem. Eng. J. 2023, 475, 146285. [Google Scholar] [CrossRef]



| Components * | a (Å) | b (Å) | c (Å) | (°) | (°) | (°) | Lattice Volume () |
|---|---|---|---|---|---|---|---|
| LiF (mp-1138) | 3.90, 4.08, 4.00 | 3.90, 4.08, 4.00 | 3.90, 4.08, 4.00 | 90, 90, 90 | 90, 90, 90 | 90, 90, 90 | 59.55, 60.09, 64.17 |
| Li2O (mp-1960) | 4.50, 4.65, 4.58 | 4.50, 4.65, 4.58 | 4.50, 4.65, 4.58 | 90, 90, 90 | 90, 90, 90 | 90, 90, 90 | 91.14, 100.78, 96.37 |
| Li2S (mp-1153) | 5.56, 5.67, 5.65 | 5.56, 5.67, 5.65 | 5.56, 5.67, 5.65 | 90, 90, 90 | 90, 90, 90 | 90, 90, 90 | 172.49, 182.32, 181.23 |
| Li2CO3 (mp-3054) | 8.22, 8.28, 8.31 | 4.94, 4.96, 4.98 | 5.89, 6.07, 6.11 | 90, 90, 90 | 113.56, 113.74, 113.94 | 90, 90, 90 | 219.66, 228.89, 231.85 |
| LiOH (mp-23856) | 4.81, 4.93, 4.94 | 4.81, 4.93, 4.94 | 4.32, 4.27, 4.32 | 90, 90, 90 | 90, 90, 90 | 90, 90, 90 | 100.04, 104.09, 105.69 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kulathuvayal, A.S.; Su, Y. Grain Size- and Temperature-Dependent Phonon-Mediated Heat Transport in the Solid Electrolyte Interphase: A First-Principles Study. Modelling 2025, 6, 89. https://doi.org/10.3390/modelling6030089
Kulathuvayal AS, Su Y. Grain Size- and Temperature-Dependent Phonon-Mediated Heat Transport in the Solid Electrolyte Interphase: A First-Principles Study. Modelling. 2025; 6(3):89. https://doi.org/10.3390/modelling6030089
Chicago/Turabian StyleKulathuvayal, Arjun S., and Yanqing Su. 2025. "Grain Size- and Temperature-Dependent Phonon-Mediated Heat Transport in the Solid Electrolyte Interphase: A First-Principles Study" Modelling 6, no. 3: 89. https://doi.org/10.3390/modelling6030089
APA StyleKulathuvayal, A. S., & Su, Y. (2025). Grain Size- and Temperature-Dependent Phonon-Mediated Heat Transport in the Solid Electrolyte Interphase: A First-Principles Study. Modelling, 6(3), 89. https://doi.org/10.3390/modelling6030089






