Calycovesicostomy, Ureterocalycostomy, and Ileocalycostomy: Rare Reconstructive Options for Transplant Ureteral Strictures
Abstract
1. Introduction
2. Case Presentation
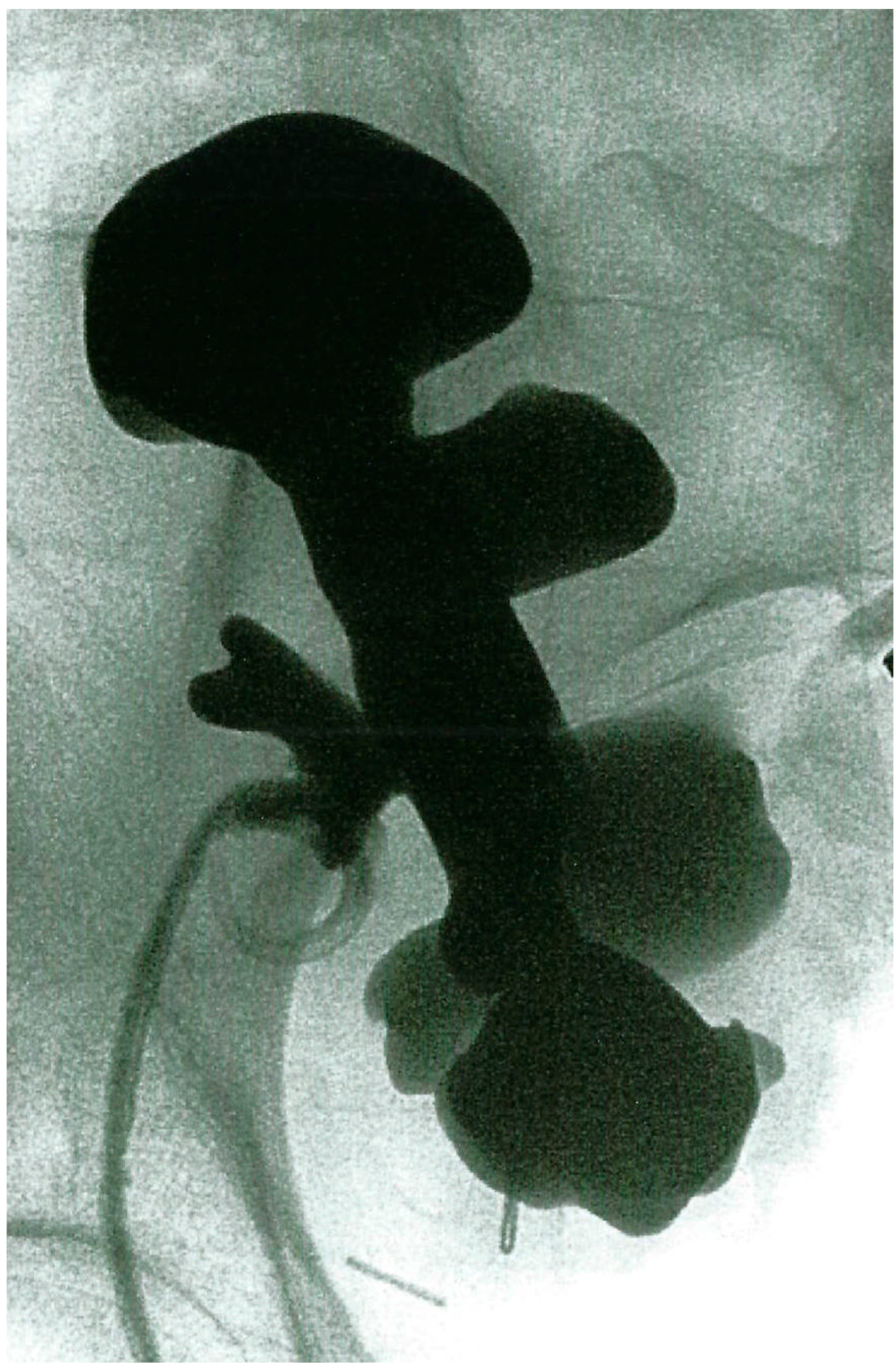
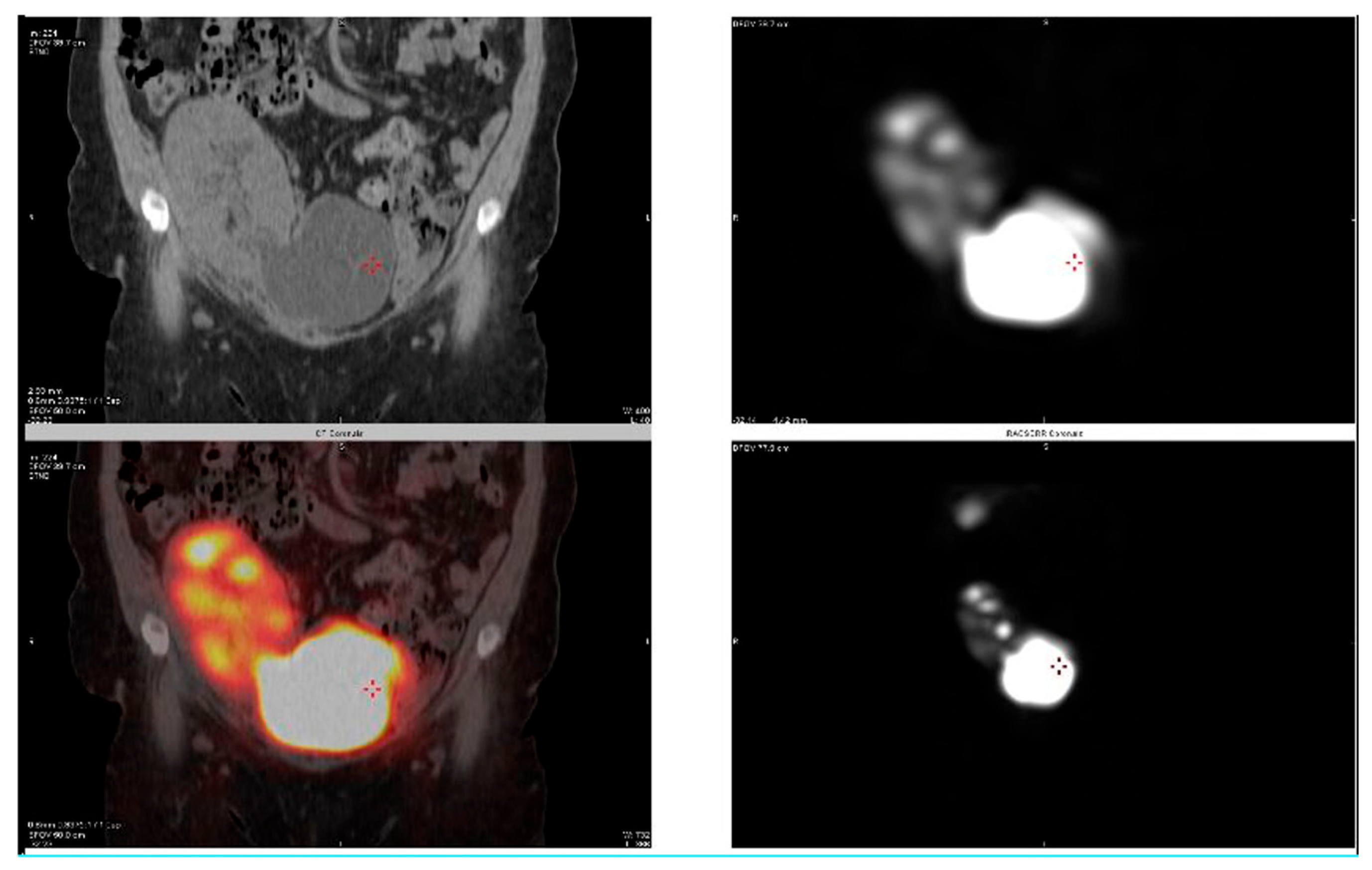
2.1. Case 1
2.2. Case 2
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| TUS | Transplant Ureteral Stricture |
| CV | Calycovesicostomy |
| UC | Ureterocalycostomy |
| IC | Ileocalycostomy |
| AKI | Acute Kidney Injury |
| CKD | Chronic Kidney Disease |
| PCN | Percutaneous Nephrostomy Tube |
| MAG3 | Mercaptoacetyltriglycine |
References
- Dalgic, A.; Boyvat, F.; Karakayali, H.; Moray, G.; Emiroglu, R.; Haberal, M. Urologic complications in 1523 renal transplantations: The Baskent University experience. Transplant. Proc. 2006, 38, 543–547. [Google Scholar] [CrossRef]
- Koch, M.; Kantas, A.; Ramcke, K.; Drabik, A.I.; Nashan, B. Surgical complications after kidney transplantation: Different impacts of immunosuppression, graft function, patient variables, and surgical performance. Clin. Transplant. 2015, 29, 252–260. [Google Scholar] [CrossRef]
- Haberal, M.; Karakayali, H.; Sevmis, S.; Moray, G.; Arslan, G. Urologic complication rates in kidney transplantation after a novel ureteral reimplantation technique. Exp. Clin. Transplant. 2006, 4, 503–505. [Google Scholar]
- Haberal, M.; Emiroglu, R.; Karakayali, H.; Torgay, A.; Moray, G.; Arslan, G.; Sozen, H.; Dalgic, A. A corner-saving ureteral reimplantation technique without stenting. Transplant. Proc. 2006, 38, 548–551. [Google Scholar] [CrossRef]
- Kwong, J.; Schiefer, D.; Aboalsamh, G.; Archambault, J.; Luke, P.P.; Sener, A. Optimal management of distal ureteric strictures following renal transplantation: A systematic review. Transpl. Int. 2016, 29, 579–588. [Google Scholar] [CrossRef]
- Arpali, E.; Al-Qaoud, T.; Martinez, E.; Redfield, R.R., III; Leverson, G.E.; Kaufman, D.B.; Odorico, J.S.; Sollinger, H.W. Impact of ureteral stricture and treatment choice on long-term graft survival in kidney transplantation. Am. J. Transplant. 2018, 18, 1977–1985. [Google Scholar] [CrossRef]
- Al-Qaoud, T.M.; Al-Adra, D.P.; Mezrich, J.D.; Fernandez, L.A.; Kaufman, D.B.; Odorico, J.S.; Sollinger, H.W. Complex Ureteral Reconstruction in Kidney Transplantation. Exp. Clin. Transplant. 2021, 19, 425–433. [Google Scholar] [CrossRef]
- Neuwirt, K. Implantation of the ureter into the lower calyx of the renal pelvis. Urol. Cutaneous Rev. 1948, 52, 351. [Google Scholar]
- Michalowski, E.; Modelski, W.; Kmak, A. End to end anastomosis between the lower renal calix and ureter (ureterocalicostomy). Z. Urol. Nephrol. 1970, 63, 1–7. [Google Scholar]
- Wesolowski, S. Ureterocalicostomy. Pol. Przegl Chir. 1975, 47, 807–820. [Google Scholar]
- Selli, C.; Carini, M.; Turini, D.; Masini, G.; Costantini, A. Experience with ureterocalyceal anastomosis. Urology 1982, 20, 7–12. [Google Scholar] [CrossRef]
- Ross, J.H.; Streem, S.B.; Novick, A.C.; Kay, R.; Montie, J. Ureterocalicostomy for reconstruction of complicated pelviureteric junction obstruction. Br. J. Urol. 1990, 65, 322–325. [Google Scholar] [CrossRef]
- Haouas, N.; Youssef, A.; Sahraoui, W.; Thabet, I.; Ben Sorba, N.; Jaidane, M.; Mosbah, A.T. Ureterocalicostomy: Indications and results based on a series of 16 patients. Prog. Urol. 2005, 15, 641–645. [Google Scholar]
- Ramanitharan, M.; Lalgudi Narayanan, D.; Sreenivasan, S.R.; Sidhartha, K.; Mehra, K.; Rajiv, K.; Khelge, V. Outcomes of Robot-Assisted Ureterocalicostomy in Secondary Ureteropelvic Junction in Adults: Initial Experience Using Da Vinci Xi System with Near-Infrared Fluorescence Imaging. J. Laparoendosc. Adv. Surg. Tech. A 2020, 30, 48–52. [Google Scholar] [CrossRef]
- So, W.Z.; Tiong, H.Y. Robotic-Assisted Laparoscopic Ureterocalicostomy for Persistent Uretero-Pelvic Junction (UPJ) Obstruction after Failed Renal Pyeloplasty. Eur. Urol. Open Sci. 2022, 37, 1–2. Available online: https://www.sciencedirect.com/science/article/pii/S2666168321033917?via%3Dihub (accessed on 1 September 2025).
- Adamic, B.L.; Lombardo, A.; Andolfi, C.; Hatcher, D.; Gundeti, M.S. Pediatric robotic-assisted laparoscopic ureterocalycostomy: Salient tips and technical modifications for optimal repair. BJUI Compass 2021, 2, 53–57. [Google Scholar] [CrossRef]
- Ansari, M.S.; Danish, N.; Yadav, P.; Kaushik, V.N.; Kakoti, S.; Kumar, A.; Banthia, R.; Srivastava, A. Role of ureterocalicostomy in management of giant hydronephrosis in children in contemporary practice: Indications, outcomes and challenges. J. Pediatr. Urol. 2021, 17, 657.e1–657.e7. [Google Scholar] [CrossRef]
- Mittal, S.; Aghababian, A.; Eftekharzadeh, S.; Saxena, S.; Janssen, K.; Lombardo, A.; Adamic, B.; Dinardo, L.; Weaver, J.; Fischer, K.; et al. Robot-Assisted Laparoscopic Ureterocalicostomy in the Setting of Ureteropelvic Junction Obstruction: A Multi-Institutional Cohort. J. Urol. 2022, 208, 180–185. [Google Scholar] [CrossRef]
- Korets, R.; Hyams, E.S.; Shah, O.D.; Stifelman, M.D. Robotic-assisted laparoscopic ureterocalicostomy. Urology 2007, 70, 366–369. [Google Scholar] [CrossRef]
- Gill, I.S.; Cherullo, E.E.; Steinberg, A.; Desai, M.M.; Abreu, S.C.; Ng, C.; Kaouk, J.H. Laparoscopic ureterocalicostomy: Initial experience. J. Urol. 2004, 171, 1227–1230. [Google Scholar] [CrossRef]
- Schimpf, M.O.; Wagner, J.R. Case report: Robotic-assisted laparoscopic ureterocalicostomy with long-term follow-up. J. Endourol. 2009, 23, 293–295. [Google Scholar] [CrossRef]
- Astolfi, R.H.; Patavino, G.A.D.; Felipe Rosso, C.; Tedesco Silva Junior, H.; Medina Pestana, J.O.; Meller, A.E.; Aguiar, W. Ureterocalicostomy with lower pole nephrectomy in a renal transplant: A case report. Am. J. Transplant. 2018, 18, 2347–2351. [Google Scholar] [CrossRef]
- Salehipour, M.; Hosseini, N.; Adib, A. Ureterocalicostomy Using Native Ureter in an Allograft Kidney: A Case Report. Exp. Clin. Transplant. 2023, 21, 361–364. [Google Scholar] [CrossRef]
- Jarowenko, M.V.; Flechner, S.M. Recipient ureterocalycostomy in a renal allograft: Case report of a transplant salvage. J. Urol. 1985, 133, 844–845. [Google Scholar] [CrossRef]
- Ehrlich, R.M.; Whitmore, K.; Fine, R.N. Calycovesicostomy for total ureteral obstruction after renal transplantation. J. Urol. 1983, 129, 818–819. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Qaoud, T.; Al-Yousef, R.; Behbehani, B.; Al-Terki, A. Calycovesicostomy, Ureterocalycostomy, and Ileocalycostomy: Rare Reconstructive Options for Transplant Ureteral Strictures. Transplantology 2025, 6, 27. https://doi.org/10.3390/transplantology6030027
Al-Qaoud T, Al-Yousef R, Behbehani B, Al-Terki A. Calycovesicostomy, Ureterocalycostomy, and Ileocalycostomy: Rare Reconstructive Options for Transplant Ureteral Strictures. Transplantology. 2025; 6(3):27. https://doi.org/10.3390/transplantology6030027
Chicago/Turabian StyleAl-Qaoud, Talal, Rawan Al-Yousef, Basma Behbehani, and Abdullatif Al-Terki. 2025. "Calycovesicostomy, Ureterocalycostomy, and Ileocalycostomy: Rare Reconstructive Options for Transplant Ureteral Strictures" Transplantology 6, no. 3: 27. https://doi.org/10.3390/transplantology6030027
APA StyleAl-Qaoud, T., Al-Yousef, R., Behbehani, B., & Al-Terki, A. (2025). Calycovesicostomy, Ureterocalycostomy, and Ileocalycostomy: Rare Reconstructive Options for Transplant Ureteral Strictures. Transplantology, 6(3), 27. https://doi.org/10.3390/transplantology6030027






