Abstract
Portal inflow modulation has become standard practice in many transplant centers performing living donor liver transplantation. This is believed to counteract the deleterious effects of excess portal flow into a small-for-size graft. A splenectomy negates the contribution of the splenic vein flow completely and thereby substantially reduces portal inflow. Although it has been adopted as a standard strategy by many Japanese centers for inflow modulation, especially while using left hemiliver grafts, there is justifiable apprehension about its usage due to potential increases in morbidity. Described here is a splenectomy performed while using a modified right hemiliver graft with a graft to recipient weight ratio of 0.49. The challenges in decision making and reasons regarding how such a small graft might have worked without manifestations of small-for-size/flow syndrome are discussed.
1. Introduction
In living donor liver transplant (LDLT), balancing the risks and benefits of using a small graft is always a challenge. With inflow modulation, smaller grafts fare better, but the acceptable minimum graft to recipient weight ratio (GRWR) depends on a complex interplay between many donor and recipient factors. Donor age, extent of macrovascular steatosis, GRWR, recipient metabolic demand and portal hemodynamic status are important considerations while planning and matching a given donor–recipient pair. Herein is a report of LDLT using a right hemiliver graft with a GRWR of 0.49 with a splenectomy for graft inflow modulation.
2. Case Report
A 41-year-old gentleman with chronic decompensated liver disease presented with a history of ascites, hepatic encephalopathy and hepatorenal syndrome. Obesity and alcohol were contributory etiological factors in this case. Patient was abstinent for over 8 months at the time of transplant. His model for end-stage liver disease (MELD) score was 23. The pre-transplant investigations were as follows: 4.1 mg/dL (bilirubin), 43/16 U/L (AST/ALT), 121/74 U/L (SAP/GGT), 7.5/3.5 mg/dL (total protein/albumin), 2.46 (INR), 138 mmol/L (sodium) and 1.14 mg/dL (creatinine). His height, weight, body mass index and body surface area were 160 cm, 114 kg, 44.5 kg/m2 and 2.28 m2, respectively. He was a bleeder status endoscopic variceal ligation; his platelet count was 100 × 109/L and his spleen was 18 cm in its longest axis. The estimated spleen volume on pre-operative CT scan was 724.5 cc. The patient was on Thyroxine tablets for hypothyroidism and on continuous positive airway pressure support for obstructive sleep apnea. He was initially considered for a dual lobe liver transplant, as a single donor would not normally be able to donate a liver with a GRWR > 0.8 (being a foreign national, he did not have the option of deceased donor LT). One of the donors was rejected due to the presence of an incidentally detected 1.5 cm space-occupying lesion in the liver, suspected to be early hepatocellular carcinoma (he was hepatitis B core antibody positive with elevated tumor markers). The second donor was a 26-year-old lady with a total liver volume of 1079 mL and an estimated right hemiliver volume of 600 mL (GRWR 0.53). A detailed informed discussion was held with the patient and family about the risks of waiting as opposed to the risk of early allograft dysfunction and increased mortality due to a small sized graft. Splenectomy was planned for portal inflow modulation, and the patient was vaccinated against Pneumococcus before the transplant. The intra operative starting portal pressure was 26 mm Hg. A splenectomy was performed before hepatectomy using LigaSure™ vessel sealer and vascular staplers for the hilum after separating the tail of the pancreas. The splenic artery was ligated in the lesser sac before commencing the dissection of the hilum. Blood loss during this phase of the operation was minimal. The dry weight of the splenectomy specimen (measured in the pathology lab) was 550 g. The total intra operative transfusion was five units of packed red blood cells, four units of fresh frozen plasma and 10 units cryoprecipitate. The actual graft weight was 550 g (GRWR 0.49). A single orifice outflow was constructed by using a polytetrafluroethylene graft sewn from the anterior sector veins to the anterior wall of the right hepatic vein. The inferior vein was implanted separately onto the recipient cava using a side clamp. The graft portal vein was anastomosed to the recipient main portal vein using 6.0 polypropylene running sutures, leaving a growth factor. The hepatic artery was reconstructed by anastomosing the recipient’s right hepatic artery to the donor’s right hepatic artery using 7.0 polypropylene under loupe magnification. After implantation, the portal flow was 210 mL/100 gm/min, the outflow was triphasic, and the hepatic artery flow was satisfactory. We did not measure the portal venous pressure after reperfusion. He was shifted to the intensive care unit with no inotropes, stable hemodynamics and a normal lactate. He did well post-operatively and was extubated on day 1. The portal flow averaged around 1.47 (±0.25) L/min in the first week. With a graft weight of 550 g, this amounted to 267 mL/100 g of liver tissue/min. The average hepatic artery resistivity index during this period was 0.67 (±0.04). Bilirubin and INR normalized on day 8 and day 10, respectively. Ascitic output was less than 500 mL on post-operative day 11. Post-operative platelet counts peaked at 594 × 103/µL on day 12 and came down to 326 × 103/µL on day 42. The patient was started on Ecosprin 150 mg OD on day 3 and low molecular weight heparin in prophylactic doses. Graft function remained excellent with a normal Doppler. He developed a bile leak with a collection, for which an ultrasound-guided percutaneous drain (PCD) was placed on post-operative day 14. The patient was discharged on post-operative day 21. Subsequently, in the fifth post-operative week, the patient underwent endoscopic retrograde cholangiography and stenting of the bile duct, followed by removal of the PCD. He remains well and stent-free at 10 months follow-up with normal graft function.
3. Discussion
Small-for-size syndrome (SFSS) is a graft dysfunction caused by insufficient critical mass of the transplanted liver to sustain metabolic functions of the recipient, in the face of portal hyperperfusion, resulting in changes in microcirculation, sinusoidal injury, and altered regeneration. It manifests as prolonged cholestasis, coagulopathy, ascites and, sometimes, encephalopathy. Typically, definitions for SFSS include both graft size and portal flow parameters [1]. The best strategy is prevention, as therapeutic options are limited.
A recent systematic review by an expert panel [2] concluded that portal inflow modulation may help reduce morbidity/mortality in LDLT recipients with small-for-size grafts. This was a strong recommendation despite the evidence from the constituent studies (largely retrospective series) being of low quality. There are three ways to decrease the portal flow to the transplanted liver: splenic artery ligation, splenectomy, and hemi-portocaval shunts. The decision making process of portal flow modulation is described in a recent review [3]. In the authors’ institution, post reperfusion splenic artery ligation is the preferred strategy if portal pressure post reperfusion is more than 20 mm Hg or if flow is more than 350 mL/100 g of the liver tissue/min in a patient with GRWR < 0.8. If the estimated GRWR is <0.7, we would consider a pre-reperfusion splenectomy in select instances. Our group is not comfortable with hemiportocaval shunts. With the estimated GRWR being less than 0.6, and the MELD score being 23, a splenectomy was felt to be the best modality for inflow modulation in this setting to ensure reliable and maximum reduction in portal flow in a controlled manner. Although a hemiportocaval shunt is preferred by many groups, as it entails no additional dissection, we opted against this as it is difficult to precisely regulate how much blood goes into the graft. The pre-operative CT scan in the portal phase was studied carefully before this decision was taken. A massively enlarged spleen, dilated splenic vein and the relative absence of peri splenic collaterals were factors considered while making this decision (Figure 1a). The decision to perform a splenectomy prior to hepatectomy was a well thought-out pre-operative decision and was conveyed to the patient and the anesthesia team at the multidisciplinary meeting. We based this on the recent paper by Fujiki et al. [4], who reported excellent outcomes in their series of 130 LDLT, with SFSS developing in only one patient despite using small grafts. Twelve patients had very small grafts (GRWR < 0.6). In this subgroup, early allograft dysfunction occurred in two patients, one of them succumbing to sepsis due to bile leak. The authors have used the strategy for pre-reperfusion splenectomy for patients with GRWR < 0.7 [4]. We have adopted the same strategy in the index patient; this prevents exposure of the graft to sinusoidal injury post reperfusion and also prevents a hit to the graft due to hypotension if bleeding were to occur during the splenectomy. One-year graft outcomes seem to be better in small grafts with a pre-reperfusion splenectomy rather than post reperfusion splenectomy [4]. The Kyushu university group has been doing splenectomy for inflow modulation, especially while using left hemiliver grafts, for many years with good results, and no increase in morbidity. In a recent study [5], it has been shown with propensity score matching, that a splenectomy has a beneficial effect on graft survival when performed for post reperfusion portal pressures of more than 20 mm Hg. We elected to do the splenectomy first and obtained a satisfactory portal flow post reperfusion. The portal flow remained consistently within the normal range without hyperperfusion in the first post operative week; therefore, the hepatic artery buffer response was not pronounced. By this time, bilirubin and INR were normalizing and ascitic output was showing a downward trend, suggesting stable graft function.
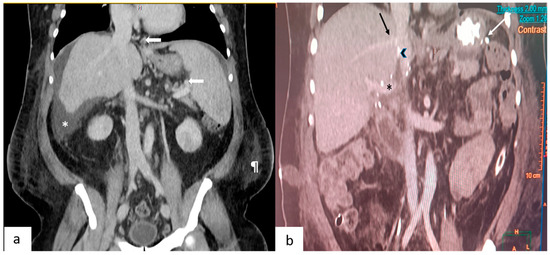
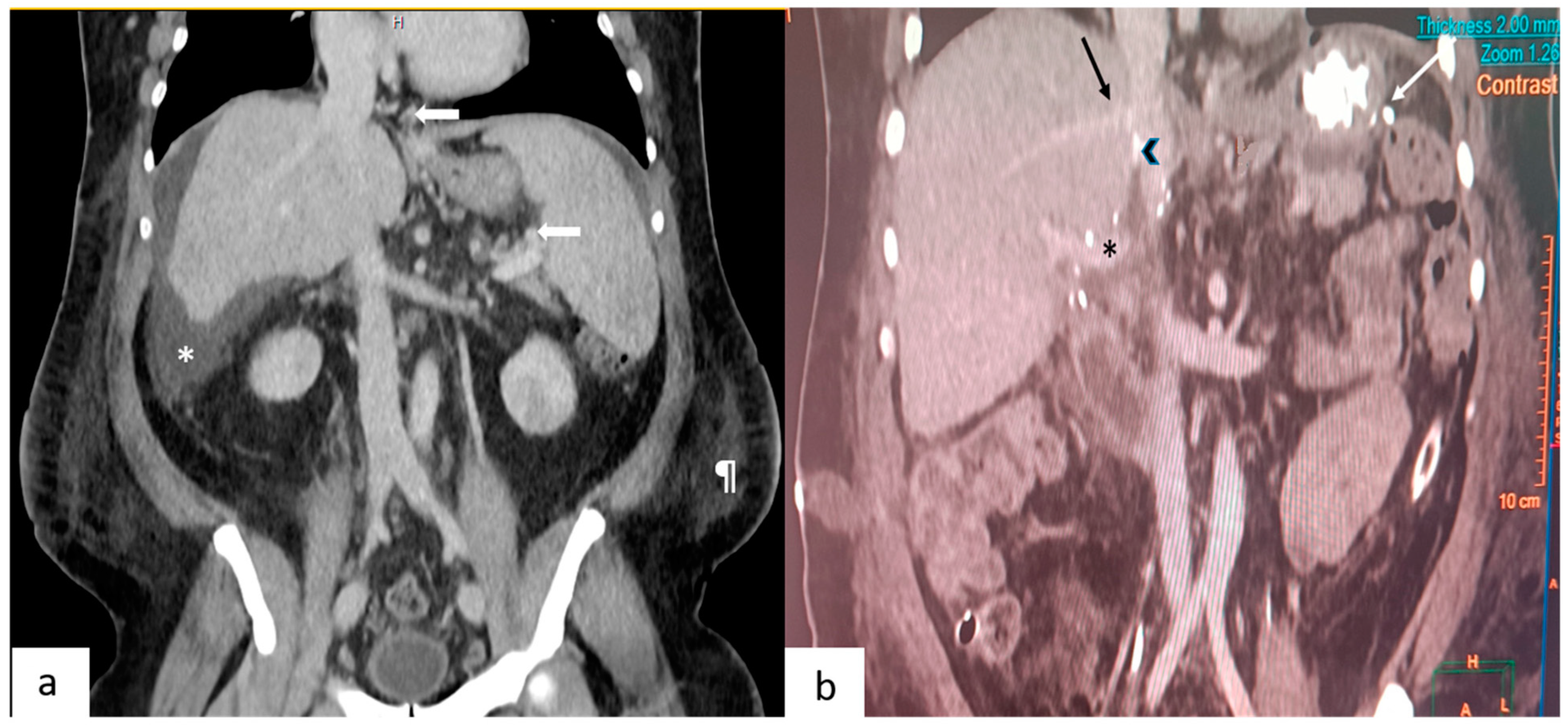
Figure 1.
(a) This depicts a coronal section of a pre-operative contrast-enhanced CT scan. Arrows point to the gastroesophageal and splenic perihilar collaterals. Also seen are ascites (*) and the prominent subcutaneous fat (¶) in the flanks. (b) This depicts a coronal section of a post-operative CT scan performed on day 7. Seen are the junction of the right hepatic vein (black arrow), polytetrafluroethylene graft (arrowhead) with the inferior vena cava, portal vein (*) and vascular staplers (white arrow) used during splenectomy.
Due to familiarity in performing splenectomy and proximal splenorenal shunts for extra-hepatic portal vein obstruction, a splenectomy could be performed without any additional morbidity. The patient did not develop pancreatitis/portal vein thrombosis/post-splenectomy infection. Generally, adult patients do not have long term complications due to splenectomy despite being on immunosuppression. In the majority of the instances, modulation with splenectomy is usually performed while using left hemiliver grafts; in this instance, we had to use it despite using a modified right hemiliver graft as the GRWR was still low (<0.6).
It is interesting to analyze how a very small graft (GRWR 0.49) worked for the patient without any features of SFSS. Firstly, the donor liver was of excellent quality. It was a 26-year-old liver without any trace of fat infiltration (not biopsiedtherefore). Secondly, the outflow was reconstructed meticulously in a standard manner [6]. Doppler waveforms of the common right with reconstructed anterior sector outflow and the inferior hepatic vein were triphasic. Figure 1b depicts a section of the post-operative CT scan. Thirdly the GRWR may have been spuriously low due to the fluid overload of the recipient. An accurate weight of the recipient is difficult to determine in the presence of cirrhosis and ascites, but some of the indicators of falsely high weight are tense ascites, fluid overload, anasarca, and renal dysfunction with oliguria. If possible, the patient should be offloaded as much as possible. The index patient was diuretic intolerant, and therefore we decided to proceed with the transplant after the creatinine normalized (peak value 1.8 mg/dL). Post-transplant, his weight reduced to 90 kg within a period of one month. Notwithstanding this, the GRWR for a 550 g liver in a 90 kg recipient is still low (0.61), and therefore making a pre-operative decision to proceed in this situation is certainly a difficult one to make. Fourthly, a second hit to a regenerating small graft might tilt the scales unfavorably. Immunosuppression should be carefully optimized to prevent rejection. This patient had a bile leak that was detected towards the end of the first week. This was picked up on a CT scan performed on a high index of suspicion as it was a ‘high risk’ biliary anastomosis. There were two anastomoses and the recipient cystic duct was anastomosed to the donor’s right posterior duct. As the bile leak occurred when the graft function had already been stabilized, it did not significantly impair patient recovery. It was managed as a controlled external biliary fistula followed by endoscopic biliary stenting after 5 weeks.
Careful consideration of donor and recipient factors, especially recipient portal hemodynamics is indispensable in LDLT, especially while using small grafts. Finally, there is the intangible element of complete trust from the patient’s family that spurred us to take on this transplant.
4. Conclusions
Pre-reperfusion splenectomy is an effective strategy to reduce portal flow in very small-sized grafts and can be used safely and successfully without the development of SFSS.
Author Contributions
Conceptualization, K.G.B. and P.K.S.; data curation, P.K.S. and K.G.B.; writing—original draft preparation, P.K.S. and K.G.B.; writing—review and editing, K.G.B. and P.K.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Ethical review and approval were waived for this study as there were no interventions made in regard to research intent. Standard clinical protocol was followed while managing the patient. Hence, IRB approval is not required.
Informed Consent Statement
Informed consent was obtained from the subjects involved in the study for publication of case details. However, no identifying information is divulged in the report.
Data Availability Statement
The relevant data generated or analysed during this study are included in this article. All data is not publicly available in the interest of patient privacy. Further enquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Hernandez-Alejandro, R.; Sharma, H. Small-for-size syndrome in liver transplantation: New horizons to cover with a good launchpad. Liver. Transpl. 2016, 22, 33–36. [Google Scholar] [CrossRef] [PubMed]
- Rammohan, A.; Rela, M.; Kim, D.S.; Soejima, Y.; Kasahara, M.; Ikegami, T.; Spiro, M.; Aristotle Raptis, D.; Humar, A.; ERAS4OLT.org Working Group. Does modification of portal pressure and flow enhance recovery of the recipient after living donor liver transplantation? A systematic review of literature and expert panel recommendations. Clin. Transplant. 2022, 36, e14657. [Google Scholar] [CrossRef] [PubMed]
- Bharathy, K.G.; Shenvi, S. Portal Hemodynamics after Living-Donor Liver Transplantation: Management for Optimal Graft and Patient Outcomes—A Narrative Review. Transplantology 2023, 4, 38–58. [Google Scholar] [CrossRef]
- Fujiki, M.; Hashimoto, K.; Quintini, C.; Aucejo, F.; Kwon, C.H.D.; Matsushima, H.; Sasaki, K.; Campos, L.; Eghtesad, B.; Diago, T.; et al. Living Donor Liver Transplantation with Augmented Venous Outflow and Splenectomy: A Promised Land for Small Left Lobe Grafts. Ann. Surg. 2022, 276, 838–845, Erratum in Ann. Surg. 2023, 277, e978. [Google Scholar] [CrossRef] [PubMed]
- Yoshizumi, T.; Itoh, S.; Shimokawa, M.; Inokuchi, S.; Harada, N.; Takeishi, K.; Mano, Y.; Yoshiya, S.; Kurihara, T.; Nagao, Y.; et al. Simultaneous splenectomy improves outcomes after adult living donor liver transplantation. J. Hepatol. 2021, 74, 372–379. [Google Scholar] [CrossRef] [PubMed]
- Pamecha, V.; Pattnaik, B.; Sinha, P.K.; Patil, N.S.; Mohapatra, N.; Sasturkar, S.V.; Sundararajan, V.B.; Thapar, S.; Sindwani, G.; Arora, M.K. Single Orifice Outflow Reconstruction: Refining the Venous Outflow in Modified Right Lobe Live Donor Liver Transplantation. J. Gastrointest. Surg. 2021, 25, 1962–1972. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).