Modelling Acid-Induced Lung Damage in Precision-Cut Lung Slices: An Ex Vivo Animal Model
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
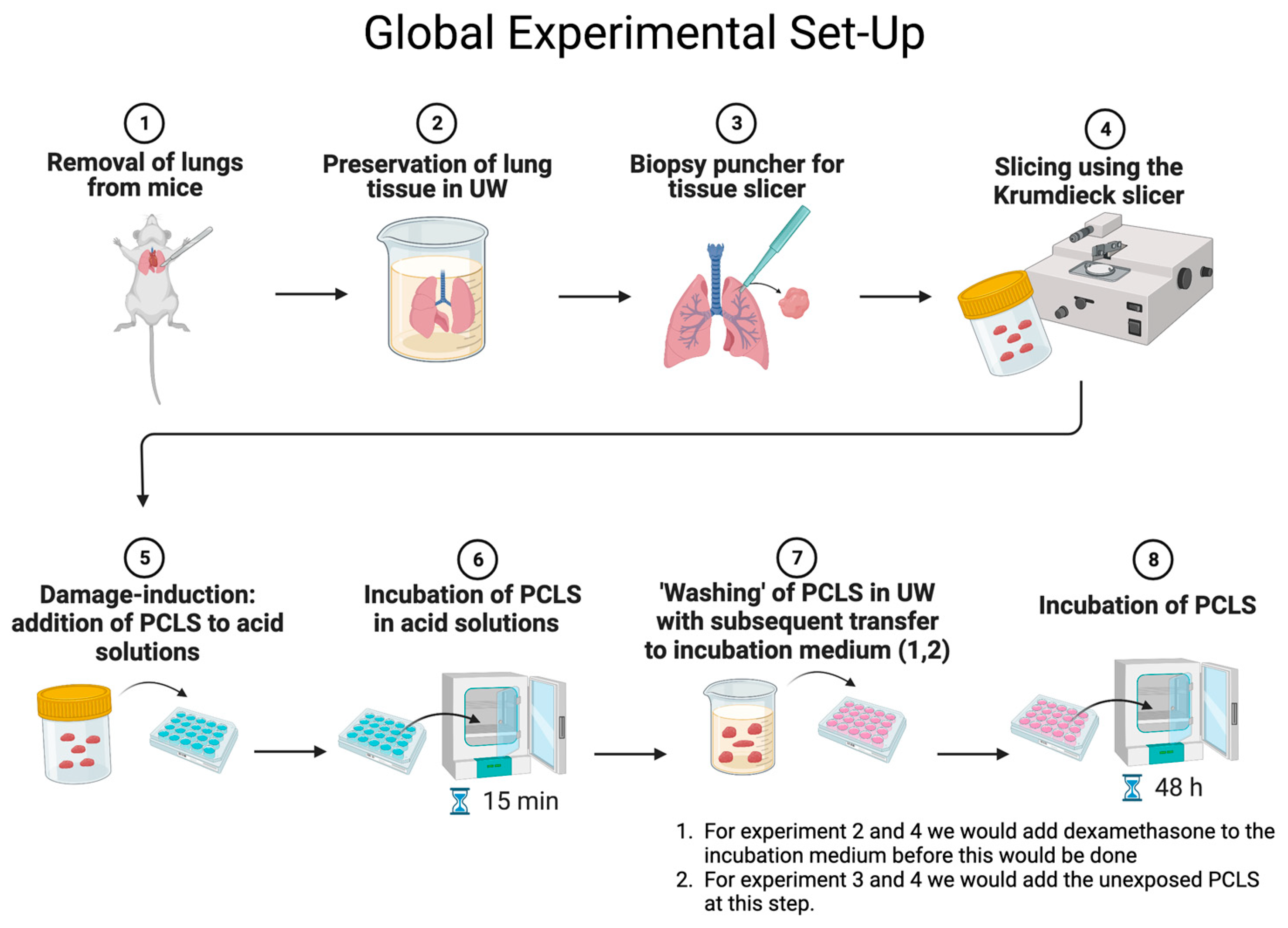
2.2. Precision-Cut Lung Slices
2.3. Experimental Set-Up
2.3.1. Evaluation of Cell Viability
2.3.2. Cytokine Release
2.3.3. Stainings
2.4. Statistics
3. Results
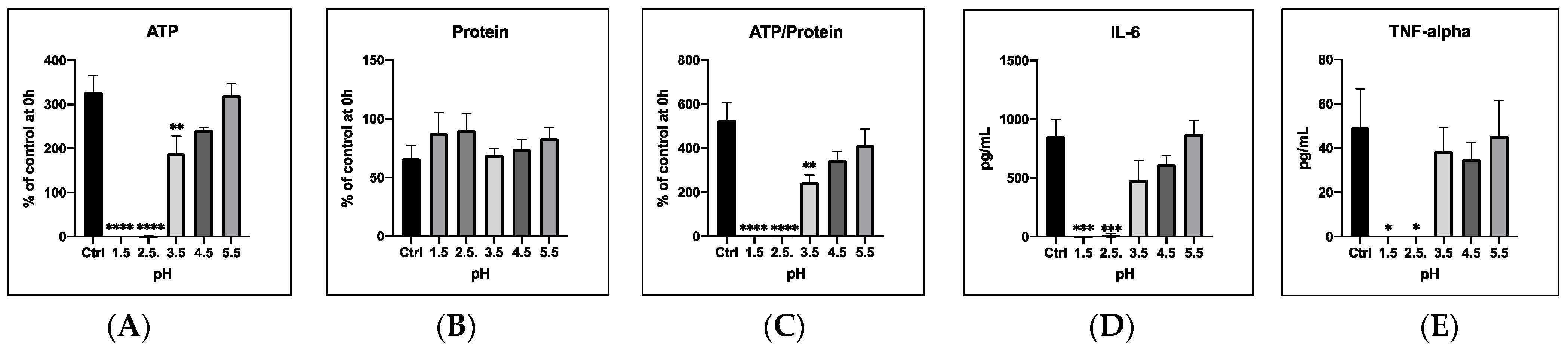
3.1. Modelling an Aspiration Event by Incubating PCLS with Acidic Saline Solutions
3.1.1. General Cell Viability
3.1.2. Cytokine Release
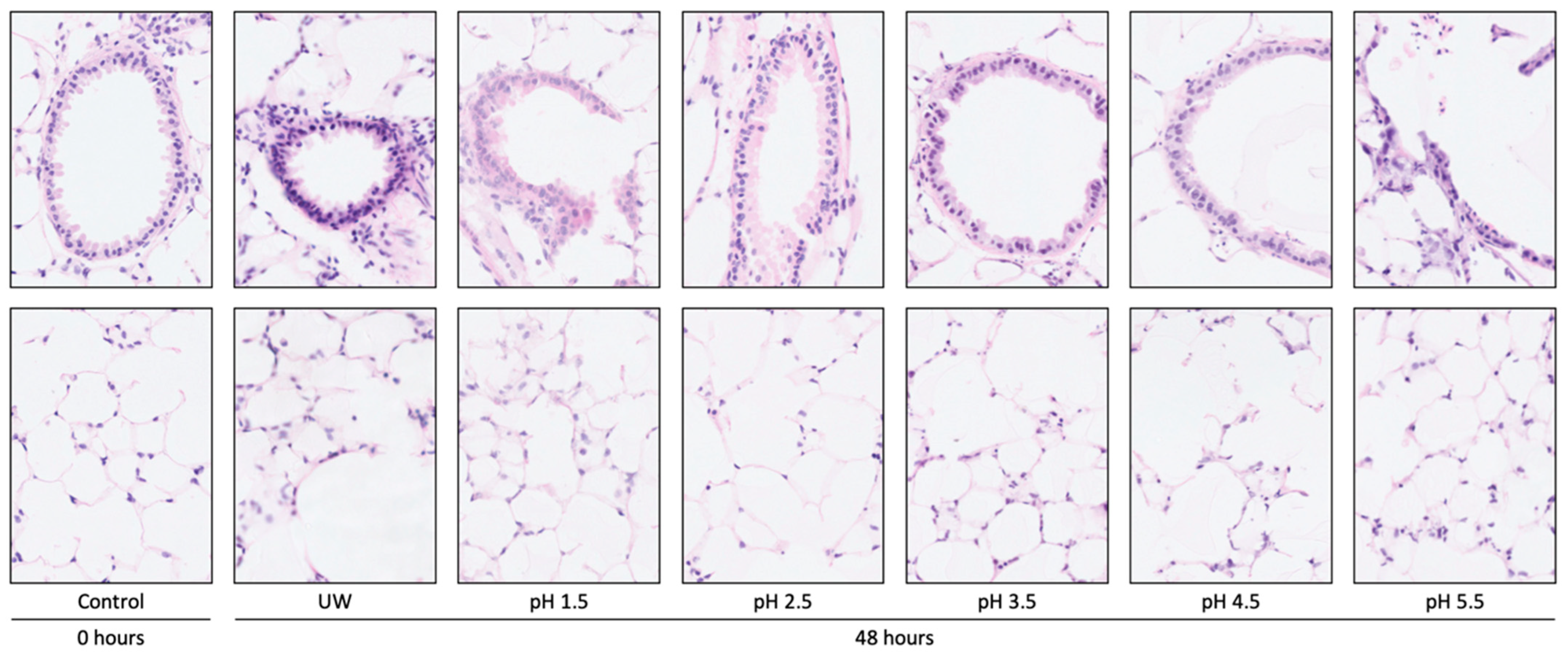
3.1.3. Tissue Damage
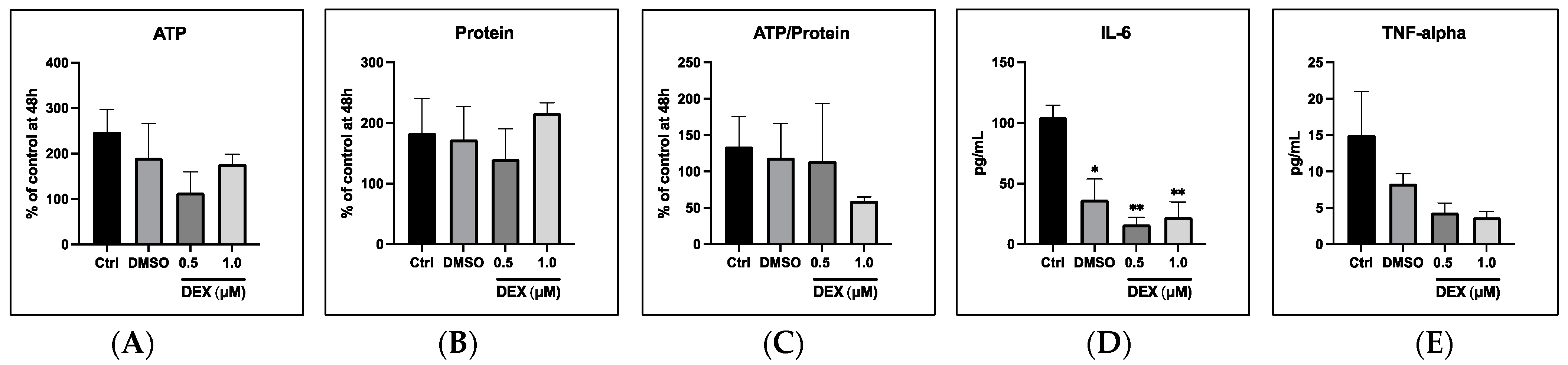
3.2. Treatment of Acid-Exposed (pH 3.5) PCLS with Dexamethasone and DMSO
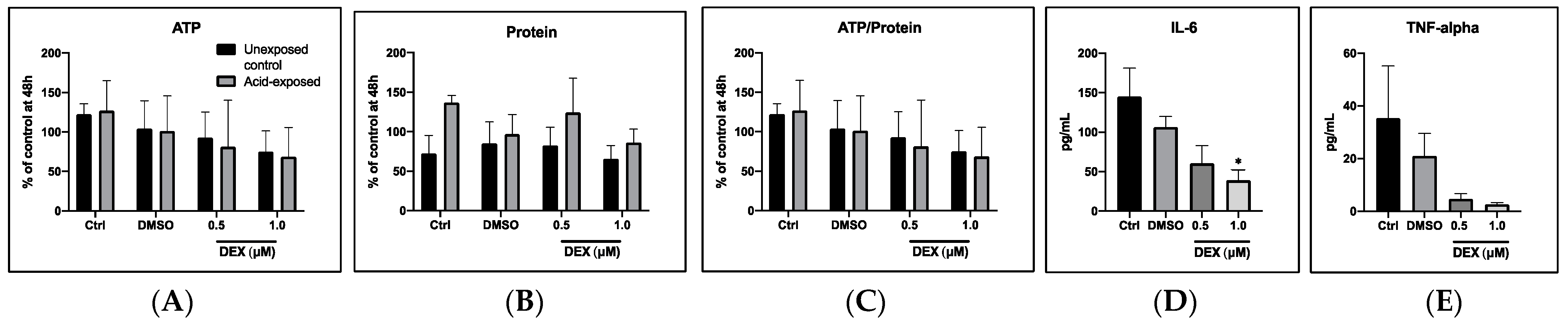
3.2.1. General Cell Viability
3.2.2. Cytokine Release
3.3. The Effects of Acid-Exposed PCLS to Unexposed Control PCLS
3.3.1. General Cell Viability
3.3.2. Cytokine Release
3.4. Dexamethasone to Prevent Damage from Acid-Exposed PCLS to Unexposed Control PCLS
3.4.1. General Cell Viability
3.4.2. Cytokine Release
4. Discussion
4.1. Incubation of PCLS with Acidic Saline Solutions Causes Cell Death
4.2. Treatment of Acid-Exposed PCLS with Dexamethasone Has No Effect on Cell Viability
4.3. Incubation of Unexposed Control PCLS with Acid-Exposed PCLS Has No Effect on Control PCLS Viability
4.4. Advantages and Disadvantages of Murine PCLS
4.5. Limitations of the Study
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Paraskeva, M.A.; Levin, K.C.; Westall, G.P.; Snell, G.I. Lung transplantation in Australia, 1986–2018: More than 30 years in the making. Med. J. Aust. 2018, 208, 445–450. Available online: https://onlinelibrary.wiley.com/doi/full/10.5694/mja17.00909 (accessed on 3 March 2022). [CrossRef] [PubMed]
- Mulvihill, M.S.; Lee, H.J.; Weber, J.; Choi, A.Y.; Cox, M.L.; Yerokun, B.A.; Bishawi, M.A.; Klapper, J.; Kuchibhatla, M.; Hartwig, M.G. Variability in donor organ offer acceptance and lung transplantation survival. J. Heart Lung Transplant. 2020, 39, 353–362. Available online: https://pubmed.ncbi.nlm.nih.gov/32029400/ (accessed on 3 March 2022). [CrossRef] [PubMed]
- Keeshan, B.C.; Rossano, J.W.; Beck, N.; Hammond, R.; Kreindler, J.; Spray, T.L.; Fuller, S.; Goldfarb, S. Lung transplant waitlist mortality: Height as a predictor of poor outcomes. Pediatr. Transplant. 2015, 19, 294–300. Available online: https://pubmed.ncbi.nlm.nih.gov/25406495/ (accessed on 3 March 2022). [CrossRef] [PubMed]
- Organizacion Nacional De Trasplantes—Newsletter 2021. ONT. 2021. Available online: http://www.ont.es/publicaciones/Paginas/Publicaciones.aspx (accessed on 3 March 2022).
- Statistics Library—Eurotransplant. Available online: https://www.eurotransplant.org/statistics/statistics-library/ (accessed on 3 March 2022).
- Organ Donation and Transplantation. Available online: https://data.hrsa.gov/topics/health-systems/organ-donation (accessed on 3 March 2022).
- Vanholder, R.; Domínguez-Gil, B.; Busic, M.; Cortez-Pinto, H.; Craig, J.C.; Jager, K.J.; Mahillo, B.; Stel, V.S.; Valentin, M.O.; Zoccali, C.; et al. Organ donation and transplantation: A multi-stakeholder call to action. Nat. Rev. Nephrol. 2021, 17, 554–568. Available online: https://www.nature.com/articles/s41581-021-00425-3 (accessed on 3 March 2022). [CrossRef]
- Snell, G.I.; Levvey, B.J.; Henriksen, A.; Whitford, H.M.; Levin, K.; Paraskeva, M.; Kotecha, S.; Williams, T.; Westall, G.P.; McGiffin, D. Donor Lung Referrals for Lung Transplantation: A “Behind The Scenes” View. Heart Lung Circ. 2020, 29, 793–799. Available online: https://pubmed.ncbi.nlm.nih.gov/31060909/ (accessed on 3 March 2022). [CrossRef]
- Okahara, S.; Levvey, B.; McDonald, M.; D’Costa, R.; Opdam, H.; Pilcher, D.V.; Snell, G.I. A Retrospective Review of Declined Lung Donors: Estimating the Potential of Ex Vivo Lung Perfusion. Ann. Thorac. Surg. 2021, 112, 443–449. Available online: http://www.annalsthoracicsurgery.org/article/S0003497520317343/fulltext (accessed on 6 November 2021). [CrossRef]
- Meers, C.; Van Raemdonck, D.; Verleden, G.M.; Coosemans, W.; Decaluwe, H.; De Leyn, P.; Nafteux, P.; Lerut, T. The number of lung transplants can be safely doubled using extended criteria donors: A single-center review. Transpl. Int. 2010, 23, 628–635. [Google Scholar] [CrossRef]
- Mojtabaee, M.; Shahryari, S.; Beigee, F.S. Reasons for Donor Lungs Unsuitability After Radiographic Selection. Exp. Clin. Transplant. 2019, 17 (Suppl. S1), 120–122. Available online: https://pubmed.ncbi.nlm.nih.gov/30777535/ (accessed on 3 March 2022). [CrossRef]
- Froio, S.; Valenza, F. Aspiration of Gastric Contents in the Critically Ill; Oxford University Press: Oxford, UK, 2016; Available online: https://oxfordmedicine.com/view/10.1093/med/9780199600830.001.0001/med-9780199600830-chapter-106 (accessed on 3 March 2022).
- Marik, P.E. Aspiration Pneumonitis and Aspiration Pneumonia. N. Engl. J. Med. 2001, 344, 665–671. [Google Scholar] [CrossRef] [PubMed]
- Raghavendran, K.; Nemzek, J.; Napolitano, L.M.; Knight, P.R. Aspiration-induced lung injury. Crit. Care Med. 2011, 39, 818–826. Available online: https://journals.lww.com/ccmjournal/Fulltext/2011/04000/Aspiration_induced_lung_injury.29.aspx (accessed on 3 March 2022). [CrossRef]
- Nakajima, D.; Liu, M.; Ohsumi, A.; Kalaf, R.; Iskender, I.; Hsin, M.; Kanou, T.; Chen, M.; Baer, B.; Coutinho, R.; et al. Lung Lavage and Surfactant Replacement During Ex Vivo Lung Perfusion for Treatment of Gastric Acid Aspiration–Induced Donor Lung Injury. J. Heart Lung Transplant. 2017, 36, 577–585. [Google Scholar] [CrossRef] [PubMed]
- Philips, M.; Zieve, D. Stomach Acid Test: MedlinePlus Medical Encyclopedia. 2020. Available online: https://medlineplus.gov/ency/article/003883.htm (accessed on 2 March 2022).
- Teabeaut, J.R., II. Aspiration of Gastric Contents: An Experimental Study. Am. J. Pathol. 1952, 28, 51. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1937292/ (accessed on 3 March 2022). [PubMed]
- Mandell, L.A.; Niederman, M.S. Aspiration pneumonia. New Engl. J. Med. 2019, 380, 651–663. [Google Scholar] [CrossRef] [PubMed]
- Raghavendran, K.P.; Abu-Elmagd, D.B.; Napolitano, N.N.; Hovda, D.J.; McKenna, J.; Rutherford, A.; Alan, D.H.; Robert, H.N.; Bruce, A.H. Progressive, Severe Lung Injury Secondary to the Interaction of Insults in Gastric Aspiration. J. Trauma. 2009, 66, 535–537. [Google Scholar] [CrossRef]
- Fernandez, S.F.; Fung, C.; Helinski, J.D.; Alluri, R.; Davidson, B.A.; Knight, P.R. Low pH environmental stress inhibits LPS and LTA-stimulated proinflammatory cytokine production in rat alveolar macrophages. Biomed. Res. Int. 2013, 2013, 742184. Available online: https://www.hindawi.com/journals/bmri/2013/267280/ (accessed on 3 March 2022). [CrossRef]
- Kennedy, T.; Johnson, K.; Kunkel, R.G.; Ward, P.A.; Knight, P.R.; Finch, J. Acute Acid Aspiration Lung Injury in the Rat: Biphasic Pathogenesis. Anesth Analg. 1989, 69, 87–92. Available online: https://journals.lww.com/anesthesia-analgesia/Abstract/1989/07000/Acute_Acid_Aspiration_Lung_Injury_in_the_Rat_.17.aspx (accessed on 6 November 2021). [CrossRef]
- Knight, P.R.; Rutter, T.; Tait, A.R.; Coleman, E.; Johnson, K. Pathogenesis of Gastric Particulate Lung Injury: A Comparison and Interaction with Acidic Pneumonitis. Anesth. Analg. 1993, 77, 754–760. Available online: https://journals.lww.com/anesthesia-analgesia/Abstract/1993/10000/Pathogenesis_of_Gastric_Particulate_Lung_Injury__A.17.aspx (accessed on 6 November 2021). [CrossRef]
- Hamelberg, W.; Bosomworth, P.P. Aspiration pneumonitis: Experimental studies and clinical observations. Anesth. Analg. 1964, 43, 669–677. Available online: https://journals.lww.com/anesthesia-analgesia/Fulltext/1964/11000/ASPIRATION_PNEUMONITIS__experimental_studies_and.11.aspx (accessed on 3 March 2022). [CrossRef]
- Graham, E.C.; Choy, D. Corticosteroids in Aspiration Pneumonia. JAMA 1963, 184, 976–977. Available online: https://jamanetwork.com/journals/jama/fullarticle/665861 (accessed on 2 March 2022). [CrossRef]
- Dudley, W.R.; Marshall, B.E. Steroid Treatment for Acid-aspiration Pneumonitis. Anesthesiology 1974, 40, 136–141. Available online: https://pubs.asahq.org/anesthesiology/article/40/2/136/23689/Steroid-Treatment-for-Acid-aspiration-Pneumonitis (accessed on 2 March 2022). [CrossRef]
- Matute-Bello, G.; Frevert, C.W.; Martin, T.R. Animal models of acute lung injury. Am. J. Physiol. Lung Cell Mol. Physiol. 2008, 295, L379. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2536793/ (accessed on 8 January 2022). [CrossRef]
- Ruigrok, M.J.R.; Tomar, J.; Frijlink, H.W.; Melgert, B.N.; Hinrichs, W.L.J.; Olinga, P. The effects of oxygen concentration on cell death, anti-oxidant transcription, acute inflammation, and cell proliferation in precision-cut lung slices. Sci. Rep. 2019, 9, 16655. Available online: https://www.nature.com/articles/s41598-019-52813-2 (accessed on 3 March 2022). [CrossRef]
- van Furth, L.A.; Leuvenink, H.G.D.; Seras, L.; de Graaf, I.A.M.; Olinga, P.; van Leeuwen, L.L. Exploring Porcine Precision-Cut Kidney Slices as a Model for Transplant-Related Ischemia-Reperfusion Injury. Transplantology 2022, 3, 139–151. [Google Scholar] [CrossRef]
- Teramoto, S. Animal Models of Aspiration Pneumonia. In Aspiration Pneumonia; Springer: Berlin/Heidelberg, Germany, 2020; p. 143. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7298540/ (accessed on 8 January 2022).
- Olinga, P. Protocol Mouse Lung Slices—Home. Available online: http://organslices.com/protocol-mouse-lung-slices/ (accessed on 2 March 2022).
- Bergman, J. ATP: The Perfect Energy Currency for the Cell. Creat. Res. Soc. Q. 1999, 36, 2–9. Available online: https://www.trueorigin.org/atp.php (accessed on 2 March 2022).
- Chen, Q.; Huang, Y.; Yang, Y.; Qiu, H. Acid-induced cell injury and death in lung epithelial cells is associated with the activation of mitogen-activated protein kinases. Mol. Med. Rep. 2013, 8, 565–570. Available online: http://www.spandidos-publications.com/10.3892/mmr.2013.1537/abstract (accessed on 2 March 2022). [CrossRef] [PubMed]
- Heuer, J.F.; Sauter, P.; Pelosi, P.; Herrmann, P.; Brück, W.; Perske, C.; Schöndube, F.; Crozier, A.T.; Bleckmann, A.; Beißbarth, T.; et al. Effects of pulmonary acid aspiration on the lungs and extra-pulmonary organs: A randomized study in pigs. Crit. Care 2012, 16, R35. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3681347/ (accessed on 2 March 2022). [CrossRef] [PubMed]
- Maniatis, N.A.; Sfika, A.; Nikitopoulou, I.; Vassiliou, A.G.; Magkou, C.; Armaganidis, A.; Roussos, C.; Kollias, G.; Orfanos, S.E.; Kotanidou, A. Acid-induced acute lung injury in mice is associated with P44/42 and c-Jun N-terminal kinase activation and requires the function of tumor necrosis factor α receptor I. Shock 2012, 38, 381–386. Available online: https://pubmed.ncbi.nlm.nih.gov/22814289/ (accessed on 2 March 2022). [CrossRef] [PubMed]
- Amigoni, M.; Bellani, G.; Scanziani, M.; Masson, S.; Bertoli, E.; Radaelli, E.; Patroniti, N.; Di Lelio, A.; Pesenti, A.; Latini, R.; et al. Lung injury and recovery in a murine model of unilateral acid aspiration: Functional, biochemical, and morphologic characterization. Anesthesiology 2008, 108, 1037–1046. Available online: https://pubmed.ncbi.nlm.nih.gov/18497604/ (accessed on 2 March 2022). [CrossRef] [PubMed]
- Hausmann, W.; Lunt, R.L. The problem of the treatment of peptic aspiration pneumonia following obstetric anaesthesia (Mendelson’s syndrome). J. Obstet. Gynaecol. Br. Emp. 1955, 62, 509–512. Available online: https://pubmed.ncbi.nlm.nih.gov/13252451/ (accessed on 2 March 2022). [CrossRef]
- Marshall, B.M.; Gordon, R.A. Vomiting, regurgitation, and aspiration in anaesthesia. II. Can. Anaesth. Soc. J. 1958, 5, 438–447. Available online: https://pubmed.ncbi.nlm.nih.gov/13585175/ (accessed on 2 March 2022).
- Bannister, W.K.; Sattilaro, A.J. Vomiting and aspiration during anesthesia. Anesthesiology 1962, 23, 251–264. Available online: https://pubmed.ncbi.nlm.nih.gov/13864692/ (accessed on 2 March 2022). [CrossRef]
- Brown, D.M.; Xie, S.; Evans, C.M. Dose-response of dexamethasone on precision-cut lung slices from rats with bleomycin-induced lung injury. J. Pharmacol. Toxicol. Methods. 2019, 96, 34–40. [Google Scholar]
- Zhao, J.N.; Liu, Y.; Li, H.C. Corticosteroids in treatment of aspiration-related acute respiratory distress syndrome: Results of a retrospective cohort study. BMC Pulm. Med. 2016, 16, 29. Available online: https://bmcpulmmed.biomedcentral.com/articles/10.1186/s12890-016-0194-4 (accessed on 2 March 2022). [CrossRef] [PubMed]
- Mokra, D.; Mikolka, P.; Kosutova, P.; Mokry, J. Corticosteroids in Acute Lung Injury: The Dilemma Continues. Int. J. Mol. Sci. 2019, 20, 4765. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.; Ju, Y.N. Budesonide Attenuates Ventilator-induced Lung Injury in a Rat Model of Inflammatory Acute Respiratory Distress Syndrome. Arch. Med. Res. 2016, 47, 275–284. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.Y.; Mongey, R.; Wang, P.; Rothery, S.; Gaboriau, D.C.A.; Hind, M.; Griffiths, M.; Dean, C.H. The acid injury and repair (AIR) model: A novel ex-vivo tool to understand lung repair. Biomaterials 2021, 267, 120480. Available online: https://pubmed.ncbi.nlm.nih.gov/33157373/ (accessed on 2 March 2022). [CrossRef]
- Morin, J.-P.; Baste, J.-M.; Gay, A.; Crochemore, C.; Corbière, C.; Monteil, C. Precision cut lung slices as an efficient tool for in vitro lung physio-pharmacotoxicology studies. Toxicology 2012, 43, 63–72. Available online: https://www.tandfonline.com/doi/abs/10.3109/00498254.2012.727043 (accessed on 6 November 2021). [CrossRef]
- Liu, G.; Betts, C.; Cunoosamy, D.M.; Åberg, P.M.; Hornberg, J.J.; Sivars, K.B.; Cohen, T.S. Use of precision cut lung slices as a translational model for the study of lung biology. Respir. Res. 2019, 20, 162. Available online: https://respiratory-research.biomedcentral.com/articles/10.1186/s12931-019-1131-x (accessed on 3 March 2022). [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moes, C.A.; Gan, C.T.; Venema, L.H.; Hoffmann, R.F.; Melgert, B.N.; Kerstjens, H.A.M.; Olinga, P.; Ruigrok, M.J.R. Modelling Acid-Induced Lung Damage in Precision-Cut Lung Slices: An Ex Vivo Animal Model. Transplantology 2023, 4, 185-196. https://doi.org/10.3390/transplantology4040018
Moes CA, Gan CT, Venema LH, Hoffmann RF, Melgert BN, Kerstjens HAM, Olinga P, Ruigrok MJR. Modelling Acid-Induced Lung Damage in Precision-Cut Lung Slices: An Ex Vivo Animal Model. Transplantology. 2023; 4(4):185-196. https://doi.org/10.3390/transplantology4040018
Chicago/Turabian StyleMoes, Carmen A., C. Tji Gan, Leonie H. Venema, Roland F. Hoffmann, Barbro N. Melgert, Huib A. M. Kerstjens, Peter Olinga, and Mitchel J. R. Ruigrok. 2023. "Modelling Acid-Induced Lung Damage in Precision-Cut Lung Slices: An Ex Vivo Animal Model" Transplantology 4, no. 4: 185-196. https://doi.org/10.3390/transplantology4040018
APA StyleMoes, C. A., Gan, C. T., Venema, L. H., Hoffmann, R. F., Melgert, B. N., Kerstjens, H. A. M., Olinga, P., & Ruigrok, M. J. R. (2023). Modelling Acid-Induced Lung Damage in Precision-Cut Lung Slices: An Ex Vivo Animal Model. Transplantology, 4(4), 185-196. https://doi.org/10.3390/transplantology4040018





