Lower Extremity Peripheral Arterial Disease and Its Relationship with Adverse Outcomes in Kidney Transplant Recipients: A Retrospective Cohort Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Kidney Transplant Technique
2.2. Study Variables
2.3. LEPAD Definition
2.4. Outcomes
2.5. Statistical Analysis
2.6. Bioethical Aspects
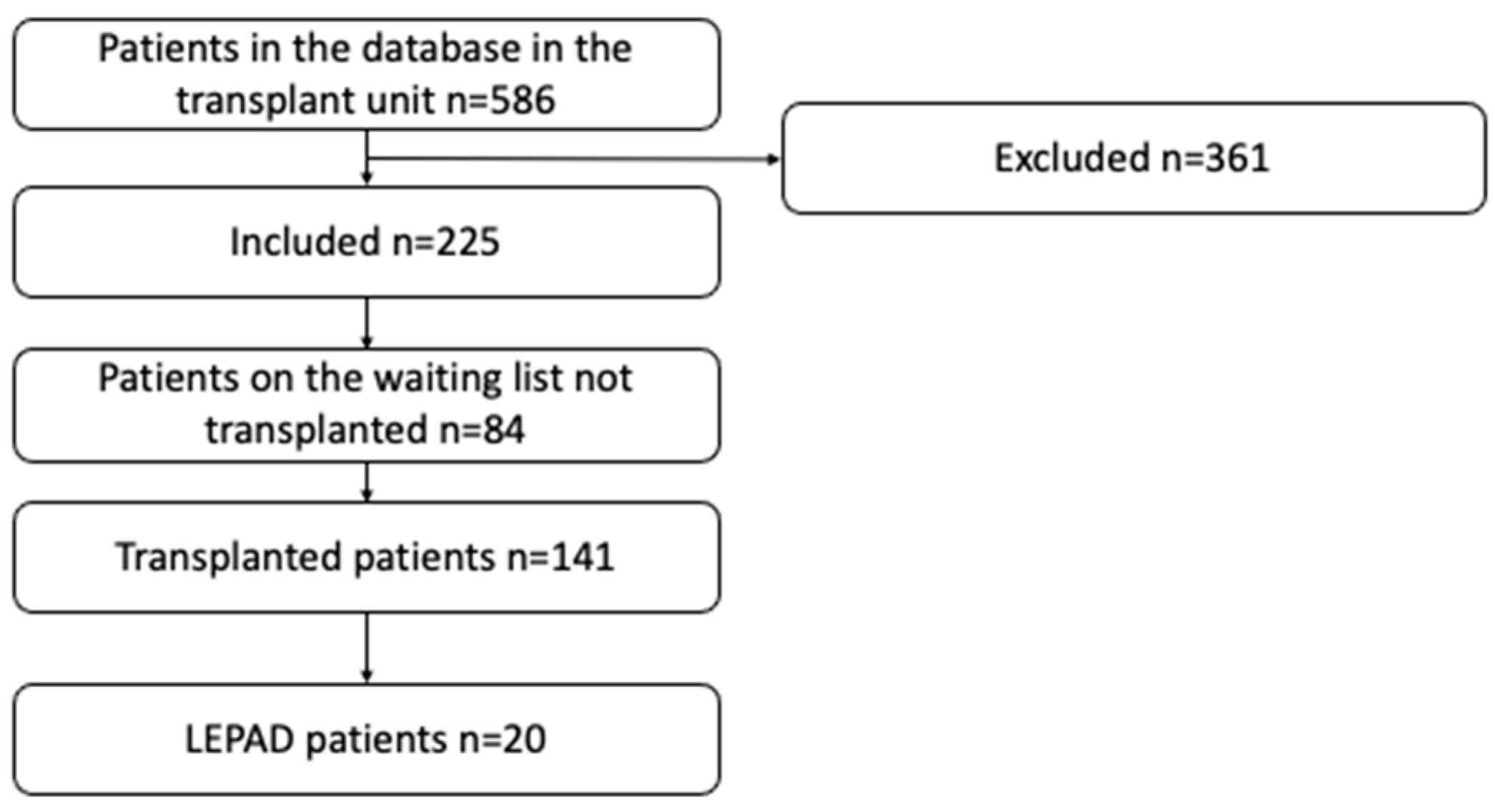
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Correction Statement
References
- Conte, M.S.; Bradbury, A.W.; Kolh, P.; White, J.V.; Dick, F.; Fitridge, R.; Mills, J.L.; Ricco, J.B.; Suresh, K.R.; Murad, M.H.; et al. Global vascular guidelines on the management of chronic limb-threatening ischemia. Eur. J. Vasc. Endovasc. Surg. 2019, 58, S1–S109. [Google Scholar] [CrossRef] [PubMed]
- Hirsch, A.T.; Haskal, Z.J.; Hertzer, N.R.; Bakal, C.W.; Creager, M.A.; Halperin, J.L.; Hiratzka, L.F.; Murphy, W.R.; Olin, J.W.; Puschett, J.B.; et al. ACC/AHA 2005 Practice Guidelines for the management of patients with peripheral arterial disease (lower extremity, renal, mesenteric, and abdominal aortic): A collaborative report from the American Association for Vascular Surgery/Society for Vascular Surgery, Society for Cardiovascular Angiography and Interventions, Society for Vascular Medicine and Biology, Society of Interventional Radiology, and the ACC/AHA Task Force on Practice Guidelines (Writing Committee to Develop Guidelines for the Management of Patients With Peripheral Arterial Disease): Endorsed by the American Association of Cardiovascular and Pulmonary Rehabilitation; National Heart, Lung, and Blood Institute; Society for Vascular Nursing; TransAtlantic Inter-Society Consensus; and Vascular Disease Foundation. Circulation 2006, 113, e463–e654. [Google Scholar]
- Gerhard-Herman, M.D.; Gornik, H.L.; Barrett, C.; Barshes, N.R.; Corriere, M.A.; Drachman, D.E.; Fleisher, L.A.; Fowkes, F.G.R.; Hamburg, N.M.; Kinlay, S.; et al. 2016 AHA/ACC Guideline on the Management of Patients With Lower Extremity Peripheral Artery Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J. Am. Coll. Cardiol. 2017, 69, e71–e126. [Google Scholar] [CrossRef]
- Fowkes, F.G.R.; Aboyans, V.; Fowkes, F.J.I.; McDermott, M.M.; Sampson, U.K.A.; Criqui, M.H. Peripheral artery disease: Epidemiology and global perspectives. Nat. Rev. Cardiol. 2017, 14, 156–170. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, M.N.; Geevar, Z.; Mohanan, P.P.; Venugopal, K.; Devika, S. Prevalence of peripheral artery disease and risk factors in the elderly: A community based cross-sectional study from northern Kerala, India. Indian Heart J. 2018, 70, 808–815. [Google Scholar] [CrossRef]
- Aboyans, V.; Ricco, J.-B.; Bartelink, M.-L.E.L.; Björck, M.; Brodmann, M.; Cohnert, T.; Collet, J.-P.; Czerny, M.; De Carlo, M.; Debus, S.; et al. 2017 ESC Guidelines on the Diagnosis and Treatment of Peripheral Arterial Diseases, in collaboration with the European Society for Vascular Surgery (ESVS): Document covering atherosclerotic disease of extracranial carotid and vertebral, mesenteric, renal, upper and lower extremity arteriesEndorsed by: The European Stroke Organization (ESO)The Task Force for the Diagnosis and Treatment of Peripheral Arterial Diseases of the European Society of Cardiology (ESC) and of the European Society for Vascular Surgery (ESVS). Eur. Heart J. 2018, 39, 763–816. [Google Scholar]
- Shabani Varaki, E.; Gargiulo, G.D.; Penkala, S.; Breen, P.P. Peripheral vascular disease assessment in the lower limb: A review of current and emerging non-invasive diagnostic methods. Biomed. Eng. Online 2018, 17, 61. [Google Scholar] [CrossRef]
- Resnick, H.E.; Lindsay, R.S.; McDermott, M.M.; Devereux, R.B.; Jones, K.L.; Fabsitz, R.R.; Howard, B.V. Relationship of high and low ankle brachial index to all-cause and cardiovascular disease mortality: The Strong Heart Study. Circulation 2004, 109, 733–739. [Google Scholar] [CrossRef] [PubMed]
- Guirguis-Blake, J.M.; Evans, C.V.; Redmond, N.; Lin, J.S. Screening for Peripheral Artery Disease Using the Ankle-Brachial Index: Updated Evidence Report and Systematic Review for the US Preventive Services Task Force. JAMA 2018, 320, 184–196. [Google Scholar] [CrossRef]
- Snyder, J.J.; Kasiske, B.L.; Maclean, R. Peripheral arterial disease and renal transplantation. J. Am. Soc. Nephrol. JASN 2006, 17, 2056–2068. [Google Scholar] [CrossRef]
- Sung, R.S.; Althoen, M.; Howell, T.A.; Merion, R.M. Peripheral vascular occlusive disease in renal transplant recipients: Risk factors and impact on kidney allograft survival. Transplantation 2000, 70, 1049–1054. [Google Scholar] [CrossRef] [PubMed]
- Aboyans, V.; Criqui, M.H.; Abraham, P.; Allison, M.A.; Creager, M.A.; Diehm, C.; Fowkes, F.G.R.; Hiatt, W.R.; Jönsson, B.; Lacroix, P.; et al. Measurement and interpretation of the ankle-brachial index: A scientific statement from the American Heart Association. Circulation 2012, 126, 2890–2909. [Google Scholar] [CrossRef]
- Ix, J.H.; Biggs, M.L.; Kizer, J.R.; Mukamal, K.J.; Djousse, L.; Zieman, S.J.; De Boer, I.H.; Nelson, T.L.; Newman, A.B.; Criqui, M.H.; et al. Association of body mass index with peripheral arterial disease in older adults: The Cardiovascular Health Study. Am. J. Epidemiol. 2011, 174, 1036–1043. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.I.; Chakkera, H.A.; Wennberg, P.W.; Liedl, D.A.; Alrabadi, F.; Cha, S.S.; Hooley, D.D.; Amer, H.; Wadei, H.M.; Shamoun, F.E. Peripheral arterial disease preoperatively may predict graft failure and mortality in kidney transplant recipients. Vasc. Med. Lond Engl. 2017, 22, 225–230. [Google Scholar] [CrossRef]
- Golomb, B.A.; Dang, T.T.; Criqui, M.H. Peripheral arterial disease: Morbidity and mortality implications. Circulation 2006, 114, 688–699. [Google Scholar] [CrossRef]
- Ranson, P. Peripheral arterial disease: Diagnosis and management. Nurs. Times 2012, 108, 23. [Google Scholar] [PubMed]
- Bunnapradist, S.; Danovitch, G.M. Evaluation of adult kidney transplant candidates. Am. J. Kidney Dis. 2007, 50, 890–898. [Google Scholar] [CrossRef]
- Lentine, K.L.; Costa, S.P.; Weir, M.R.; Robb, J.F.; Fleisher, L.A.; Kasiske, B.L.; Carithers, R.L.; Ragosta, M.; Bolton, K.; Auerbach, A.D.; et al. Cardiac disease evaluation and management among kidney and liver transplantation candidates: A scientific statement from the American Heart Association and the American College of Cardiology Foundation: Endorsed by the American Society of Transplant Surgeons, American Society of Transplantation, and National Kidney Foundation. Circulation 2012, 126, 617–663. [Google Scholar]
- Bundó, M.; Urrea, M.; Muñoz, L.; Llussà, J.; Forés, R.; Torán, P. Correlation between toe-brachial index and ankle-brachial index in patients with diabetes mellitus type 2. Med. Clin. 2013, 140, 390–394. [Google Scholar] [CrossRef]
- Norgren, L.; Hiatt, W.R.; Dormandy, J.A.; Nehler, M.R.; Harris, K.A.; Fowkes, F.G.R.; Tasc II Working Group. Inter-Society Consensus for the Management of Peripheral Arterial Disease (TASC II). J. Vasc. Surg. 2007, 45 (Suppl. S), S5–S67. [Google Scholar] [CrossRef]
- Hopley, C.W.; Kavanagh, S.; Patel, M.R.; Ostrom, C.; Baumgartner, I.; Berger, J.S.; Blomster, J.I.; Fowkes, F.G.R.; Jones, W.S.; Katona, B.G.; et al. Chronic kidney disease and risk for cardiovascular and limb outcomes in patients with symptomatic peripheral artery disease: The EUCLID trial. Vasc. Med. 2019, 24, 422–430. [Google Scholar] [CrossRef]
- Berger, J.S.; Katona, B.G.; Jones, W.S.; Patel, M.R.; Norgren, L.; Baumgartner, I.; Blomster, J.; Mahaffey, K.W.; Held, P.; Millegård, M.; et al. Design and rationale for the Effects of Ticagrelor and Clopidogrel in Patients with Peripheral Artery Disease (EUCLID) trial. Am. Heart J. 2016, 175, 86–93. [Google Scholar] [CrossRef]
- Rijkse, E.; Kimenai, H.J.A.N.; Roodnat, J.I.; Ten Raa, S.; Bijdevaate, D.C.; van Dam, J.L.; Muller, K.; IJzermans, J.N.; van der Zijden, M.A.; Minnee, R.C. Impact of Aortoiliac Stenosis on Graft and Patient Survival in Kidney Transplant Recipients Using the TASC II Classification. Transplantation 2019, 103, 2164–2172. [Google Scholar] [CrossRef] [PubMed]
- Rijkse, E.; van Dam, J.L.; Roodnat, J.I.; Kimenai, H.J.A.N.; IJzermans, J.N.M.; Minnee, R.C. The prognosis of kidney transplant recipients with aorto-iliac calcification: A systematic review and meta-analysis. Transpl. Int. 2020, 33, 483–496. [Google Scholar] [CrossRef]
- Wang, W.; Zhao, T.; Geng, K.; Yuan, G.; Chen, Y.; Xu, Y. Smoking and the Pathophysiology of Peripheral Artery Disease. Front. Cardiovasc. Med. 2021, 8, 704106. [Google Scholar] [CrossRef]
- Ceresa, C.D.L.; Aitken, E.; Dempster, N.J.; Kingsmore, D. Outcomes of renal transplantation in patients with major lower limb amputation. Transplant. Proc. 2014, 46, 115–120. [Google Scholar] [CrossRef] [PubMed]
- Droupy, S.; Eschwège, P.; Hammoudi, Y.; Durrbach, A.; Charpentier, B.; Benoit, G. Consequences of iliac arterial atheroma on renal transplantation. J. Urol. 2006, 175, 1036–1039. [Google Scholar] [CrossRef]
- Goldsmith, P.J.; Fraser, S.M.; Fitzpatrick, M.; Scott, D.J.; Ahmad, N. Acute lower limb ischemia following pediatric renal transplantation. Pediatr. Transplant. 2010, 14, E93–E95. [Google Scholar] [CrossRef] [PubMed]
- Northcutt, A.; Zibari, G.; Tan, T.-W.; Coulter, A.H.; Zhang, W.W. Does kidney transplantation to iliac artery deteriorate ischemia in the ipsilateral lower extremity with peripheral arterial disease? Vascular 2015, 23, 490–493. [Google Scholar] [CrossRef]
- Baumann, D.S.; McGraw, D.; Rubin, B.G.; Allen, B.T.; Anderson, C.B.; Sicard, G.A. An institutional experience with arterial atheroembolism. Ann. Vasc. Surg. 1994, 8, 258–265. [Google Scholar] [CrossRef]
- Sucher, R.; Rademacher, S.; Jahn, N.; Brunotte, M.; Wagner, T.; Alvanos, A.; Sucher, E.; Seehofer, D.; Scheuermann, U.; Hau, H.M. Effects of simultaneous pancreas-kidney transplantation and kidney transplantation alone on the outcome of peripheral vascular diseases. BMC Nephrol. 2019, 20, 453. [Google Scholar] [CrossRef] [PubMed]
- Arhuidese, I.; Nejim, B.; Aji, E.A.; Canner, J.; Malas, M.B. Survival after major lower extremity amputation in patients with end-stage renal disease. J. Vasc. Surg. 2019, 70, 1291–1298. [Google Scholar] [CrossRef] [PubMed]
- Krishnamurthy, V.; Munir, K.; Rectenwald, J.E.; Mansour, A.; Hans, S.; Eliason, J.L.; Escobar, G.A.; Gallagher, K.A.; Grossman, P.M.; Gurm, H.S.; et al. Contemporary outcomes with percutaneous vascular interventions for peripheral critical limb ischemia in those with and without poly-vascular disease. Vasc. Med. 2014, 19, 491–499. [Google Scholar] [CrossRef]
- Akl, A.; Elshayeb, M.; Rahim, M.A.; Refaie, A.F.; Ghoneim, M.A. Evaluation of Antithymocyte Globulin Efficacy in Reversing Refractory Graft Rejection Using Retrospective Event-Based Sequential Graft Biopsy Analysis in Living Related Donor Renal Transplant. Exp. Clin. Transplant. 2023, 21, 428–433. [Google Scholar] [CrossRef] [PubMed]
- Ali, H.; Mohammed, M.; Fülöp, T.; Malik, S. Outcomes of thymoglobulin versus basiliximab induction therapies in living donor kidney transplant recipients with mild to moderate immunological risk—A retrospective analysis of UNOS database. Ann. Med. 2023, 55, 2215536. [Google Scholar] [CrossRef]

| Baseline Clinical Characteristics | LEPAD (n = 20) | Non-LEPAD (n = 121) | Total (n = 141) | p Value | ||||
|---|---|---|---|---|---|---|---|---|
| N | % | N | % | N | % | |||
| Gender | Male | 17 | (85) | 82 | (68) | 99 | (70.2) | 0,19 |
| Female | 3 | (15) | 39 | (32) | 42 | (29.8) | ||
| Age | - | 58 | IQR (10.5) | 58 | IQR (10.5) | 58 | IQR (10.5) | 0.97 |
| Days of hospitalization | - | 9 | IQR (6) | 9 | IQR (6) | 9 | IQR (6) | 0.56 |
| Coronary heart disease | Yes | 6 | (30.0) | 14 | (11.6) | 20 | (14.2) | 0.22 |
| Not | 14 | (70) | 107 | (88.4) | 121 | (85.8%) | ||
| Arterial hypertension | Yes | 20 | (100) | 109 | (90) | 129 | (91.5%) | 0.13 |
| Not | - | (-) | 12 | (10) | 12 | (8.5%) | ||
| Diabetes mellitus | Yes | 12 | (60) | 51 | (52) | 63 | (44.7) | 0.92 |
| Not | 8 | (40) | 70 | (48) | 78 | (55.3) | ||
| Chronic heart failure | Yes | 1 | (5) | 3 | (2.1) | 4 | (2.8) | 0.86 |
| Not | 19 | (95) | 118 | (97.9) | 137 | (97.2) | ||
| Smoking | Yes | 9 | (45) | 29 | (24) | 38 | (27) | 0.05 |
| Not | 11 | (55) | 92 | (76) | 103 | (73) | ||
| Retinopathy | Yes | 9 | (45.0) | 29 | (23.9) | 38 | (27) | 0.74 |
| Not | 11 | (55.0) | 92 | (61.2) | 103 | (73) | ||
| Dyslipidemia | Yes | 7 | (35) | 47 | (38.8) | 54 | (38.2) | >0.99 |
| Not | 13 | (65) | 74 | (61.2) | 87 | (61.8) | ||
| Cerebrovascular disease | Yes | 1 | (5.0) | 5 | (4.1) | 6 | (4) | 1.00 |
| Not | 19 | (95.0) | 116 | (95.9) | 135 | (96.4) | ||
| Body Mass Index | Underweight < 18.5 | 1 | (5.0) | 1 | (0.8) | 2 | (2) | 0.46 |
| Normal 18.5–24.9 | 9 | (45.0) | 48 | (39.7) | 57 | (40) | ||
| Overweight 25–29.9 | 8 | (40.0) | 54 | (44.6) | 62 | (44) | ||
| Obesity > 30 | 2 | (5.0) | 18 | (14.9) | 20 | (14) | ||
| Variable | LEPAD (n = 20) | ||
|---|---|---|---|
| N | % | ||
| Claudication | Yes | 4 | (20) |
| Not | 16 | (80) | |
| Compromised limb | Right | 4 | (20) |
| Left | 7 | (35) | |
| Bilateral | 9 | (45) | |
| Aortoiliac right | Yes | - | - |
| Not | 20 | (100) | |
| Iliofemoral right | Yes | 1 | (5) |
| Not | 19 | (95) | |
| Femoropopliteus right | Yes | 2 | (10) |
| Not | 18 | (90) | |
| Infrapopliteal right | Yes | 11 | (55) |
| Not | 9 | (45) | |
| Metatarsus right | Yes | 10 | (50) |
| Not | 10 | (50) | |
| Aortoiliac left | Yes | - | - |
| Not | 20 | (100) | |
| Iliofemoral left | Yes | - | - |
| Not | 20 | (100) | |
| Femoropopliteus left | Yes | 2 | (10) |
| Not | 18 | (90) | |
| Infrapopliteal left | Yes | 9 | (45) |
| Not | 11 | (55) | |
| Ankle-brachial index right | Altered high ≥ 1.3 | 4 | (20) |
| Normal 1.0–1.29 | 5 | (25) | |
| Bordering 0.91–0.99 | 3 | (15) | |
| Altered low ≤ 0.9 | 8 | (40) | |
| Ankle-brachial index right | Altered high ≥ 1.3 | 4 | (20) |
| Normal 1.0–1.29 | 9 | (45) | |
| Bordering 0.91–0.99 | 2 | (10) | |
| Altered low ≤ 0.9 | 5 | (25) | |
| Index toe-brachial right | <0.7 abnormal | 12 | (66.7) |
| ≥0.7 normal | 6 | (33.3) | |
| Index toe-brachial left | <0.7 abnormal | 13 | (72.2) |
| ≥0.7 normal | 5 | (27.8) | |
| Baseline Clinical Characteristics | LEPAD (n = 20) | Non-LEPAD (n = 121) | Total (n = 141) | p-Value | RR CI 95% | ||||
|---|---|---|---|---|---|---|---|---|---|
| N | % | N | % | N | % | ||||
| Perioperative Outcomes | |||||||||
| Major cardiovascular event | Yes | 1 | (5.0) | 1 | (0.8) | 2 | (1.4) | NA | NA |
| Not | 19 | (95.0) | 120 | (99.2) | 139 | (98.6) | |||
| Yes | - | - | - | NA | NA | ||||
| Not | 20 | (100) | 121 | (100) | 141 | (100) | |||
| Cerebrovascular disease | Yes | - | - | - | |||||
| Not | 20 | (100) | 121 | (100) | 141 | (100) | NA | NA | |
| In-hospital mortality | Yes | 1 | (5) | - | - | (0.7) | NA | NA | |
| Not | 19 | (95) | 121 | (100) | 140 | (99.3) | |||
| Functionality graft | Excellent | 16 | (80) | 89 | (73.6) | 105 | (74.5) | 0.74 | NA |
| Retarded | 4 | (20) | 30 | (24.8) | 34 | (24.1) | |||
| Lost | - | - | 2 | (1.7) | 2 | (1.4) | |||
| Posthospital Outcomes | |||||||||
| Graft loss | Yes | 1 | (5) | 7 | (5.8) | 8 | (5.7) | >0.99 | 0.86 (0.11–6.65) |
| Not | 19 | (95) | 114 | (94.2) | 133 | (94.3) | |||
| Amputation | Yes | 3 | (15) | 2 | (1.7) | 5 | (3.5) | 0.04 | 9.1 (1.6–51) |
| Not | 17 | (85) | 119 | (98.3) | 136 | (96.5) | |||
| Death | Yes | - | 3 | (2.5) | 3 | (2.1) | 1.00 | NA | |
| Not | 20 | (100) | 118 | (97.5) | 138 | (97.9) | |||
| Factor | Coefficient | Hazard Ratio | 95% Confidence Interval | p-Value |
|---|---|---|---|---|
| Age | 0.063 | 1.065 | 1.004–1.130 | 0.036 |
| Gender | −1.983 | 0.138 | 0.031–0.605 | 0.009 |
| Weight | −0.081 | 0.922 | 0.873–0.973 | 0.003 |
| Diabetes Mellitus | −0.826 | 0.438 | 0.169–1.133 | 0.089 |
| Smoking | −0.006 | 0.994 | 0.383–2.580 | 0.990 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alvarez-Perdomo, L.C.; Cataño-Bedoya, J.U.; Plaza-Tenorio, M.; Botero-Mora, A.M.; Cardozo-Moreno, I.d.P.; Barrera-Lozano, L.M.; Ramírez-Arbeláez, J.A.; Ardila, C.M. Lower Extremity Peripheral Arterial Disease and Its Relationship with Adverse Outcomes in Kidney Transplant Recipients: A Retrospective Cohort Study. Transplantology 2023, 4, 111-123. https://doi.org/10.3390/transplantology4030012
Alvarez-Perdomo LC, Cataño-Bedoya JU, Plaza-Tenorio M, Botero-Mora AM, Cardozo-Moreno IdP, Barrera-Lozano LM, Ramírez-Arbeláez JA, Ardila CM. Lower Extremity Peripheral Arterial Disease and Its Relationship with Adverse Outcomes in Kidney Transplant Recipients: A Retrospective Cohort Study. Transplantology. 2023; 4(3):111-123. https://doi.org/10.3390/transplantology4030012
Chicago/Turabian StyleAlvarez-Perdomo, Luis Carlos, John Ubeimar Cataño-Bedoya, Maribel Plaza-Tenorio, Ana María Botero-Mora, Isabel del Pilar Cardozo-Moreno, Luis Manuel Barrera-Lozano, Jaime Alberto Ramírez-Arbeláez, and Carlos M. Ardila. 2023. "Lower Extremity Peripheral Arterial Disease and Its Relationship with Adverse Outcomes in Kidney Transplant Recipients: A Retrospective Cohort Study" Transplantology 4, no. 3: 111-123. https://doi.org/10.3390/transplantology4030012
APA StyleAlvarez-Perdomo, L. C., Cataño-Bedoya, J. U., Plaza-Tenorio, M., Botero-Mora, A. M., Cardozo-Moreno, I. d. P., Barrera-Lozano, L. M., Ramírez-Arbeláez, J. A., & Ardila, C. M. (2023). Lower Extremity Peripheral Arterial Disease and Its Relationship with Adverse Outcomes in Kidney Transplant Recipients: A Retrospective Cohort Study. Transplantology, 4(3), 111-123. https://doi.org/10.3390/transplantology4030012







