Discrete-Event Simulation in Healthcare Settings: A Review
Abstract
1. Introduction
1.1. Background
1.2. The Need for DES in Healthcare
1.3. Advantages
1.4. Disadvantages
2. Materials and Methods
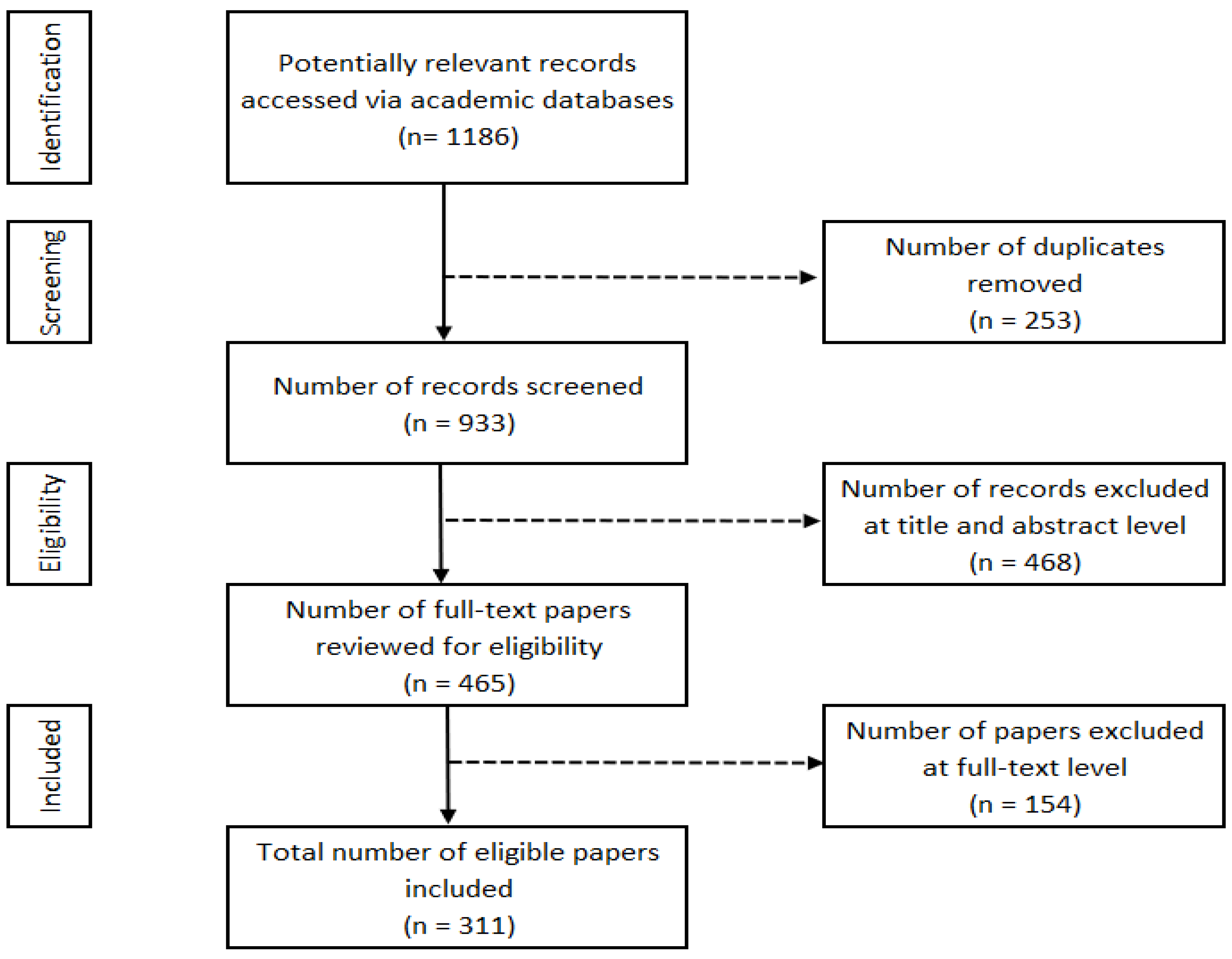
2.1. Literature Search Strategy
- (1)
- have healthcare delivery as the primary system of interest,
- (2)
- use discrete-event simulation as the primary modeling tool,
- (3)
- (4)
- quantify the inputs to and results from the study, and
- (5)
- appear in peer-reviewed academic journals.
2.2. Search Results
3. Results
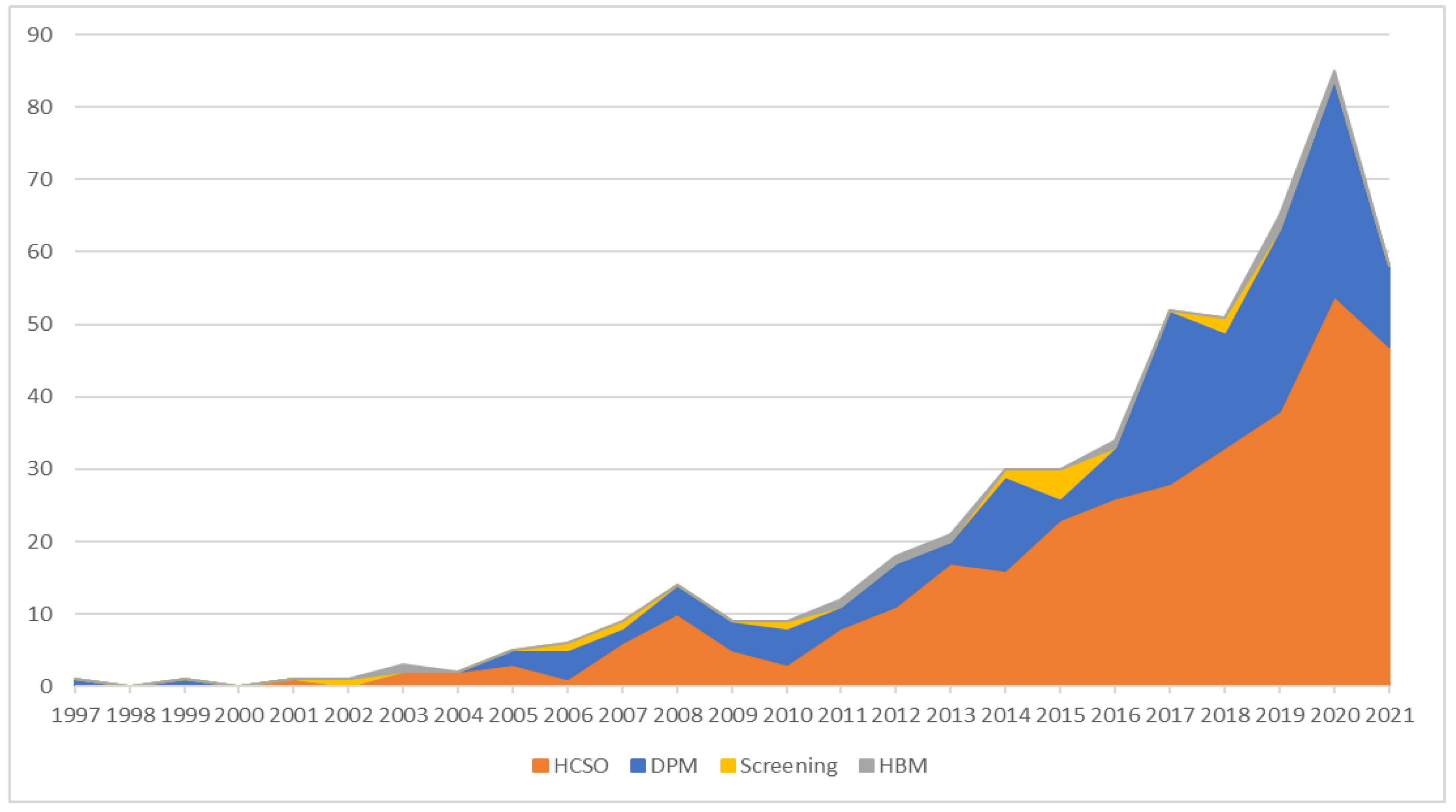
3.1. Healthcare DES over Time
3.2. Studies by Disease Process
3.3. Disease Progression Modeling
3.4. Healthcare System Operation
HCSO Sub-Categories
3.5. Screening Modeling
3.6. Human Behavior Modeling
3.7. Location Distribution
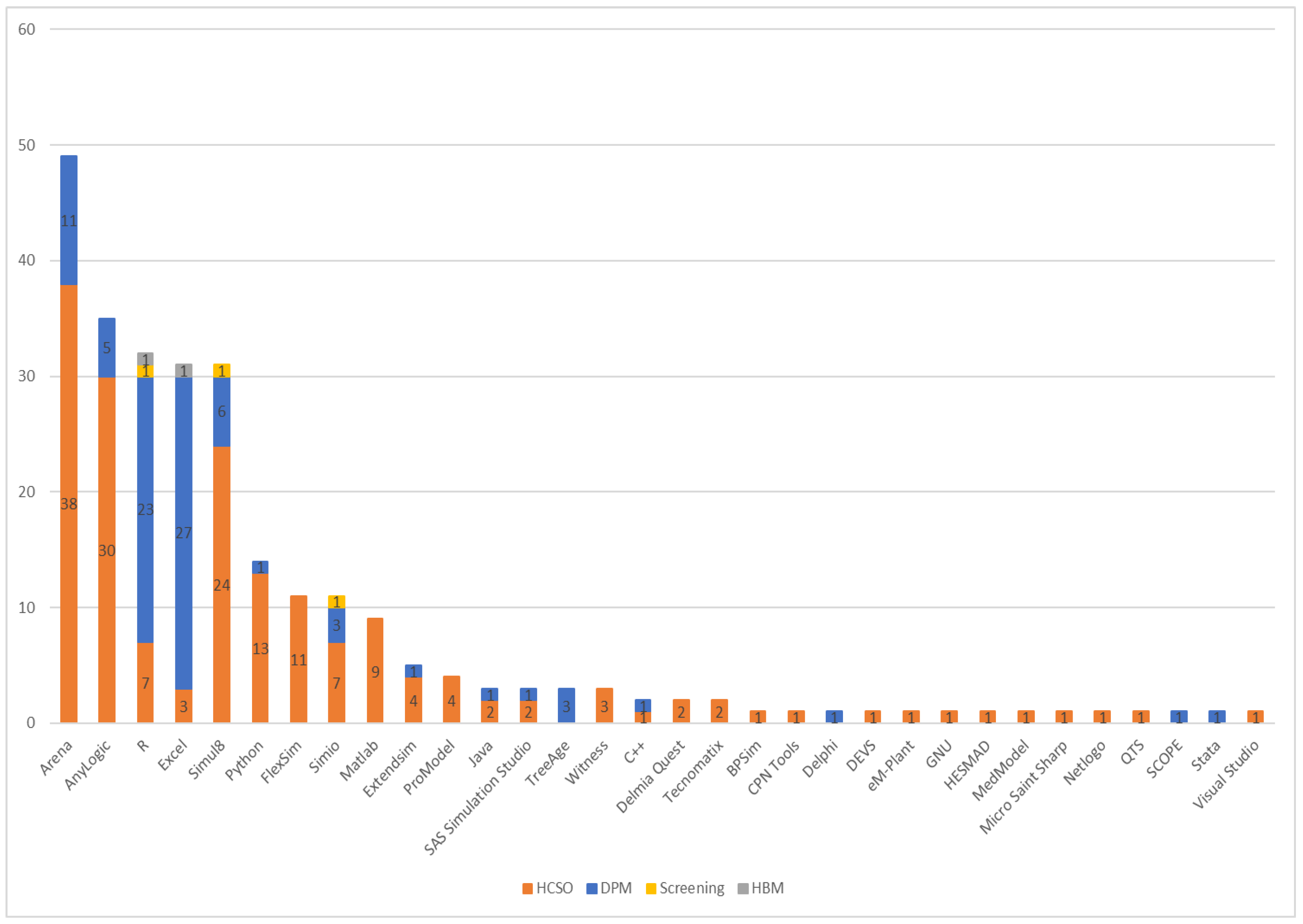
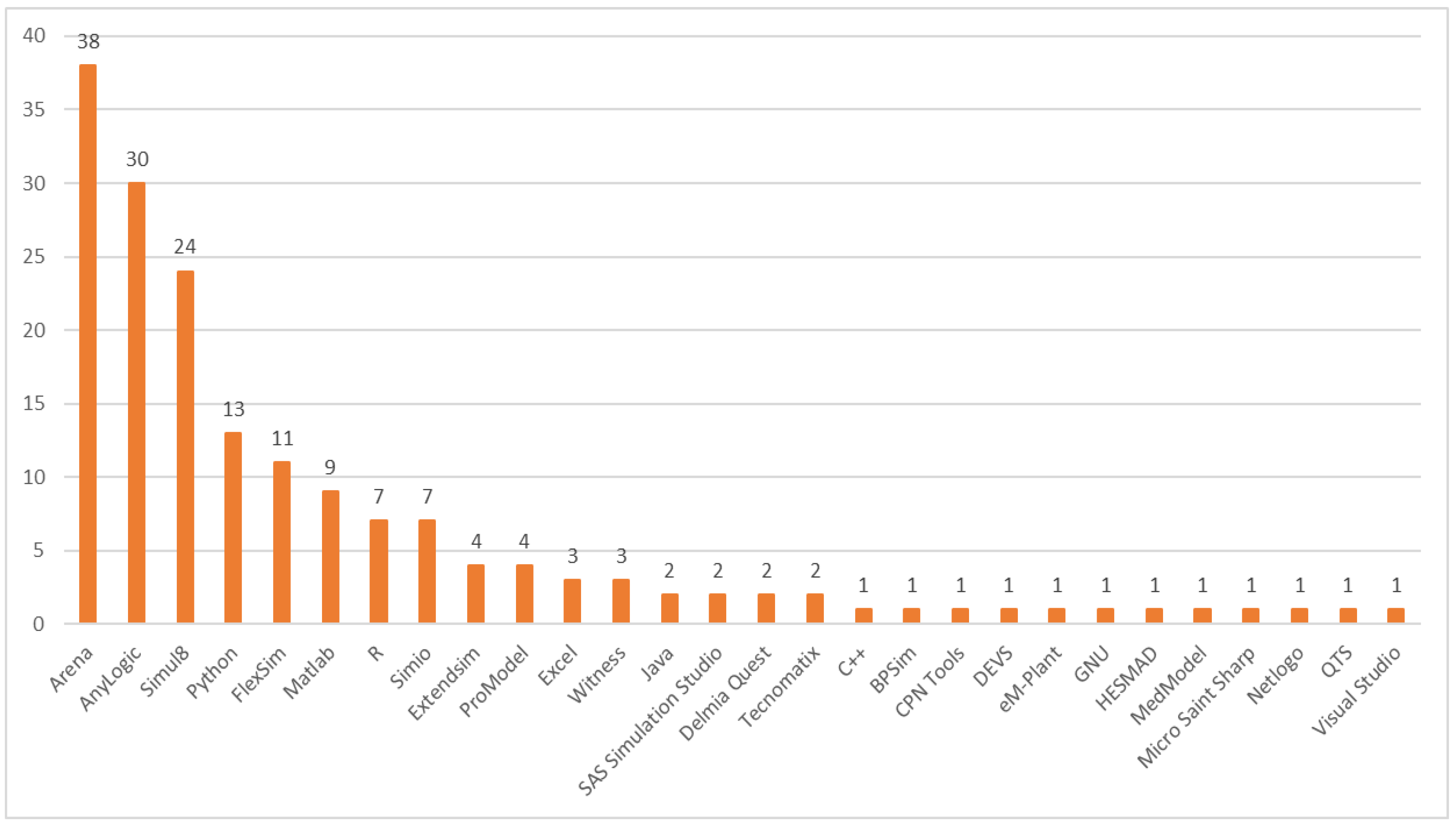
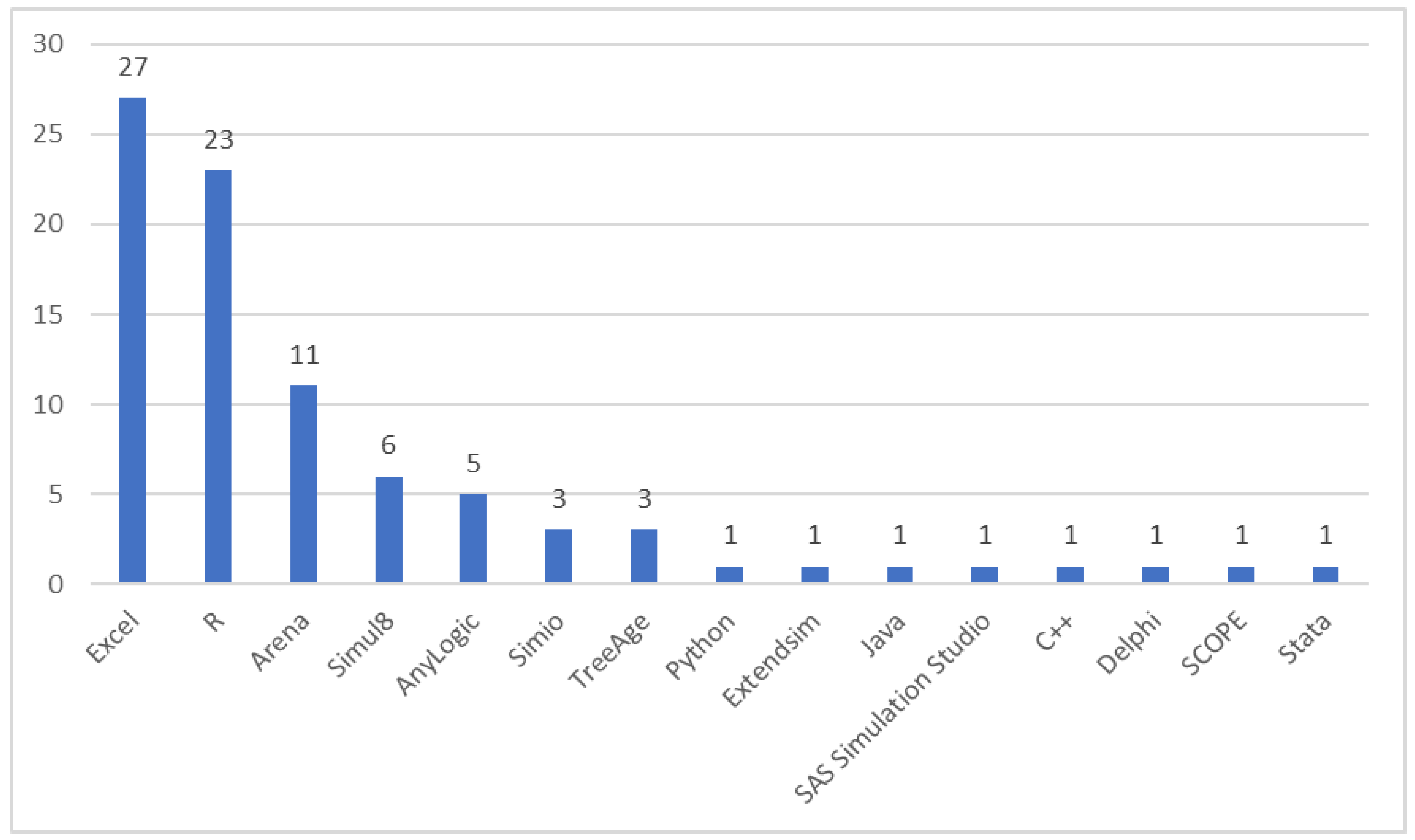
3.8. Software Usage
- a.
- Random number generator to represent stochastic uncertainty
- b.
- Process transformers to convert random numbers to statistical distributions
- c.
- List processors to add, delete, and manipulate sets and set members
- d.
- Statistical analysis routines to summarize model behavior
- e.
- Report generators to present large data outputs
- f.
- Timing mechanism to explicitly represent the flow of time
3.9. Simulation Size
3.10. Patient Demographics
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ABM | Agent-Based Model |
| DES | Discrete Event Simulation |
| DPM | Disease Progression Management |
| ED | Emergency Department |
| EMT | Emergency Medical Transport |
| GPSS | General Purpose Simulation System |
| HBM | Health Behavior Modeling |
| HCSO | Health Care System Operations |
| ISPOR-SMDM | The Professional Society for Health Economics and Outcomes Research (formerly International Society for Pharmacoeconomics and Outcomes Research) Society for Medical Decision Making |
| PDSA | Plan-Do-Study-Act |
| PRISMA | Preferred Reporting Items for Systematic Reviews and MetaAnalyses |
| QALY | Quality-Adjusted Life Years |
References
- Banks, J. Handbook of Simulation: Principles, Methodology, Advances, Applications and Practice; Wiley: Hoboken, NJ, USA, 1998. [Google Scholar]
- Allen, T.T. Introduction to Discrete Event Simulation and Agent-Based Modeling: Voting Systems, Health Care, Military, and Manufacturing; Springer: London, UK, 2011. [Google Scholar]
- Robinson, S. Discrete-Event Simulation: From the Pioneers to the Present, What Next? J. Oper. Res. Soc. 2005, 56, 619–629. [Google Scholar] [CrossRef]
- Crane, M.A.; Iglehart, D.L. Simulating stable stochastic systems: III. Regenerative Processes and Discrete-Event Simulations. Oper. Res. 1975, 23, 33–45. [Google Scholar] [CrossRef]
- Babulak, E.; Wang, M. Discrete Event Simulation: State of the Art. Discret. Event Simul. 2010, 4, 60–63. [Google Scholar] [CrossRef]
- Schroer, B.J.; Tseng, F.T. Modelling Complex Manufacturing Systems Using Discrete Event Simulation. Comput. Ind. Eng. 1988, 14, 455–464. [Google Scholar] [CrossRef]
- Marcinko, D.E. Recognizing the Differences Between Healthcare and Other Industries. The Leading Business Education Network for Doctors, Financial Advisors and Health Industry Consultants. 31 October 2014. Available online: http://medicalexecutivepost.com/2011/01/19/recognizing-the-differences-between-healthcare-and-other-industries/ (accessed on 4 August 2022).
- Nguyen, L.K.N.; Megiddo, I.; Howick, S. Simulation Models for Transmission of Health Care-Associated Infection: A Systematic Review. Am. J. Infect. Control. 2020, 48, 810–821. [Google Scholar] [CrossRef]
- Lockner, A.M.; Walcker, C.A. INSIGHT: The Healthcare Industry’s Shift from Fee-for-Service to Value-Based Reimbursement. Bloomberg BNA News. 26 September 2018. Available online: http://news.bloomberglaw.com/health-law-and-business/insight-the-healthcare-industrys-shift-from-fee-for-service-to-value-based-reimbursement (accessed on 4 August 2022).
- Dall, T.M.; Gallo, P.D.; Chakrabarti, R.; West, T.; Semilla, A.P.; Storm, M.V. An Aging Population and Growing Disease Burden Will Require a Large and Specialized Health Care Workforce by 2025. Health Aff. 2013, 32, 2013–2020. [Google Scholar] [CrossRef] [PubMed]
- de Koning, H.; Verver, J.P.; van den Heuvel, J.; Bisgaard, S.; Does, R.J. Lean Six Sigma in Healthcare. J. Healthc. Qual. 2006, 28, 4–11. [Google Scholar] [CrossRef]
- Taylor, M.J.; McNicholas, C.; Nicolay, C.; Darzi, A.; Bell, D.; Reed, J.E. Systematic Review of the Application of the Plan-Do-Study-Act Method to Improve Quality in Healthcare. BMJ Qual. Saf. 2013, 23, 290–298. [Google Scholar] [CrossRef] [PubMed]
- Donnelly, P.; Kirk, P. Use the PDSA Model for Effective Change Management. Educ. Prim. Care 2015, 26, 279–281. [Google Scholar] [CrossRef]
- Zhao, Y.; Peng, Q.; Strome, T.; Weldon, E.; Zhang, M.; Chochinov, A. Bottleneck Detection for Improvement of Emergency Department Efficiency. Bus. Process Manag. J. 2015, 21, 564–585. [Google Scholar] [CrossRef]
- Zhang, X. Application of Discrete Event Simulation in Health Care: A Systematic Review. BMC Health Serv. Res. 2018, 18, 687. [Google Scholar] [CrossRef] [PubMed]
- Caro, J.J.; Möller, J. Advantages and Disadvantages of Discrete-Event Ssimulation for Health Economic Analyses. Expert Rev. Pharm. Outcomes Res. 2016, 16, 327–329. [Google Scholar] [CrossRef]
- Standfield, L.B.; Comans, T.A.; Scuffham, P.A. An Empirical Comparison of Markov Cohort Modeling and Discrete Event Simulation in a Capacity-Constrained Health Care Setting. Eur. J. Health Econ. 2015, 18, 33–47. [Google Scholar] [CrossRef]
- Sharma, P. Discrete-Event Simulation. Int. J. Sci. Technol. Res. 2015, 4, 136–140. [Google Scholar]
- Marshall, D.A.; Burgos-Liz, L.; IJzerman, M.J.; Crown, W.; Padula, W.V.; Wong, P.; Pasupathy, K.S.; Higashi, M.K.; Osgood, N. Selecting a Dynamic Simulation Modeling Method for Health Care Delivery Research—Part 2: Report of the ISPOR Dynamic Simulation Modeling Emerging Good Practices Task Force. Value Health 2015, 18, 147–160. [Google Scholar] [CrossRef]
- Marshall, D.A.; Burgos-Liz, L.; IJzerman, M.J.; Osgood, N.; Padula, W.V.; Higashi, M.; Wong, P.; Pasupathy, K.; Crown, W. Applying Dynamic Simulation Modeling Methods in Health Care Delivery Research—The SIMULATE Checklist: Report of the ISPOR Simulation Modeling Emerging Good Practices Task Force. Value Health 2015, 18, 5–16. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; The PRISMA Group. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. Phys. Ther. 2009, 89, 873–880. [Google Scholar] [CrossRef]
- Bardet, A.; Fraslin, A.M.; Marghadi, J.; Borget, I.; Faron, M.; Honoré, C.; Delaloge, S.; Albiges, L.; Planchard, D.; Ducreux, M.; et al. Impact of COVID-19 on Healthcare organisation and Cancer Outcomes. Eur. J. Cancer 2021, 153, 123–132. [Google Scholar] [CrossRef]
- Wood, R.M.; Murch, B.J.; Moss, S.J.; Tyler, J.M.B.; Thompson, A.L.; Vasilakis, C. Operational Research for the Safe and Effective Design of COVID-19 Mass Vaccination Centres. Vaccine 2021, 39, 3537–3540. [Google Scholar] [CrossRef]
- Willis, M.; Asseburg, C.; Slee, A.; Nilsson, A.; Neslusan, C. Development and Internal Validation of a Discrete Event Simulation Model of Diabetic Kidney Disease Using CREDENCE Trial Data. Diabetes Ther. 2020, 11, 2657–2676. [Google Scholar] [CrossRef]
- Dutta, D.; Parry, F.; Obaid, M.; Ramadurai, G. Mechanical Thrombectomy in Stroke—Planning for Service Expansion Using Discrete Event Simulation. Future Healthc. J. 2020, 7, 65–71. [Google Scholar] [CrossRef]
- Lahr, M.M.H.; Maas, W.J.; van der Zee, D.-J.; Uyttenboogaart, M.; Buskens, E. Rationale and Design for Studying Organisation of Care for Intra-arterial Thrombectomy in The Netherlands: Simulation Modelling Study. BMJ Open 2020, 10, e037084. [Google Scholar] [CrossRef] [PubMed]
- Ambavane, A.; Yang, S.; Atkins, M.B.; Rao, S.; Shah, A.; Regan, M.M.; McDermott, D.F.; Michaelson, M.D. Clinical and economic outcomes of treatment sequences for intermediate- to poor-risk advanced renal cell carcinoma. Immunotherapy 2020, 12, 37–51. [Google Scholar] [CrossRef] [PubMed]
- Bae, S.; Karnon, J.; Crane, G.; Bessen, T.; Desai, J.; Crowe, P.; Neuhaus, S. Cost-effectiveness analysis of imaging surveillance in stage II and III extremity soft tissue sarcoma: An Australian perspective. Cost Eff. Resour. Alloc. 2020, 18, 5. [Google Scholar] [CrossRef] [PubMed]
- Dieleman, J.M.; Myles, P.S.; Bulfone, L.; Younie, S.; Van Zaane, B.; McGiffin, D.; Moodie, M.; Gao, L. Cost-effectiveness of routine transoesophageal echocardiography during cardiac surgery: A discrete-event simulation study. Br. J. Anaesth 2019, 124, 136–145. [Google Scholar] [CrossRef]
- Soorapanth, S.; Young, T. Assessing the Value of Modelling and Simulation in Health Care: An example Based on Increasing Access to Stroke Treatment. J. Oper. Res. Soc. 2019, 70, 226–236. [Google Scholar] [CrossRef]
- Nawaz, R.; Maqsood, S.; Baber, A.R. Analysis of Emergency Medical Response Service in shawar through Simulation. Mehran Univ. Res. J. Eng. Technol. 2019, 38, 1033–1044. [Google Scholar] [CrossRef]
- Furushima, D.; Yamada, H.; Kido, M.; Ohno, Y. The Impact of One-Dose Package of Medicines on Patient Waiting Time in Dispensing Pharmacy: Application of a Discrete Event Simulation Model. Biolog. Pharm. Bull. 2018, 41, 409–418. [Google Scholar] [CrossRef]
- Al-Fandi, L.M.; Obaid, A.A.B.; Alfailakawi, B.I.; Alsubaiei, H.A.; Khudhair, S.A. A simulation study to determine the param-eters of medicine inventory policy. Proc. Est. Acad. Sci. 2019, 68, 376–382. [Google Scholar] [CrossRef]
- Sewell, B.; Jones, M.; Gray, H.; Wilkes, H.; Lloyd-Bennett, C.; Beddow, K.; Bevan, M.; Fitzsimmons, D. Rapid Cancer Diagnosis For Patients With Vague Symptoms: A Cost-effectiveness Study. Br. J. Gen. Pract. 2020, 70, e186–e192. [Google Scholar] [CrossRef]
- Campbell, J.R.; Johnston, J.C.; Cook, V.J.; Sadatsafavi, M.; Elwood, R.K.; Marra, F. Cost-effectiveness of Latent Tuberculosis Infection Screening before Immigration to Low-Incidence Countries. Emerg. Infect. Dis. 2019, 25, 661–671. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Graves, J.A.; Garbett, S.P.; Zhou, Z.; Marathi, R.; Wang, X.; Harrell, F.E.; Lasko, T.A.; Denny, J.C.; Roden, D.M.; et al. A Decision-Theoretic Approach to Panel-Based, Preemptive Genotyping. MDM Policy Pract. 2019, 4, 2381468319864337. [Google Scholar] [CrossRef] [PubMed]
- Nau, C.; Kumanyika, S.; Gittelsohn, J.; Adam, A.; Wong, M.S.; Mui, Y.; Lee, B.Y. Identifying Financially Sustainable Pricing Interventions to Promote Healthier Beverage Purchases in Small Neighborhood Stores. Prev. Chronic. Dis. 2018, 15, 160611. [Google Scholar] [CrossRef] [PubMed]
- Pennington, B.; Filby, A.; Owen, L.; Taylor, M. Smoking Cessation: A Comparison of Two Model Structures. Pharmacoeconomics 2018, 36, 1101–1112. [Google Scholar] [CrossRef] [PubMed]
- Nance, R. A history of discrete event simulation programming languages. In History of Programming Languages; Bergin, T., Ed.; ACM Press: New York, NY, USA, 1996; Chapter VIII. [Google Scholar]
- Dias, L.M.S.; Vieira, A.A.C.; Pereira, G.A.B.; Oliveira, J.A. Discrete simulation software ranking—A top list of the worldwide most popular and used tools. In Proceedings of the 2016 Winter Simulation Conference, Washington, DC, USA, 11–14 December 2016. [Google Scholar]
- Tofighi, M.; Asgary, A.; Merchant, A.A.; Shafiee, M.A.; Najafabadi, M.M.; Nadri, N.; Aarabi, M.; Heffernan, J.; Wu, J.; Provenzano, M. Modelling COVID-19 Transmission in a Hemodialysis Centre Using Simulation Generated Contacts Matrices. PLoS ONE 2021, 16, e0259970. [Google Scholar] [CrossRef]
- Zehrouni, A.; Augusto, V.; Garaix, T.; Phan, R.; Xie, X.; Denis, S.; Gentile, M. Hospital Flood Emergency Management Planning Using Markov Models and Discrete-Event Simulation. Oper. Res. Health Care 2021, 30, 100310. [Google Scholar] [CrossRef]
- Ni, W.; Jiang, Y. Evaluation on the Cost-Effective Threshold of Osteoporosis Treatment on Elderly Women in China Using Discrete Event Simulation Model. Osteoporos. Int. 2016, 28, 529–538. [Google Scholar] [CrossRef]
- Gray, E.; Donten, A.; Karssemeijer, N.; van Gils, C.; Evans, D.G.; Astley, S.; Payne, K. Evaluation of a Stratified National Breast Screening Program in the United Kingdom: An Early Model-Based Cost-Effectiveness Analysis. Value Health 2017, 20, 1100–1109. [Google Scholar] [CrossRef]
- Husereau, D.; Drummond, M.; Augustovski, F.; de Bekker-Grob, E.; Briggs, A.H.; Carswell, C.; Caulley, L.; Chaiyakunapruk, N.; Greenberg, D.; Loder, E.; et al. Consolidated Health Economic Evaluation Reporting Standards 2022 (CHEERS 2022) Explanation and Elaboration: A report of the ISPOR CHEERS II Good Practices Task Force. Value Health 2022, 25, 10–31. [Google Scholar] [CrossRef]






| Outpatient clinic | 63 | 31.50% | Inpatient hospital | 62 | 31.00% | ||
| General | 12 | 6.00% | Multi-department | 17 | 8.50% | ||
| Hematology Oncology | 8 | 4.00% | Surgery | 13 | 6.50% | ||
| Ophthalmology/Optometry | 8 | 4.00% | MedSurg unit | 6 | 3.00% | ||
| Primary care | 6 | 3.00% | ICU | 5 | 2.50% | ||
| Vaccine | 4 | 2.00% | Radiology | 5 | 2.50% | ||
| Ob/Gyn | 3 | 1.50% | General | 4 | 2.00% | ||
| Vascular | 3 | 1.50% | Acute care | 1 | 0.50% | ||
| Ambulatory surgery | 2 | 1.00% | Administration | 1 | 0.50% | ||
| Cardiac | 2 | 1.00% | Cardiac | 1 | 0.50% | ||
| Orthopedics | 2 | 1.00% | Gerontology | 1 | 0.50% | ||
| Telemedicine | 2 | 1.00% | Hospice care | 1 | 0.50% | ||
| Urgent care | 2 | 1.00% | NICU | 1 | 0.50% | ||
| Laboratory | 2 | 1.00% | Oncology | 1 | 0.50% | ||
| Dermatology | 1 | 0.50% | Pathology lab | 1 | 0.50% | ||
| Dentistry | 1 | 0.50% | Pediatric | 1 | 0.50% | ||
| Maternal/child health | 1 | 0.50% | Pharmacy | 1 | 0.50% | ||
| Neurology | 1 | 0.50% | Psychiatric ward | 1 | 0.50% | ||
| Pediatric | 1 | 0.50% | Dialysis | 1 | 0.50% | ||
| Radiology | 1 | 0.50% | |||||
| Sexual health | 1 | 0.50% | Pharmacy (Stand-alone) | 1 | 0.50% | ||
| Emergency Department | 49 | 24.50% | Emergency Medical Service | 1 | 0.50% | ||
| Multi-facility provider | 12 | 6.00% | Distributor (Medical Warehouse) | 1 | 0.50% | ||
| Healthcare System | 11 | 5.50% | |||||
| Age | |||
|---|---|---|---|
| Focus | Adult | Pediatric | Family |
| HCSO | 190 | 9 | 1 |
| DPM | 103 | 3 | |
| Screening | 3 | ||
| HBM | 2 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Forbus, J.J.; Berleant, D. Discrete-Event Simulation in Healthcare Settings: A Review. Modelling 2022, 3, 417-433. https://doi.org/10.3390/modelling3040027
Forbus JJ, Berleant D. Discrete-Event Simulation in Healthcare Settings: A Review. Modelling. 2022; 3(4):417-433. https://doi.org/10.3390/modelling3040027
Chicago/Turabian StyleForbus, John J., and Daniel Berleant. 2022. "Discrete-Event Simulation in Healthcare Settings: A Review" Modelling 3, no. 4: 417-433. https://doi.org/10.3390/modelling3040027
APA StyleForbus, J. J., & Berleant, D. (2022). Discrete-Event Simulation in Healthcare Settings: A Review. Modelling, 3(4), 417-433. https://doi.org/10.3390/modelling3040027





