Electrochemical Model-Based Investigation of Thick LiFePO4 Electrode Design Parameters
Abstract
:1. Introduction
2. Model Development
2.1. Electrochemical Model
2.2. Electrolyte Equations
2.3. Electrode Equations
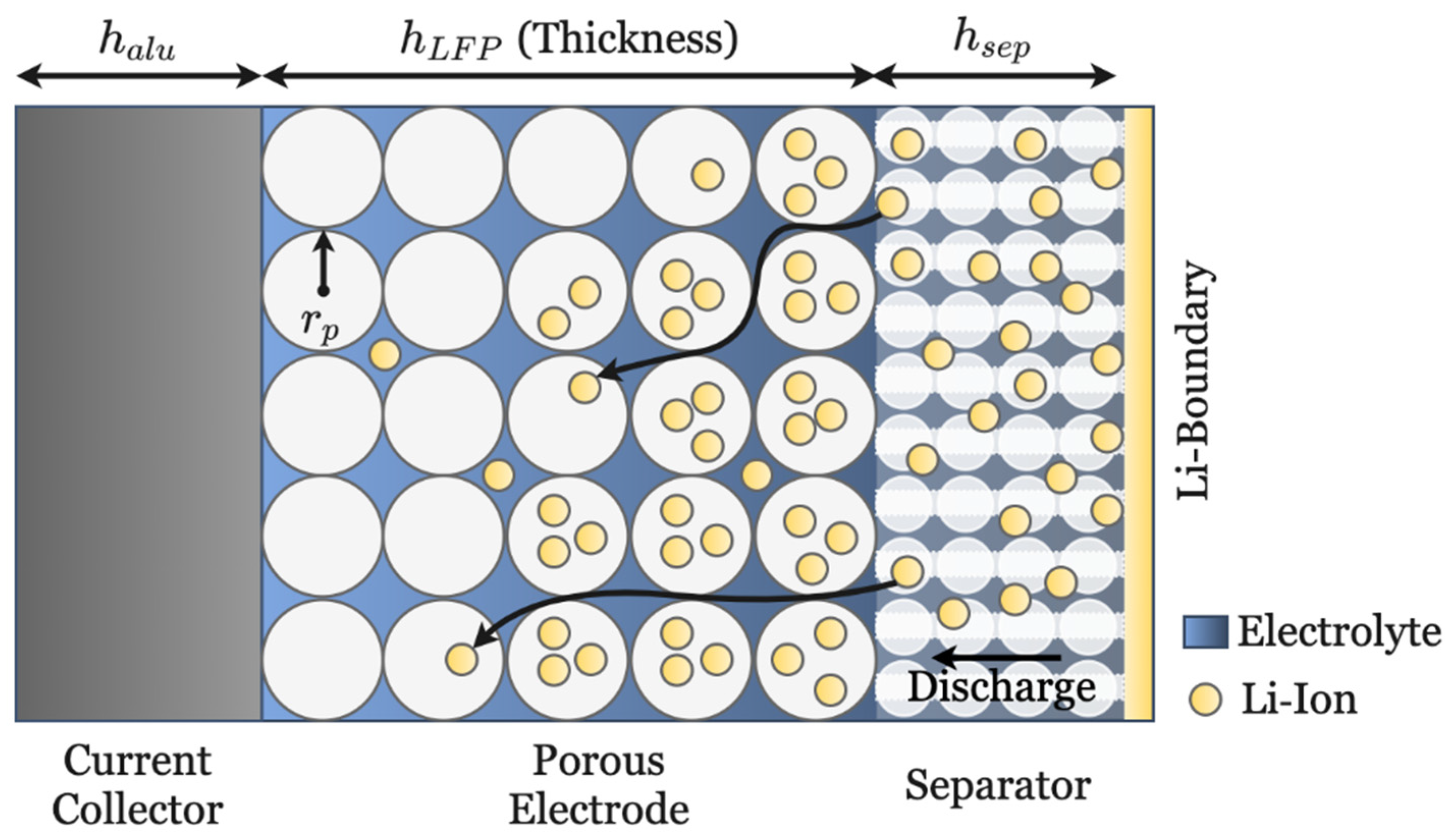
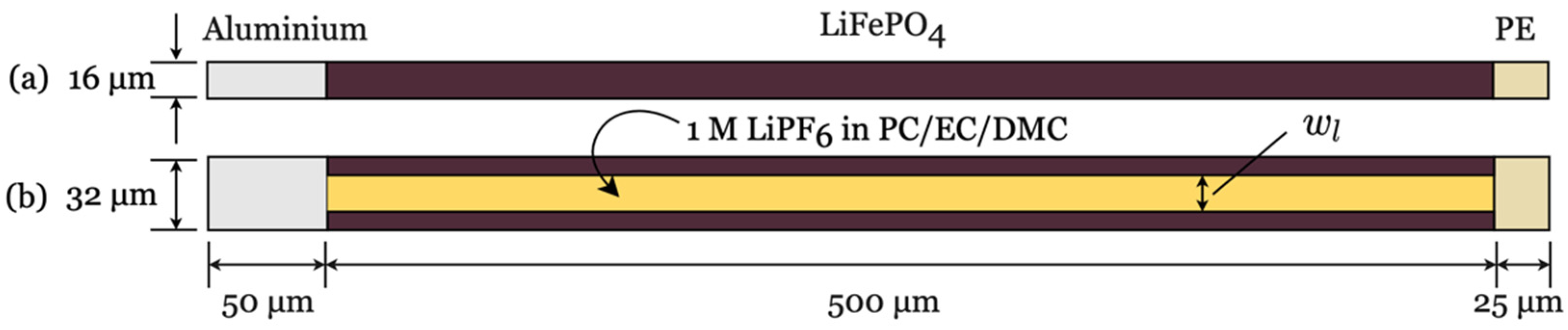
2.4. Material and Geometric Properties
3. Results and Discussion
3.1. Investigation of Electrode Design Parameters
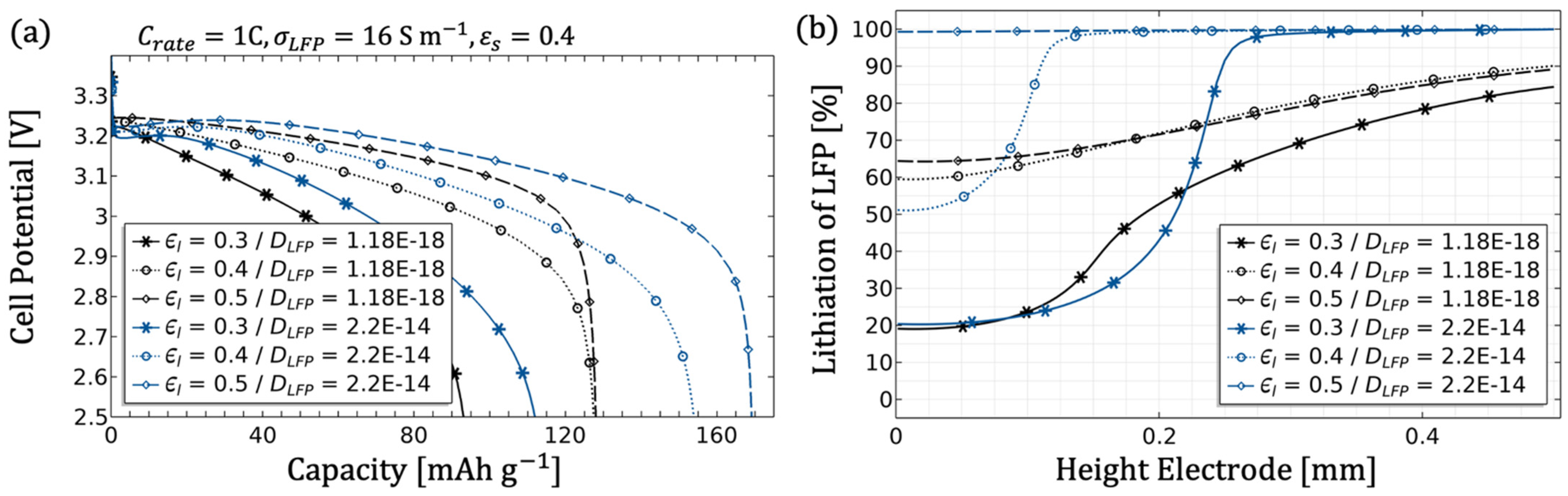
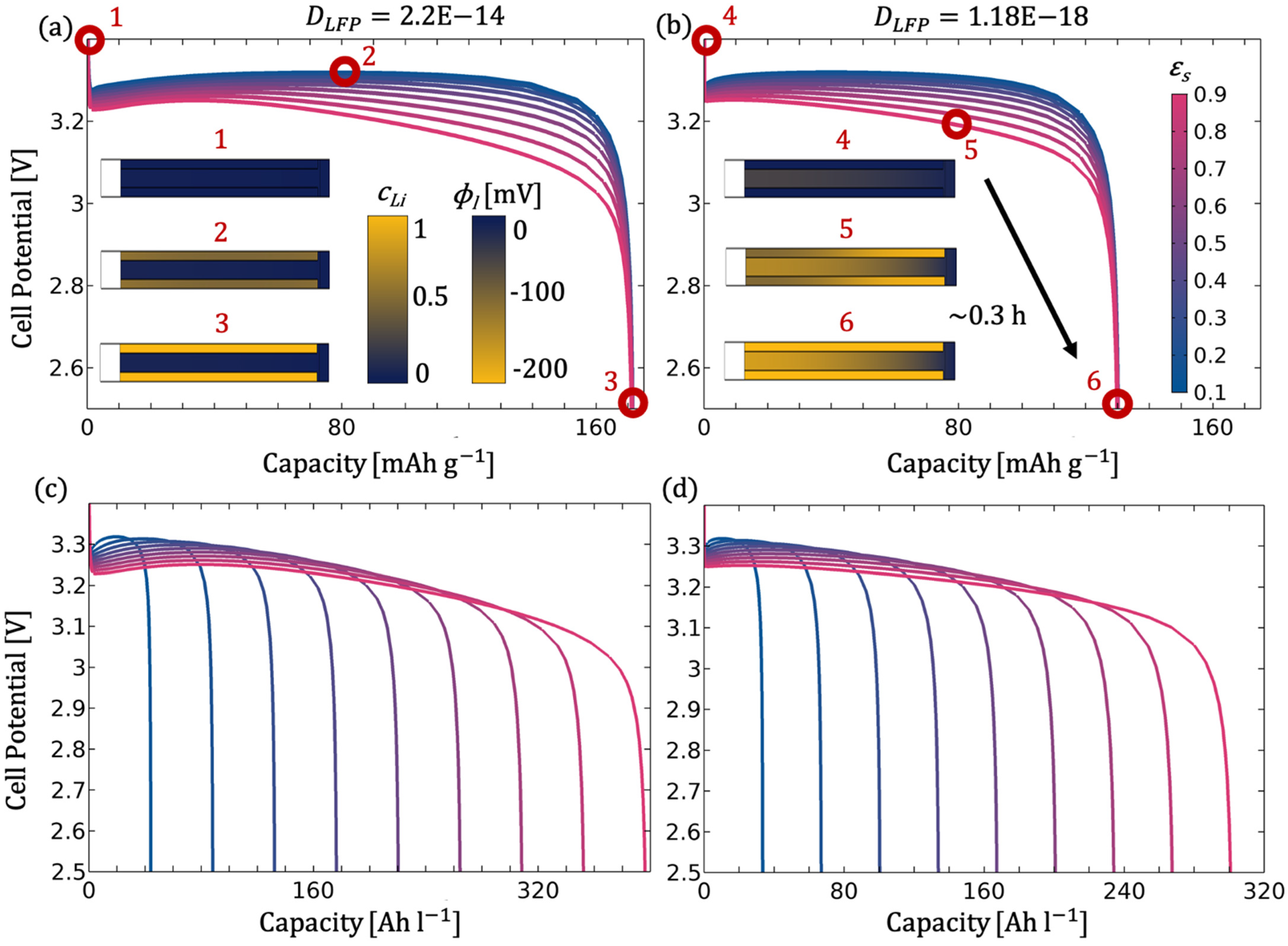
3.1.1. Influence of the Electrode Volume Fraction
3.1.2. Influence of the Electrode Conductivity
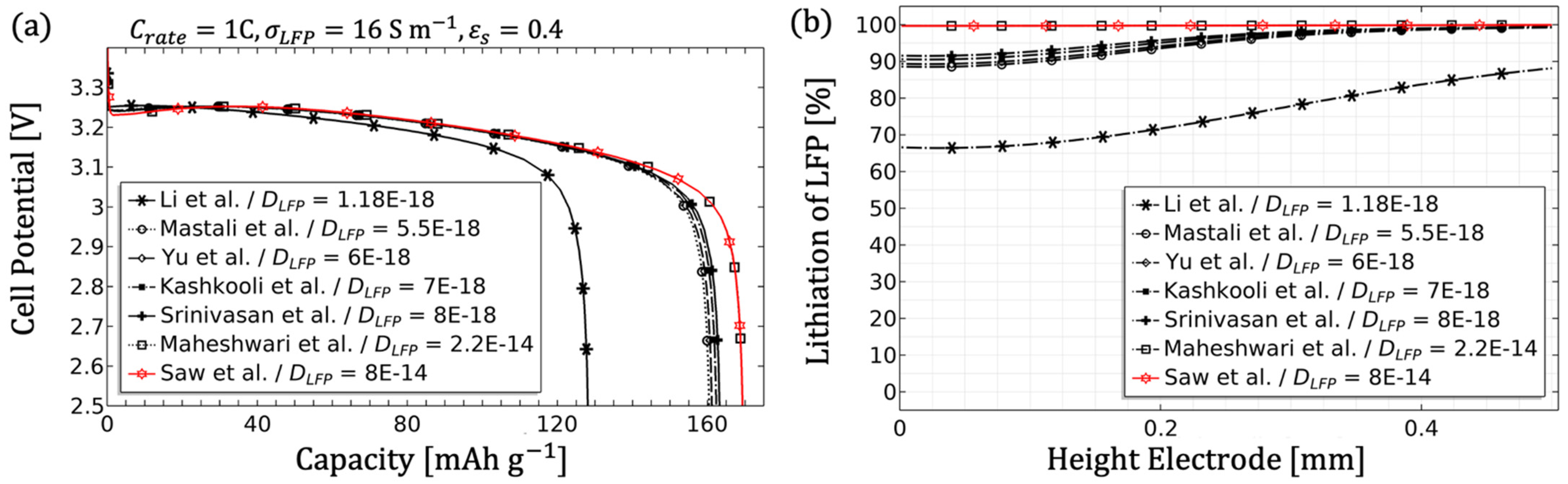
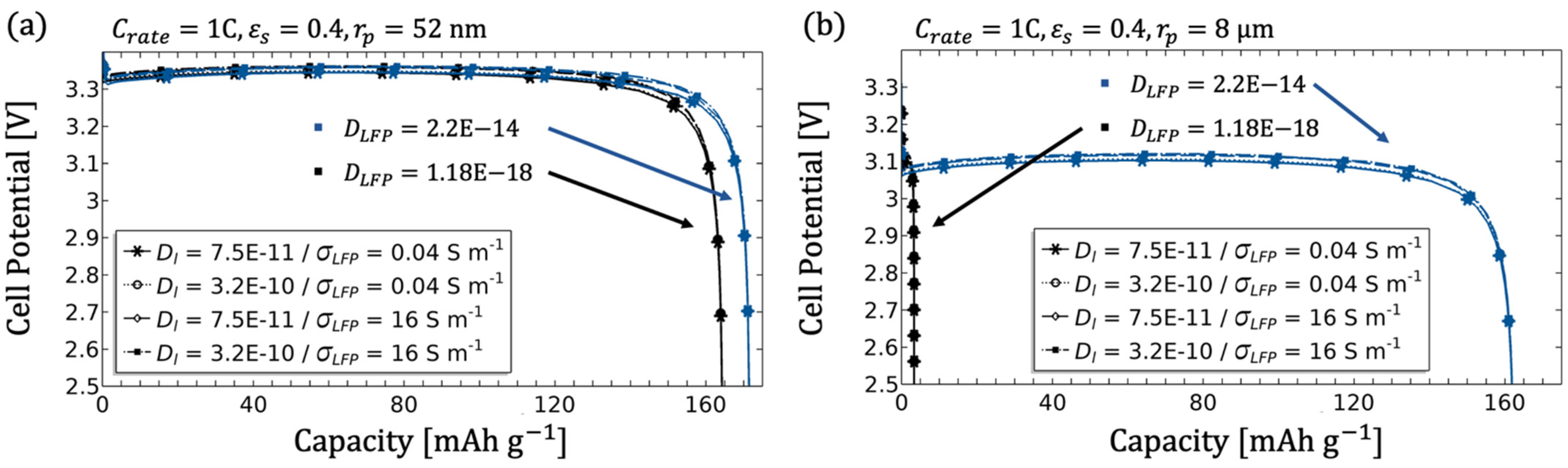
3.1.3. Influence of the Electrode Diffusion Coefficient
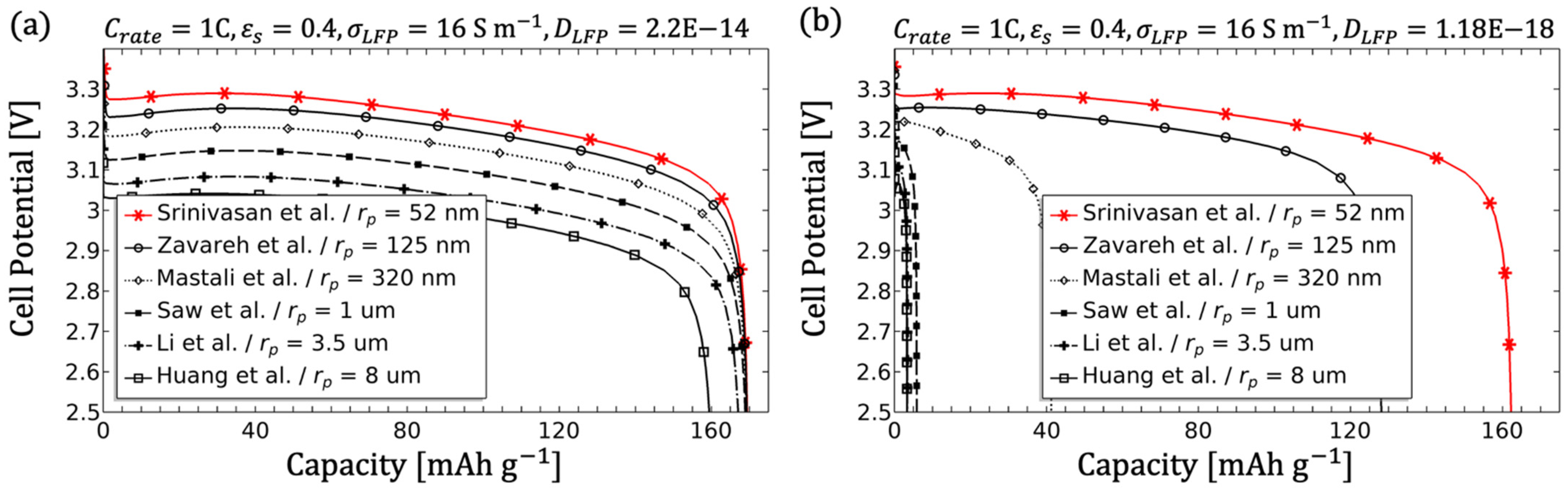
3.1.4. Influence of the Particle Radius
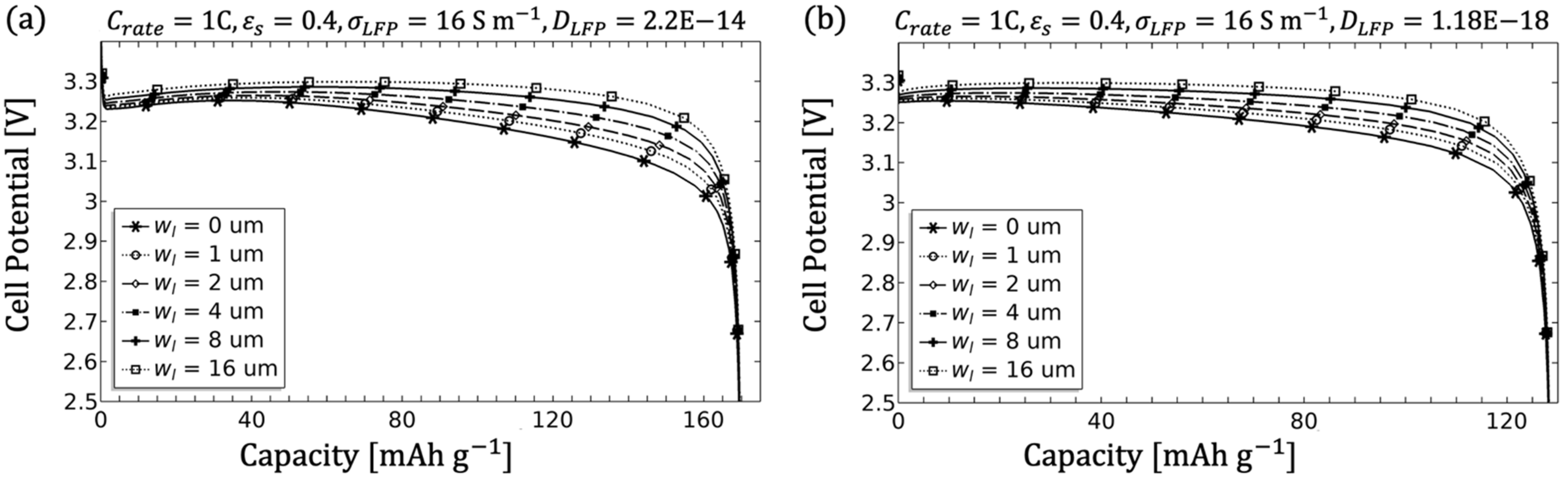
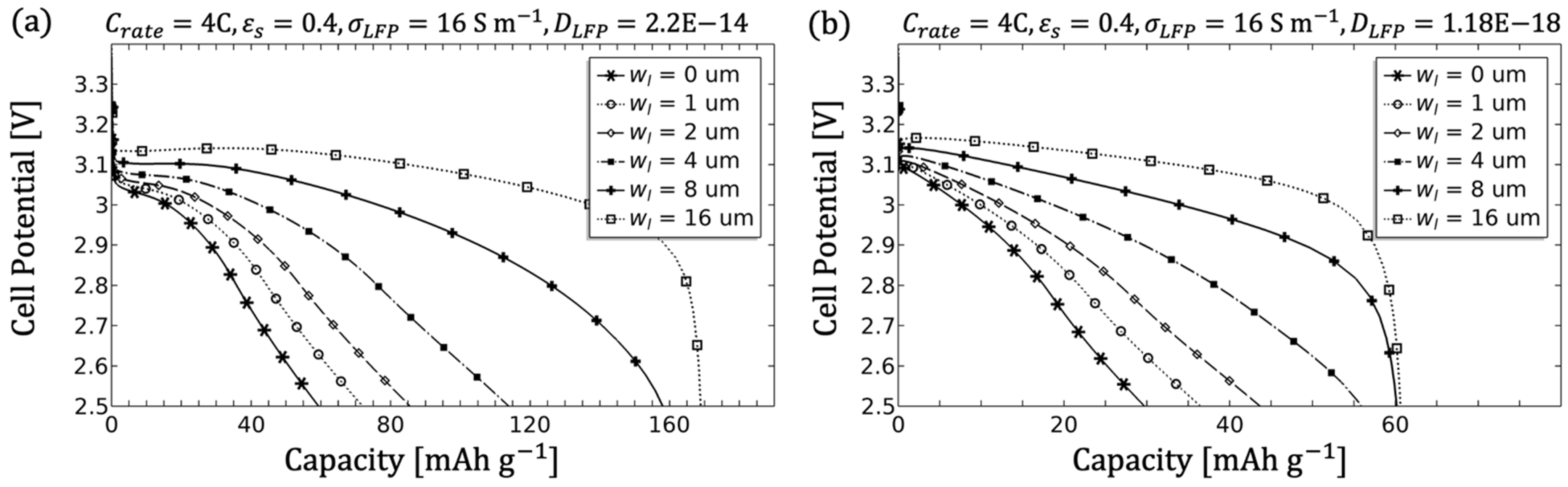
3.1.5. Influence of the Filler Content
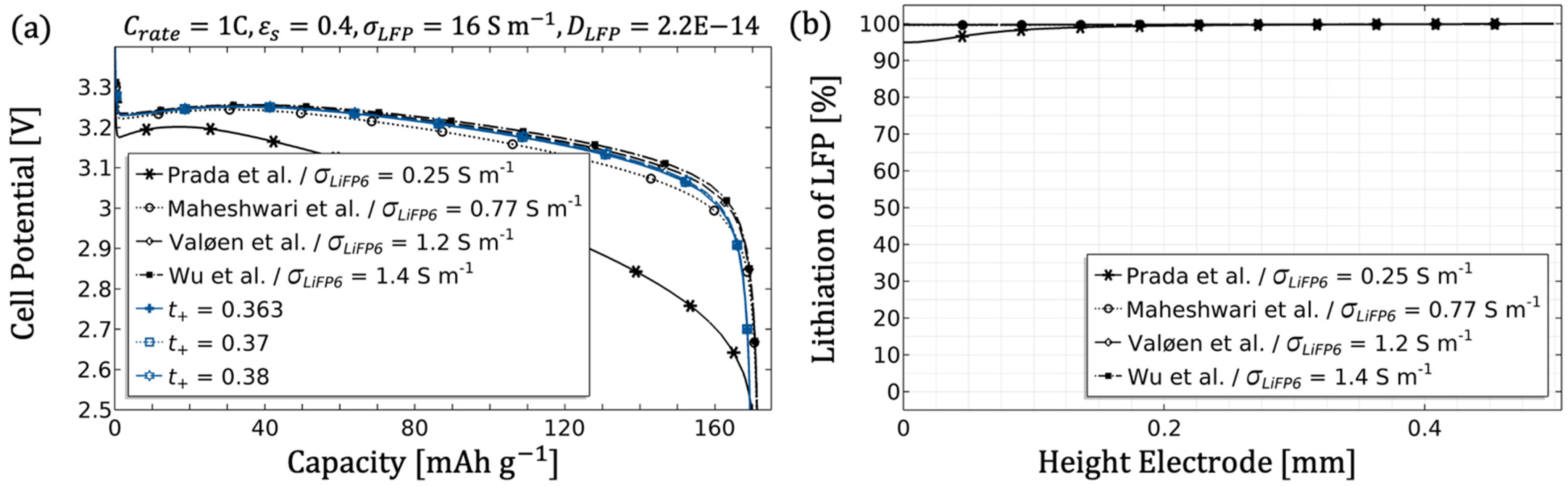
3.1.6. Influence of the Electrolyte
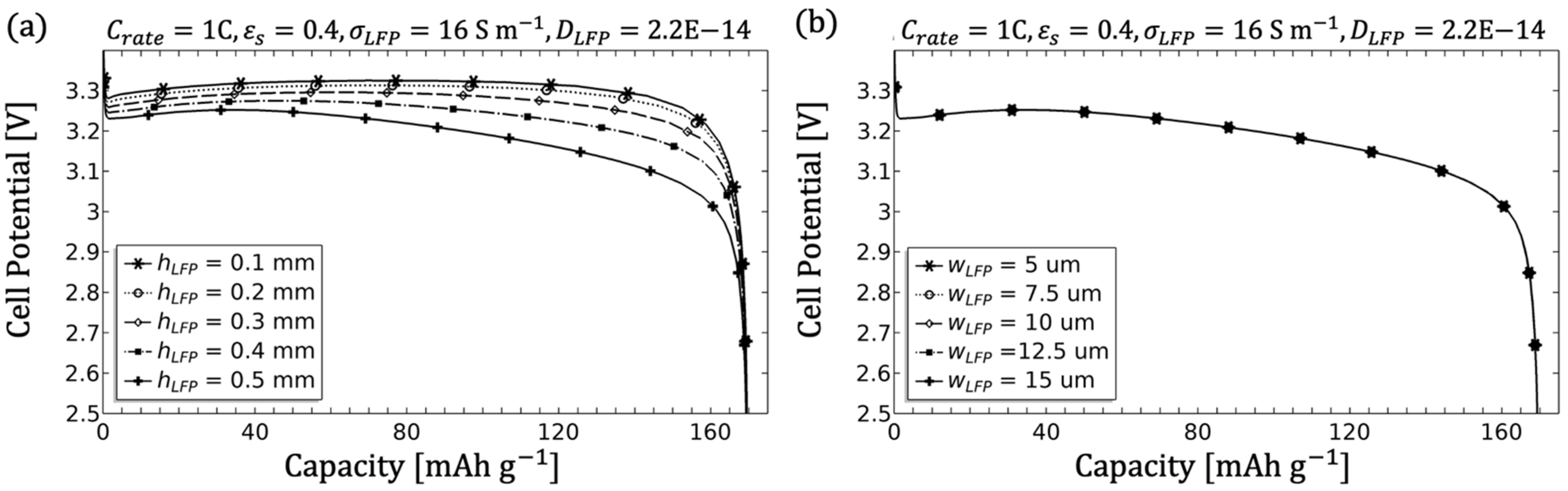
3.1.7. Influence of the Geometry
3.1.8. Influence of the Tortuosity
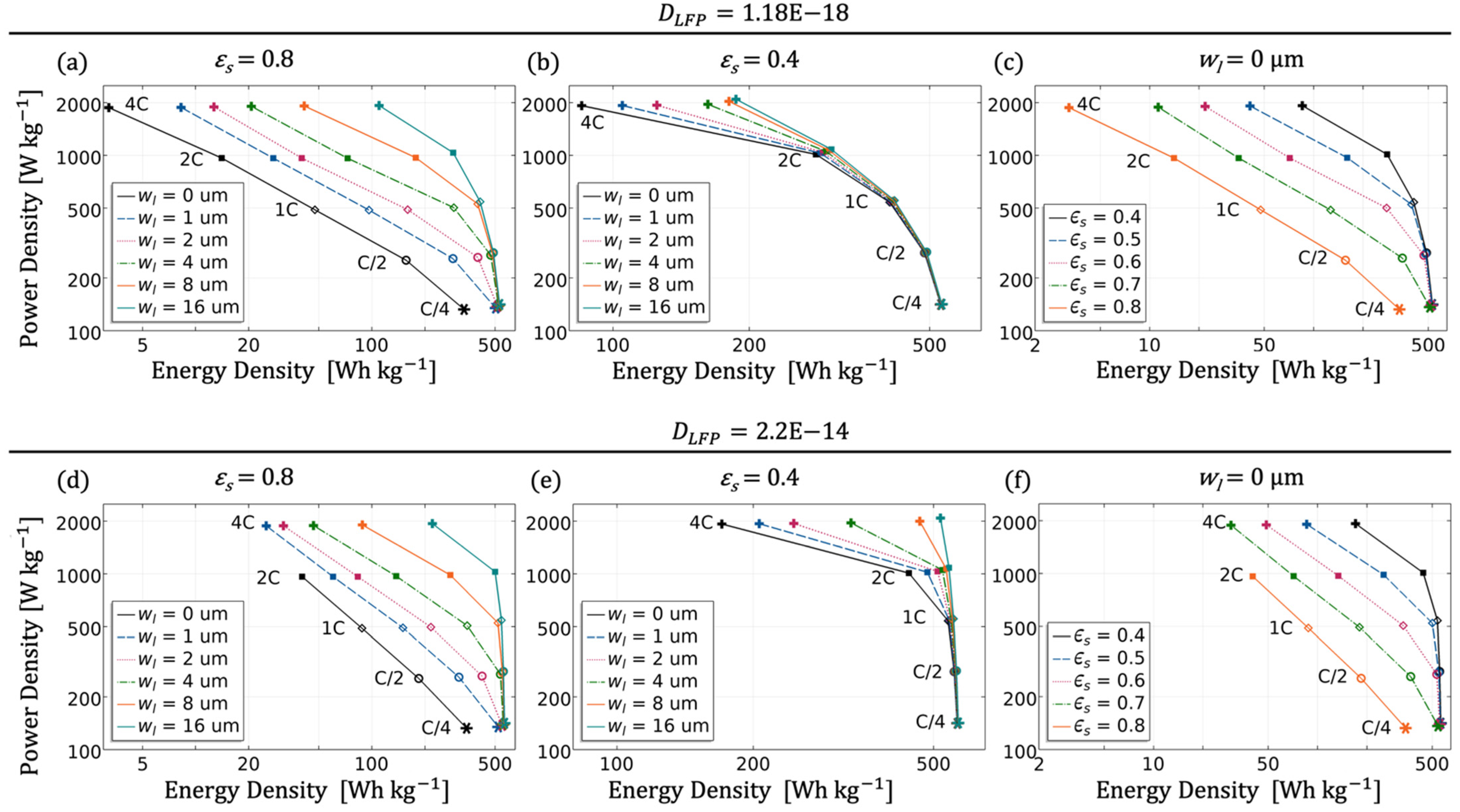
3.2. Performance Tests
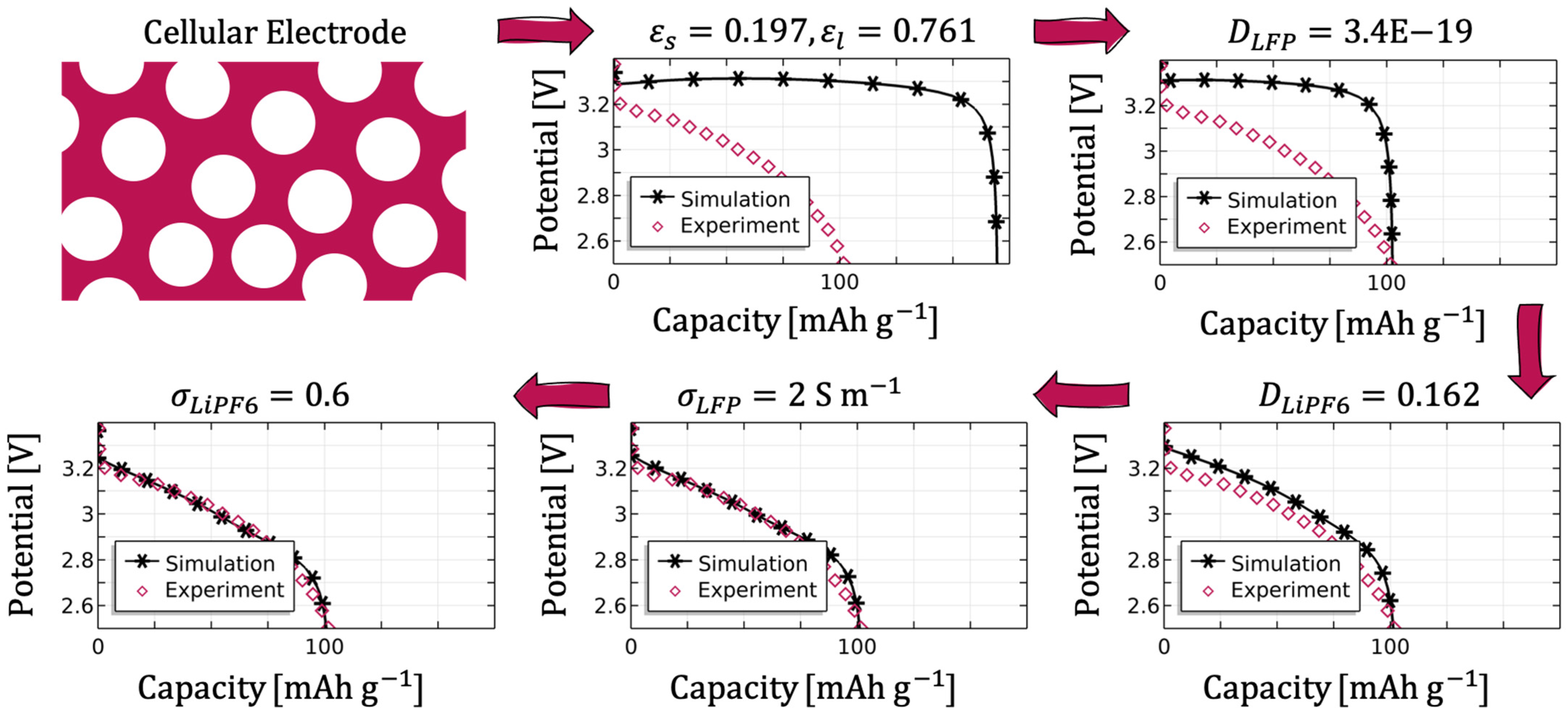
3.3. Validation and Usability
- All physical parameters that are verified, e.g., by the manufacturer’s specifications or experiments, must be taken into account.
- The best parameter set related to the theoretical study should be chosen for the undetermined parameters in order to start without any limitations.
- The external circumstances such as the discharge or charging current and the temperature must be clearly defined.
- The electrode diffusion coefficient should be determined to scale the achievable capacity (see Figure 5).
- An adjustment of the inhomogeneities can be done by the diffusivity as well as the conductivity of the electrolyte. Since in the porous electrode these two parameters are influenced by the microstructure (see Equations (8) and (9)), an adjustment of the microstructure would likewise be target-oriented (see Figure 7, Figure 8 and Figure 9).
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| LFP | Lithium iron phosphate |
| SoL | State-of-Lithiation |
| P2D | Pseudo two-dimensional model |
| DoD | Depth-of-Discharge |
Appendix A
| Name Identifier | Parameter | Value | Source |
|---|---|---|---|
| Haverkort | 0.4 | [36] | |
| Saw et al. | 0.04 S m−1 | [24] | |
| 1 µm | |||
| Li et al. | 0.5 S m−1 | [27] | |
| 3.5 µm | |||
| m2 s−1 | |||
| Mastali et al. | 6.75 S m−1 | [31] | |
| 320 nm | |||
| Yu et al. | 11.8 S m−1 | [23] | |
| m2 s−1 | |||
| Huang et al. | 91 S m−1 | [49] | |
| 8 µm | |||
| m2 s−1 | |||
| Delacourt et al. | 16 S m−1 | [51] | |
| Kashkooli et al. | [30] | ||
| Srinivasan et al. | [34] | ||
| 52 nm | |||
| Maheshwari et al. | [28] | ||
| m2 s−1 | |||
| 0.77 S m−1 | |||
| Zavareh et al. | 125 nm | [39] | |
| Thorat et al. | m2 s−1 | [26] | |
| Prada et al. | 0.25 S m−1 | [65] | |
| Valøen et al. | 1.2 S m−1 | [41] | |
| Wu et al. | 1.4 S m−1 | [57] |

References
- Linden, D.; Reddy, T.B. Linden’s Handbook of Batteries, 3rd ed.; McGraw-Hill Education: New York, NY, USA, 2010. [Google Scholar]
- Chen, X.; Shen, W.; Vo, T.T.; Cao, Z.; Kapoor, A. An Overview of Lithium-Ion Batteries for Electric Vehicles. In Proceedings of the 10th International Power & Energy Conference (IPEC), Ho Chi Minh City, Vietnam, 12–14 December 2012; pp. 230–235. [Google Scholar] [CrossRef]
- Quartarone, E.; Mustarelli, P. Review—Emerging Trends in the Design of Electrolytes for Lithium and Post-Lithium Batteries. J. Electrochem. Soc. 2020, 167, 050508. [Google Scholar] [CrossRef]
- Jang, J.; Oh, J.; Jeong, H.; Kang, W.; Jo, C. A Review of Functional Separators for Lithium Metal Battery Applications. Materials 2020, 13, 4625. [Google Scholar] [CrossRef]
- Yang, C.; Han, D.; Zhang, M. Progress in Separators for Rechargeable Batteries. Recharg. Batter. Hist. Prog. Appl. 2020, 1–16. [Google Scholar] [CrossRef]
- Cheng, X.B.; Zhang, R.; Zhao, C.Z.; Zhang, Q. Toward Safe Lithium Metal Anode in Rechargeable Batteries: A Review. Chem. Rev. 2017, 117, 10403–10473. [Google Scholar] [CrossRef]
- Vijayaraghavan, B.; Ely, D.R.; Chiang, Y.M.; García-García, R.; García, R.E. An Analytical Method to Determine Tortuosity in Rechargeable Battery Electrodes. J. Electrochem. Soc. 2012, 159, A548–A552. [Google Scholar] [CrossRef]
- Hamamoto, K.; Fukushima, M.; Mamiya, M.; Yoshizawa, Y.; Akimoto, J.; Suzuki, T.; Fujishiro, Y. Morphology Control and Electrochemical Properties of LiFePO 4/C Composite Cathode for Lithium Ion Batteries. Solid State Ion. 2012, 225, 560–563. [Google Scholar] [CrossRef]
- Regli, W.; Rossignac, J.; Shapiro, V.; Srinivasan, V. The New Frontiers in Computational Modeling of Material Structures. Comput. Aided Des. 2016, 77, 73–85. [Google Scholar] [CrossRef] [Green Version]
- Dong, T.K.; Kirchev, A.; Mattera, F.; Kowal, J.; Bultel, Y. Dynamic Modeling of Li-Ion Batteries Using an Equivalent Electrical Circuit. J. Electrochem. Soc. 2011, 158, A326. [Google Scholar] [CrossRef]
- Ferraz, P.K.P.; Schmidt, R.; Kober, D.; Kowal, J. A High Frequency Model for Predicting the Behavior of Lithium-Ion Batteries Connected to Fast Switching Power Electronics. J. Energy Storage 2018, 18, 40–49. [Google Scholar] [CrossRef]
- Neupert, S.; Kowal, J. Inhomogeneities in Battery Packs. World Electr. Veh. J. 2018, 9, 20. [Google Scholar] [CrossRef] [Green Version]
- Jokar, A.; Rajabloo, B.; Désilets, M.; Lacroix, M. Review of Simplified Pseudo-Two-Dimensional Models of Lithium-Ion Batteries. J. Power Sources 2016, 327, 44–55. [Google Scholar] [CrossRef]
- Doyle, M.; Fuller, T.F.; Newman, J. Modeling of Galvanostatic Charge and Discharge of the Lithi-um/Polymer/Insertion Cell. J. Electrochem. Soc. 1993, 140, 1526–1533. [Google Scholar] [CrossRef]
- Fuller, T.F.; Doyle, M.; Newman, J. Simulation and Optimization of the Dual Lithium Ion Insertion Cell. J. Electrochem. Soc. 1994, 141, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Doyle, M.; Newman, J. The use of mathematical modeling in the design of lithium/polymer battery systems. Electrochim. Acta 1995, 40, 2191–2196. [Google Scholar] [CrossRef]
- Newman, J.; Tiedemann, W. Porous-Electrode Theory with Battery Applications. AIChE J. 1975, 21, 25–41. [Google Scholar] [CrossRef] [Green Version]
- Han, X.; Ouyang, M.; Lu, L.; Li, J. Simplification of Physics-Based Electrochemical Model for Lithium Ion Battery on Electric Vehicle. Part I: Diffusion Simplification and Single Particle Model. J. Power Sources 2015, 278, 802–813. [Google Scholar] [CrossRef]
- Xia, L.; Najafi, E.; Bergveld, H.J.; Donkers, M.C.F. A Computationally Efficient Implementation of an Electro-Chemistry-Based Model for Lithium-Ion Batteries. IFAC-PapersOnLine 2017, 50, 2169–2174. [Google Scholar] [CrossRef]
- Yamanaka, T.; Kihara, D.; Takagishi, Y.; Yamaue, T. Multi-Physics Equivalent Circuit Models for a Cooling System of a Lithium Ion Battery Pack. Batteries 2020, 6, 44. [Google Scholar] [CrossRef]
- Geng, Z.; Wang, S.; Lacey, M.J.; Brandell, D.; Thiringer, T. Bridging Physics-Based and Equivalent Circuit Models for Lithium-ion Batteries. Electrochim. Acta 2021, 372, 137829. [Google Scholar] [CrossRef]
- Li, Y.; Vilathgamuwa, M.; Farrell, T.; Choi, S.S.; Tran, N.T.; Teague, J. A Physics-Based Distributed-Parameter Equivalent Circuit Model for Lithium-Ion Batteries. Electrochim. Acta 2019, 299, 451–469. [Google Scholar] [CrossRef]
- Yu, S.; Kim, S.; Kim, T.Y.; Nam, J.H.; Cho, W.I. Model Prediction and Experiments for the Electrode Design Opti-Mization of LiFePo4/Graphite Electrodes in High Capacity Lithium-Ion Batteries. Bull. Korean Chem. Soc. 2013, 34, 79–88. [Google Scholar] [CrossRef] [Green Version]
- Saw, L.H.; Ye, Y.; Tay, A.A.O. Electrochemical-Thermal Analysis of 18650 Lithium Iron Phosphate Cell. Energy Convers. Manag. 2013, 75, 162–174. [Google Scholar] [CrossRef]
- Thorat, I.V.; Stephenson, D.E.; Zacharias, N.A.; Zaghib, K.; Harb, J.N.; Wheeler, D.R. Quantifying Tortuosity in Porous Li-ion Battery Materials. J. Power Sources 2009, 188, 592–600. [Google Scholar] [CrossRef]
- Thorat, I.V.; Joshi, T.; Zaghib, K.; Harb, J.N.; Wheeler, D.R. Understanding Rate-Limiting Mechanisms in LiFePO4 Cathodes for Li-Ion Batteries. J. Electrochem. Soc. 2011, 158, A1185. [Google Scholar] [CrossRef]
- Li, J.; Cheng, Y.; Jia, M.; Tang, Y.; Lin, Y.; Zhang, Z.; Liu, Y. An Electrochemical-Thermal Model Based on Dynamic Responses for Lithium Iron Phosphate Battery. J. Power Sources 2014, 255, 130–143. [Google Scholar] [CrossRef]
- Maheshwari, A.; Dumitrescu, M.A.; Destro, M.; Santarelli, M. Inverse Parameter Determination in the Development of an Optimized Lithium Iron Phosphate-Graphite Battery Discharge Model. J. Power Sources 2016, 307, 160–172. [Google Scholar] [CrossRef]
- Maheshwari, A.; Dumitrescu, M.A.; Destro, M.; Santarelli, M. A Modelling Approach To Understand Charge Discharge Differences in Thermal Behaviour in Lithium Iron Phosphate–Graphite Battery. Electrochim. Acta 2017, 243, 129–141. [Google Scholar] [CrossRef]
- Kashkooli, A.G.; Farhad, S.; Lee, D.; Feng, K.; Litster, S.; Babu, S.K.; Zhu, L.; Chen, Z. Multiscale Modeling of Lithium-Ion Battery Electrodes Based on Nano-Scale X-ray Computed Tomography. J. Power Sources 2016, 307, 496–509. [Google Scholar] [CrossRef]
- Mastali, M.; Farkhondeh, M.; Farhad, S.; Fraser, R.A.; Fowler, M. Electrochemical Modeling of Commercial LiFePO 4 and Graphite Electrodes: Kinetic and Transport Properties and Their Temperature Dependence. J. Electrochem. Soc. 2016, 163, A2803–A2816. [Google Scholar] [CrossRef]
- Safari, M.; Delacourt, C. Mathematical Modeling of Lithium Iron Phosphate Electrode: Galvanostatic Charge/Discharge and Path Dependence. J. Electrochem. Soc. 2011, 158, A63. [Google Scholar] [CrossRef]
- Safari, M.; Farkhondeh, M.; Pritzker, M.; Fowler, M.; Han, T.; Chen, S.K. Simulation of Lithium Iron Phosphate Lithiation/Delithiation: Limitations of the Core-Shell Model. Electrochim. Acta 2014, 115, 352–357. [Google Scholar] [CrossRef]
- Srinivasan, V.; Newman, J. Design and Optimization of a Natural Graphite/Iron Phosphate Lithium-Ion Cell. J. Electrochem. Soc. 2004, 151, A1530. [Google Scholar] [CrossRef] [Green Version]
- Hosseinzadeh, E.; Marco, J.; Jennings, P. The Impact of Multi-Layered Porosity Distribution on the Performance of a Lithium Ion Battery. Appl. Math. Model. 2018, 61, 107–123. [Google Scholar] [CrossRef]
- Haverkort, J.W. A Theoretical Analysis of the Optimal Electrode Thickness and Porosity. Electrochim. Acta 2018, 295, 846–860. [Google Scholar] [CrossRef]
- García, R.E.; Chiang, Y.-M.; Carter, W.C.; Limthongkul, P.; Bishop, C.M. Microstructural Modeling and Design of Rechargeable Lithium-Ion Batteries. J. Electrochem. Soc. 2005, 152, A255. [Google Scholar] [CrossRef]
- Lu, X.; Bertei, A.; Finegan, D.P.; Tan, C.; Daemi, S.R.; Weaving, J.S.; O’Regan, K.B.; Heenan, T.M.M.; Hinds, G.; Kendrick, E.; et al. 3D Microstructure Design of Lithium-Ion Battery Electrodes Assisted by X-ray Nano-Computed Tomography and Modelling. Nat. Commun. 2020, 11, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Zavareh, S.; Hilger, A.; Hirselandt, K.; Goerke, O.; Manke, I.; Banhart, J.; Gurlo, A. Fabrication of Cellular and Lamellar LiFePO4/C Cathodes for Li-Ion Batteries by Unidirectional Freeze-Casting Method. J. Ceram. Soc. Jpn. 2016, 124, 1067–1071. [Google Scholar] [CrossRef] [Green Version]
- Tjaden, B.; Cooper, S.J.; Brett, D.J.; Kramer, D.; Shearing, P.R. On the Origin and Application of the Bruggeman Correlation for Analysing Transport Phenomena in Electrochemical Systems. Curr. Opin. Chem. Eng. 2016, 12, 44–51. [Google Scholar] [CrossRef] [Green Version]
- Valøen, L.O.; Reimers, J.N. Transport Properties of LiPF[sub 6]-Based Li-Ion Battery Electrolytes. J. Electrochem. Soc. 2005, 152, A882. [Google Scholar] [CrossRef]
- Ashwin, T.R.; McGordon, A.; Jennings, P.A. A Mass Transfer Based Variable Porosity Model with Particle Radius Change for a Lithium-Ion Battery. Electrochim. Acta 2017, 232, 203–214. [Google Scholar] [CrossRef]
- White, M.A.; Conrad, J.; Ellis, S.N.; Chen, R. Investigations of Ice-Structuring Agents in Ice-Templated Ceramics. J. Am. Ceram. Soc. 2017, 100, 5066–5074. [Google Scholar] [CrossRef]
- Gaudillere, C.; Serra, J.M. Freeze-Casting: Fabrication of Highlyporous and Hierarchical Ceramic Supports for Energy Applications. Bol. Soc. Esp. Ceram. Vidr. 2016, 55, 45–54. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Bunch, J.; Li, T.; Mao, Z.; Chen, F. Novel Functionally Graded Acicular Electrode for Solid Oxide Cells Fab-Ricated by the Freeze-Tape-Casting Process. J. Power Sources 2012, 213, 93–99. [Google Scholar] [CrossRef]
- Scotti, K.L.; Dunand, D.C. Freeze Casting–A Review of Processing, Microstructure and Properties via the Open Data Repository, FreezeCasting.net. Prog. Mater. Sci. 2018, 94, 243–305. [Google Scholar] [CrossRef] [Green Version]
- Fan, G.; Pan, K.; Canova, M.; Marcicki, J.; Yang, X.G. Modeling of Li-Ion Cells for Fast Simulation of High C-Rate and Low Temperature Operations. J. Electrochem. Soc. 2016, 163, A666–A676. [Google Scholar] [CrossRef]
- Zahn, R.; Lagadec, M.F.; Hess, M.; Wood, V. Improving Ionic Conductivity and Lithium-Ion Transference Number in Lithium-Ion Battery Separators. ACS Appl. Mater. Interfaces 2016, 8, 32637–32642. [Google Scholar] [CrossRef]
- Huang, H.H.; Chen, H.Y.; Liao, K.C.; Young, H.T.; Lee, C.F.; Tien, J.Y. Thermal-Electrochemical Coupled Simulations for Cell-to-Cell Imbalances in Lithium-Iron-Phosphate Based Battery Packs. Appl. Therm. Eng. 2017, 123, 584–591. [Google Scholar] [CrossRef]
- Li, J.; Lotfi, N.; Landers, R.G.; Park, J. A Single Particle-Based Battery Degradation Model Including Chemical and Mechanical Degradation Physics. ECS Trans. 2017, 77, 1003–1014. [Google Scholar] [CrossRef]
- Delacourt, C.; Laffont, L.; Bouchet, R.; Wurm, C.; Leriche, J.B.; Morcrette, M.; Tarascon, J.M.; Masquelier, C. Toward Understanding of Electrical Limitations (Electronic, Ionic) in LiMPO[sub 4] (M=Fe, Mn) Electrode Materials. J. Electrochem. Soc. 2005, 152, A913. [Google Scholar] [CrossRef]
- Byles, B.W.; Palapati, N.K.R.; Subramanian, A.; Pomerantseva, E. The Role of Electronic and Ionic Conductivities in the Rate Performance of Tunnel Structured Manganese Oxides in Li-Ion Batteries. APL Mater. 2016, 4, 046108. [Google Scholar] [CrossRef]
- Srinivasan, V.; Newman, J. Discharge Model for the Lithium Iron-Phosphate Electrode. J. Electrochem. Soc. 2004, 151, A1517. [Google Scholar] [CrossRef]
- Farkhondeh, M.; Delacourt, C. Mathematical Modeling of Commercial LiFePO 4 Electrodes Based on Variable Solid-State Diffusivity. J. Electrochem. Soc. 2011, 159, A177–A192. [Google Scholar] [CrossRef]
- Nickol, A.; Schied, T.; Heubner, C.; Schneider, M.; Michaelis, A.; Bobeth, M.; Cuniberti, G. GITT Analysis of Lithium Insertion Cathodes for Determining the Lithium Diffusion Coefficient at Low Temperature: Challenges and Pitfalls. J. Electrochem. Soc. 2020, 167, 090546. [Google Scholar] [CrossRef]
- Wang, C.; Kasavajjula, U.S.; Arce, P.E. A Discharge Model for Phase Transformation Electrodes: Formulation, Experimental Validation, and Analysis. J. Phys. Chem. C 2007, 111, 16656–16663. [Google Scholar] [CrossRef]
- Wu, W.; Xiao, X.; Huang, X. The Effect of Battery Design Parameters on Heat Generation and Utilization in a Li-Ion Cell. Electrochim. Acta 2012, 83, 227–240. [Google Scholar] [CrossRef]
- Landesfeind, J.; Eldiven, A.; Gasteiger, H.A. Influence of the Binder on Lithium Ion Battery Electrode Tortuosity and Performance. J. Electrochem. Soc. 2018, 165, A1122–A1128. [Google Scholar] [CrossRef]
- Hu, S.; Li, Y.; Yin, J.; Wang, H.; Yuan, X.; Li, Q. Effect of Different Binders on Electrochemical Properties of LiFePO4/C Cathode Material in Lithium Ion Batteries. Chem. Eng. J. 2014, 237, 497–502. [Google Scholar] [CrossRef]
- Daemi, S.R.; Tan, C.; Volkenandt, T.; Cooper, S.J.; Palacios-Padros, A.; Cookson, J.; Brett, D.J.L.; Shearing, P.R. Visualizing the Carbon Binder Phase of Battery Electrodes in Three Dimensions. ACS Appl. Energy Mater. 2018, 1, 3702–3710. [Google Scholar] [CrossRef]
- Lundgren, H.; Behm, M.; Lindbergh, G. Electrochemical Characterization and Temperature Dependency of Mass-Transport Properties of LiPF 6 in EC:DEC. J. Electrochem. Soc. 2015, 162, A413–A420. [Google Scholar] [CrossRef]
- Safari, M.; Delacourt, C. Modeling of a Commercial Graphite/LiFePO4 Cell. J. Electrochem. Soc. 2011, 158, A562. [Google Scholar] [CrossRef]
- Berhaut, C.L.; Porion, P.; Timperman, L.; Schmidt, G.; Lemordant, D.; Anouti, M. LiTDI as Electrolyte Salt for Li-Ion Batteries: Transport Properties in EC/DMC. Electrochim. Acta 2015, 180, 778–787. [Google Scholar] [CrossRef]
- Tenney, C.M.; Cygan, R.T. Analysis of Molecular Clusters in Simulations of Lithium-Ion Battery Electrolytes. J. Phys. Chem. C 2013, 117, 24673–24684. [Google Scholar] [CrossRef]
- Prada, E.; di Domenico, D.; Creff, Y.; Bernard, J.; Sauvant-Moynot, V.; Huet, F. Simplified Electrochemical and Thermal Model of LiFePO 4 -Graphite Li-Ion Batteries for Fast Charge Applications. J. Electrochem. Soc. 2012, 159, A1508–A1519. [Google Scholar] [CrossRef] [Green Version]
- Delattre, B.; Amin, R.; Sander, J.; de Coninck, J.; Tomsia, A.P.; Chiang, Y.-M. Impact of Pore Tortuosity on Electrode Kinetics in Lithium Battery Electrodes: Study in Directionally Freeze-Cast LiNi 0.8 Co 0.15 Al 0.05 O 2 (NCA). J. Electrochem. Soc. 2018, 165, A388–A395. [Google Scholar] [CrossRef] [Green Version]
- Zheng, H.; Li, J.; Song, X.; Liu, G.; Battaglia, V.S. A Comprehensive Understanding of Electrode Thickness Effects on the Electrochemical Performances of Li-Ion Battery Cathodes. Electrochim. Acta 2012, 71, 258–265. [Google Scholar] [CrossRef]
- le Houx, J.; Kramer, D. Physics Based Modelling of Porous Lithium Ion Battery Electrodes—A Review. Energy Rep. 2020, 6, 1–9. [Google Scholar] [CrossRef]
- Forouzan, M.M.; Mazzeo, B.A.; Wheeler, D.R. Modeling the Effects of Electrode Microstructural Heterogeneities on Li-Ion Battery Performance and Lifetime. J. Electrochem. Soc. 2018, 165, A2127–A2144. [Google Scholar] [CrossRef]
- Zhang, X.; Ju, Z.; Housel, L.M.; Wang, L.; Zhu, Y.; Singh, G.; Sadique, N.; Takeuchi, K.J.; Takeuchi, E.S.; Marschilok, A.C.; et al. Promoting Transport Kinetics in Li-Ion Battery with Aligned Porous Electrode Architectures. Nano Lett. 2019, 19, 8255–8261. [Google Scholar] [CrossRef]
- Kumaresan, K.; Sikha, G.; White, R.E. Thermal Model for a Li-Ion Cell. J. Electrochem. Soc. 2008, 155, A164. [Google Scholar] [CrossRef]
- Ma, S.; Jiang, M.; Tao, P.; Song, C.; Wu, J.; Wang, J.; Deng, T.; Shang, W. Temperature Effect and Thermal Impact in Lithium-Ion Batteries: A Review. Prog. Nat. Sci. Mater. Int. 2018, 28, 653–666. [Google Scholar] [CrossRef]






| 1 | −1.239 | −7.903 | 0.3821 |
| 2 | 21.12 | 30.37 | |
| 3 | 22.39 | 1.56 |
| 0.0032158 | −0.046272 | 0.28857 | −0.98716 | 1.9635 | −2.2585 | 1.3902 | −0.35376 |
| Parameter | Value | Unit | Reference |
|---|---|---|---|
| electrolyte | |||
| (5) | cm2 s−1 | [41] | |
| 1 | - | [41] | |
| (3) | mS cm−1 | [41] | |
| 1 | - | [41] | |
| (4) | - | [41] | |
| 0.38 | - | [41] | |
| 1000 | mol m−3 | [47] | |
| electrode | |||
| (27) | m2 s−1 | [27] | |
| - | [32] | ||
| 0.5 | S m−1 | [27] | |
| (12) | V | [31] | |
| 16,481 | mol m−3 | calculated | |
| 1 | mol m−3 | assumed | |
| 0.01 | - | assumed | |
| 0.99 | - | assumed | |
| m | [39] | ||
| 0.4 | - | calculated | |
| 0.6 | - | [36] | |
| 1.5 (spherical) | - | [40] | |
| 2.6 | g cm−3 | [39] | |
| electrode kinetics | |||
| 0.5 | - | [32] | |
| 0.5 | - | [32] | |
| (17) | m s−1 | [27] | |
| (18) | m s−1 | [27] | |
| m s−1 | assumed | ||
| 30 | kJ mol−1 | [27] | |
| 20 | kJ mol−1 | [27] | |
| 35 | kJ mol−1 | [27] | |
| z | 1 | - | assumed |
| m | spherical: = 3 | - | assumed |
| separator | |||
| 0.724 | - | [24] | |
| current collector | |||
| S cm−1 | [29] |
| Set | ||||
|---|---|---|---|---|
| A | 0.162 | 0.4 | 0.35 | |
| B | 0.3 | 0.6 | 0.35 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Franke-Lang, R.; Kowal, J. Electrochemical Model-Based Investigation of Thick LiFePO4 Electrode Design Parameters. Modelling 2021, 2, 259-287. https://doi.org/10.3390/modelling2020014
Franke-Lang R, Kowal J. Electrochemical Model-Based Investigation of Thick LiFePO4 Electrode Design Parameters. Modelling. 2021; 2(2):259-287. https://doi.org/10.3390/modelling2020014
Chicago/Turabian StyleFranke-Lang, Robert, and Julia Kowal. 2021. "Electrochemical Model-Based Investigation of Thick LiFePO4 Electrode Design Parameters" Modelling 2, no. 2: 259-287. https://doi.org/10.3390/modelling2020014
APA StyleFranke-Lang, R., & Kowal, J. (2021). Electrochemical Model-Based Investigation of Thick LiFePO4 Electrode Design Parameters. Modelling, 2(2), 259-287. https://doi.org/10.3390/modelling2020014







