Absence of Exogenous Glucose in the Perfusate During Kidney Hypothermic Machine Perfusion Does Not Affect Mitochondrial Function
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Model
2.2. Experimental Design
2.3. Hypothermic Machine Perfusion
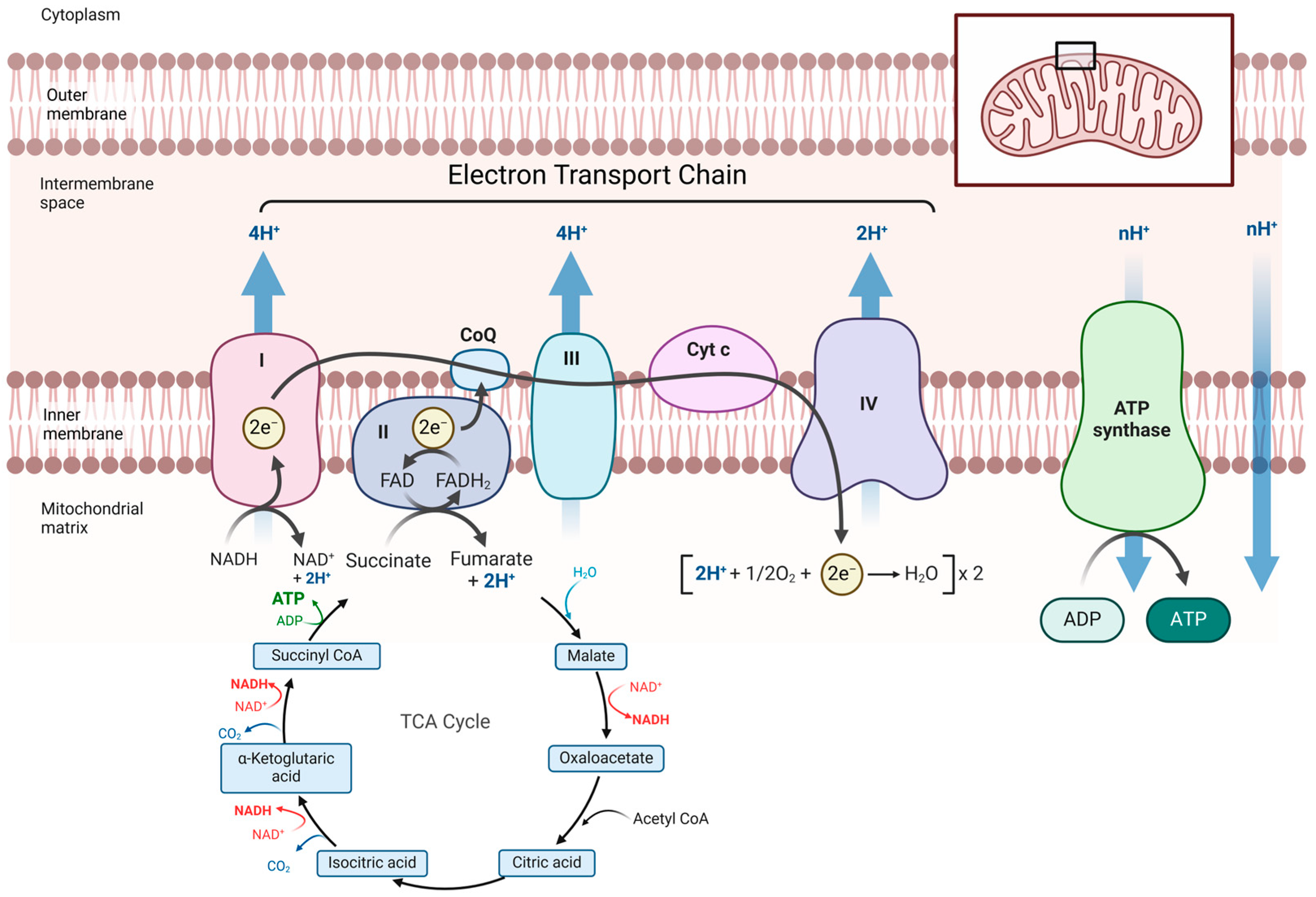
2.4. Mitochondrial Function Measurements
2.5. Oxidative Stress
2.6. Perfusate Samples
2.7. Flow and HMP Resistance
2.8. Histological Analysis
2.9. Statistical Analysis
3. Results
3.1. Mitochondrial Function During Hypothermic Machine Perfusion
3.2. ATP Concentration and Oxidative Stress
3.3. Glucose and Lactate Released into the Perfusion Solution
3.4. Flow and Intrarenal Vascular Resistance
3.5. Histological Assessment
4. Discussion and Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Brat, A.; Pol, R.A.; Leuvenink, H.G.D. Novel Preservation Methods to Increase the Quality of Older Kidneys. Curr. Opin. Organ Transplant. 2015, 20, 438–443. [Google Scholar] [CrossRef] [PubMed]
- Giwa, S.; Lewis, J.K.; Alvarez, L.; Langer, R.; Roth, A.E.; Church, G.M.; Markmann, J.F.; Sachs, D.H.; Chandraker, A.; Wertheim, J.A.; et al. The Promise of Organ and Tissue Preservation to Transform Medicine. Nat. Biotechnol. 2017, 35, 530–542. [Google Scholar] [CrossRef] [PubMed]
- Moers, C.; Leuvenink, H.G.D.; Ploeg, R.J. Non-Heart Beating Organ Donation: Overview and Future Perspectives. Transpl. Int. 2007, 20, 567–575. [Google Scholar] [CrossRef] [PubMed]
- Heylen, L.; Jochmans, I.; Samuel, U.; Tieken, I.; Naesens, M.; Pirenne, J.; Sprangers, B. The Duration of Asystolic Ischemia Determines the Risk of Graft Failure after Circulatory-Dead Donor Kidney Transplantation: A Eurotransplant Cohort Study. Am. J. Transplant. 2018, 18, 881–889. [Google Scholar] [CrossRef]
- Jochmans, I.; Moers, C.; Smits, J.M.; Leuvenink, H.G.D.; Treckmann, J.; Paul, A.; Rahmel, A.; Squifflet, J.-P.; van Heurn, E.; Monbaliu, D.; et al. Machine Perfusion Versus Cold Storage for the Preservation of Kidneys Donated After Cardiac Death. Ann. Surg. 2010, 252, 756–764. [Google Scholar] [CrossRef]
- Salvadori, M.; Rosso, G.; Bertoni, E. Update on Ischemia-Reperfusion Injury in Kidney Transplantation: Pathogenesis and Treatment. World J. Transplant. 2015, 5, 52. [Google Scholar] [CrossRef]
- Deng, R.; Gu, G.; Wang, D.; Tai, Q.; Wu, L.; Ju, W.; Zhu, X.; Guo, Z.; He, X. Machine Perfusion versus Cold Storage of Kidneys Derived from Donation after Cardiac Death: A Meta-Analysis. PLoS ONE 2013, 8, e56368. [Google Scholar] [CrossRef]
- Jiao, B.; Liu, S.; Liu, H.; Cheng, D.; Cheng, Y.; Liu, Y. Hypothermic Machine Perfusion Reduces Delayed Graft Function and Improves One-Year Graft Survival of Kidneys from Expanded Criteria Donors: A Meta-Analysis. PLoS ONE 2013, 8, e81826. [Google Scholar] [CrossRef]
- Foguenne, M.; MacMillan, S.; Kron, P.; Nath, J.; Devresse, A.; De Meyer, M.; Michel, M.; Hosgood, S.; Darius, T. Current Evidence and Future Perspectives to Implement Continuous and End-Ischemic Use of Normothermic and Oxygenated Hypothermic Machine Perfusion in Clinical Practice. J. Clin. Med. 2023, 12, 3207. [Google Scholar] [CrossRef]
- Watson, C.J.E.; Wells, A.C.; Roberts, R.J.; Akoh, J.A.; Friend, P.J.; Akyol, M.; Calder, F.R.; Allen, J.E.; Jones, M.N.; Collett, D.; et al. Cold Machine Perfusion Versus Static Cold Storage of Kidneys Donated After Cardiac Death: A UK Multicenter Randomized Controlled Trial. Am. J. Transplant. 2010, 10, 1991–1999. [Google Scholar] [CrossRef]
- Venema, L.H.; Brat, A.; Moers, C.; ‘t Hart, N.A.; Ploeg, R.J.; Hannaert, P.; Minor, T.; Leuvenink, H.G.D. Effects of Oxygen During Long-Term Hypothermic Machine Perfusion in a Porcine Model of Kidney Donation After Circulatory Death. Transplantation 2019, 103, 2057–2064. [Google Scholar] [CrossRef] [PubMed]
- Jochmans, I.; Brat, A.; Davies, L.; Hofker, H.S.; van de Leemkolk, F.E.M.; Leuvenink, H.G.D.; Knight, S.R.; Pirenne, J.; Ploeg, R.J.; Abramowicz, D.; et al. Oxygenated versus Standard Cold Perfusion Preservation in Kidney Transplantation (Compare): A Randomised, Double-Blind, Paired, Phase 3 Trial. Lancet 2020, 396, 1653–1662. [Google Scholar] [CrossRef] [PubMed]
- Moers, C.; Smits, J.M.; Maathuis, M.-H.J.; Treckmann, J.; van Gelder, F.; Napieralski, B.P.; van Kasterop-Kutz, M.; van der Heide, J.J.H.; Squifflet, J.-P.; van Heurn, E.; et al. Machine Perfusion or Cold Storage in Deceased-Donor Kidney Transplantation. N. Engl. J. Med. 2009, 360, 7–19. [Google Scholar] [CrossRef]
- Husen, P.; Boffa, C.; Jochmans, I.; Krikke, C.; Davies, L.; Mazilescu, L.; Brat, A.; Knight, S.; Wettstein, D.; Cseprekal, O.; et al. Oxygenated End-Hypothermic Machine Perfusion in Expanded Criteria Donor Kidney Transplant. JAMA Surg. 2021, 156, 517. [Google Scholar] [CrossRef]
- Hendriks, K.D.W.; Brüggenwirth, I.M.A.; Maassen, H.; Gerding, A.; Bakker, B.; Porte, R.J.; Henning, R.H.; Leuvenink, H.G.D. Renal Temperature Reduction Progressively Favors Mitochondrial ROS Production over Respiration in Hypothermic Kidney Preservation. J. Transl. Med. 2019, 17, 265. [Google Scholar] [CrossRef]
- Nath, J.; Smith, T.; Hollis, A.; Ebbs, S.; Canbilen, S.W.; Tennant, D.A.; Ready, A.R.; Ludwig, C. 13C Glucose Labelling Studies Using 2D NMR Are a Useful Tool for Determining Ex Vivo Whole Organ Metabolism during Hypothermic Machine Perfusion of Kidneys. Transplant. Res. 2016, 5, 7. [Google Scholar] [CrossRef]
- Patel, K.; Smith, T.B.; Neil, D.A.H.; Thakker, A.; Tsuchiya, Y.; Higgs, E.B.; Hodges, N.J.; Ready, A.R.; Nath, J.; Ludwig, C. The Effects of Oxygenation on Ex Vivo Kidneys Undergoing Hypothermic Machine Perfusion. Transplantation 2019, 103, 314–322. [Google Scholar] [CrossRef]
- Nath, J.; Smith, T.B.; Patel, K.; Ebbs, S.R.; Hollis, A.; Tennant, D.A.; Ludwig, C.; Ready, A.R. Metabolic Differences between Cold Stored and Machine Perfused Porcine Kidneys: A 1 H NMR Based Study. Cryobiology 2017, 74, 115–120. [Google Scholar] [CrossRef]
- Liu, R.X.; Koyawala, N.; Thiessen-Philbrook, H.R.; Doshi, M.D.; Reese, P.P.; Hall, I.E.; Mohan, S.; Parikh, C.R. Untargeted Metabolomics of Perfusate and Their Association with Hypothermic Machine Perfusion and Allograft Failure. Kidney Int. 2023, 103, 762–771. [Google Scholar] [CrossRef]
- Guy, A.J.; Nath, J.; Cobbold, M.; Ludwig, C.; Tennant, D.A.; Inston, N.G.; Ready, A.R. Metabolomic Analysis of Perfusate During Hypothermic Machine Perfusion of Human Cadaveric Kidneys. Transplantation 2015, 99, 754–759. [Google Scholar] [CrossRef]
- Brand, M.D.; Nicholls, D.G. Assessing Mitochondrial Dysfunction in Cells. Biochem. J. 2011, 435, 297–312. [Google Scholar] [CrossRef] [PubMed]
- van Furth, L.A.; Efraimoglou, D.; Gerding, A.; Bakker, B.M.; Olinga, P.; Leuvenink, H.G.D.; Venema, L.H. Macronutrient Supplementation during Prolonged Normothermic Incubation Increases Mitochondrial Function of Precision-Cut Kidney Slices after Ischemia. Transplantology 2024. submitted. [Google Scholar]
- Divakaruni, A.S.; Jastroch, M. A Practical Guide for the Analysis, Standardization and Interpretation of Oxygen Consumption Measurements. Nat. Metab. 2022, 4, 978–994. [Google Scholar] [CrossRef] [PubMed]
- Mahboub, P.; Ottens, P.; Seelen, M.; ’t Hart, N.; Van Goor, H.; Ploeg, R.; Martins, P.N.; Leuvenink, H. Gradual Rewarming with Gradual Increase in Pressure during Machine Perfusion after Cold Static Preservation Reduces Kidney Ischemia Reperfusion Injury. PLoS ONE 2016, 11, e0152006. [Google Scholar] [CrossRef]
- Pool, M.B.F.; Hamelink, T.L.; Van Goor, H.; Van Den Heuvel, M.C.; Leuvenink, H.G.D.; Moers, C. Prolonged Ex-Vivo Normothermic Kidney Perfusion: The Impact of Perfusate Composition. PLoS ONE 2021, 16, e0251595. [Google Scholar] [CrossRef]
- Casanova, A.; Wevers, A.; Navarro-Ledesma, S.; Pruimboom, L. Mitochondria: It Is All about Energy. Front. Physiol. 2023, 14, 1114231. [Google Scholar] [CrossRef]
- Bon, D.; Claire, B.; Thuillier, R.; Hebrard, W.; Boildieu, N.; Celhay, O.; Irani, J.; Seguin, F.; Hauet, T. Analysis of Perfusates During Hypothermic Machine Perfusion by NMR Spectroscopy. Transplantation 2014, 97, 810–816. [Google Scholar] [CrossRef]
- Darius, T.; Vergauwen, M.; Smith, T.; Gerin, I.; Joris, V.; Mueller, M.; Aydin, S.; Muller, X.; Schlegel, A.; Nath, J.; et al. Brief O2 Uploading during Continuous Hypothermic Machine Perfusion Is Simple yet Effective Oxygenation Method to Improve Initial Kidney Function in a Porcine Autotransplant Model. Am. J. Transplant. 2020, 20, 2030–2043. [Google Scholar] [CrossRef]
- Patel, K.; Nath, J.; Smith, T.; Darius, T.; Thakker, A.; Dimeloe, S.; Inston, N.; Ready, A.; Ludwig, C. Metabolic Characterization of Deceased Donor Kidneys Undergoing Hypothermic Machine Perfusion Before Transplantation Using 13C-Enriched Glucose. Transplant. Direct 2024, 11, e1736. [Google Scholar] [CrossRef]
- Verstraeten, L.; Den Abt, R.; Ghesquière, B.; Jochmans, I. Current Insights into the Metabolome during Hypothermic Kidney Perfusion—A Scoping Review. J. Clin. Med. 2023, 12, 3613. [Google Scholar] [CrossRef]
- Legouis, D.; Faivre, A.; Cippà, P.E.; de Seigneux, S. Renal Gluconeogenesis: An Underestimated Role of the Kidney in Systemic Glucose Metabolism. Nephrol. Dial. Transplant. 2022, 37, 1417–1425. [Google Scholar] [CrossRef] [PubMed]
- King, M.W. Carbohydrates: Galactose Metabolism. In Integrative Medical Biochemistry Examination and Board Review; McGraw-Hill Education: New York, NY, USA, 2014. [Google Scholar]
- t Hart, N.A.; der van Plaats, A.; Leuvenink, H.G.D.; van Goor, H.; Wiersema-Buist, J.; Verkerke, G.J.; Rakhorst, G.; Ploeg, R.J. Determination of an Adequate Perfusion Pressure for Continuous Dual Vessel Hypothermic Machine Perfusion of the Rat Liver. Transpl. Int. 2007, 20, 343–352. [Google Scholar] [CrossRef] [PubMed]
- Gao, A.W.; Cantó, C.; Houtkooper, R.H. Mitochondrial Response to Nutrient Availability and Its Role in Metabolic Disease. EMBO Mol. Med. 2014, 6, 580–589. [Google Scholar] [CrossRef] [PubMed]
- Akdogan, M.; Demirbakan, K.; Baydilek, Y.; Yuksel, Y. Lactated Ringer as Preservation Solution in Living Donor Renal Transplantation. Transplant. Proc. 2023, 55, 1134–1139. [Google Scholar] [CrossRef]
- Tan, H.P.; Vyas, D.; Basu, A.; Randhawa, P.; Shah, N.; Donaldson, J.; Marcos, A.; Simmons, R.L.; Starzl, T.E.; Shapiro, R. Cold Heparinized Lactated Ringers with Procaine (HeLP) Preservation Fluid in 266 Living Donor Kidney Transplantations. Transplantation 2007, 83, 1134–1136. [Google Scholar] [CrossRef]
- Nath, J.; Guy, A.; Smith, T.B.; Cobbold, M.; Inston, N.G.; Hodson, J.; Tennant, D.A.; Ludwig, C.; Ready, A.R. Metabolomic Perfusate Analysis during Kidney Machine Perfusion: The Pig Provides an Appropriate Model for Human Studies. PLoS ONE 2014, 9, e114818. [Google Scholar] [CrossRef]
- Darius, T.; Nath, J.; Mourad, M. Simply Adding Oxygen during Hypothermic Machine Perfusion to Combat the Negative Effects of Ischemia-Reperfusion Injury: Fundamentals and Current Evidence for Kidneys. Biomedicines 2021, 9, 993. [Google Scholar] [CrossRef]
- Ravaioli, M.; Baldassare, M.; Vasuri, F.; Pasquinelli, G.; Laggetta, M.; Valente, S.; De Pace, V.; Neri, F.; Siniscalchi, A.; Zanfi, C.; et al. Strategies to Restore Adenosine Triphosphate (ATP) Level After More than 20 Hours of Cold Ischemia Time in Human Marginal Kidney Grafts. Ann. Transplant. 2018, 23, 34–44. [Google Scholar] [CrossRef]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Efraimoglou, D.; van Furth, L.A.; Gerding, A.; Bakker, B.M.; Hillebrands, J.-L.; Leuvenink, H.G.D.; Venema, L.H. Absence of Exogenous Glucose in the Perfusate During Kidney Hypothermic Machine Perfusion Does Not Affect Mitochondrial Function. Transplantology 2025, 6, 8. https://doi.org/10.3390/transplantology6020008
Efraimoglou D, van Furth LA, Gerding A, Bakker BM, Hillebrands J-L, Leuvenink HGD, Venema LH. Absence of Exogenous Glucose in the Perfusate During Kidney Hypothermic Machine Perfusion Does Not Affect Mitochondrial Function. Transplantology. 2025; 6(2):8. https://doi.org/10.3390/transplantology6020008
Chicago/Turabian StyleEfraimoglou, Dafni, L. Annick van Furth, Albert Gerding, Barbara M. Bakker, Jan-Luuk Hillebrands, Henri G. D. Leuvenink, and Leonie H. Venema. 2025. "Absence of Exogenous Glucose in the Perfusate During Kidney Hypothermic Machine Perfusion Does Not Affect Mitochondrial Function" Transplantology 6, no. 2: 8. https://doi.org/10.3390/transplantology6020008
APA StyleEfraimoglou, D., van Furth, L. A., Gerding, A., Bakker, B. M., Hillebrands, J.-L., Leuvenink, H. G. D., & Venema, L. H. (2025). Absence of Exogenous Glucose in the Perfusate During Kidney Hypothermic Machine Perfusion Does Not Affect Mitochondrial Function. Transplantology, 6(2), 8. https://doi.org/10.3390/transplantology6020008





