Portal Hemodynamics after Living-Donor Liver Transplantation: Management for Optimal Graft and Patient Outcomes—A Narrative Review
Abstract
1. Introduction
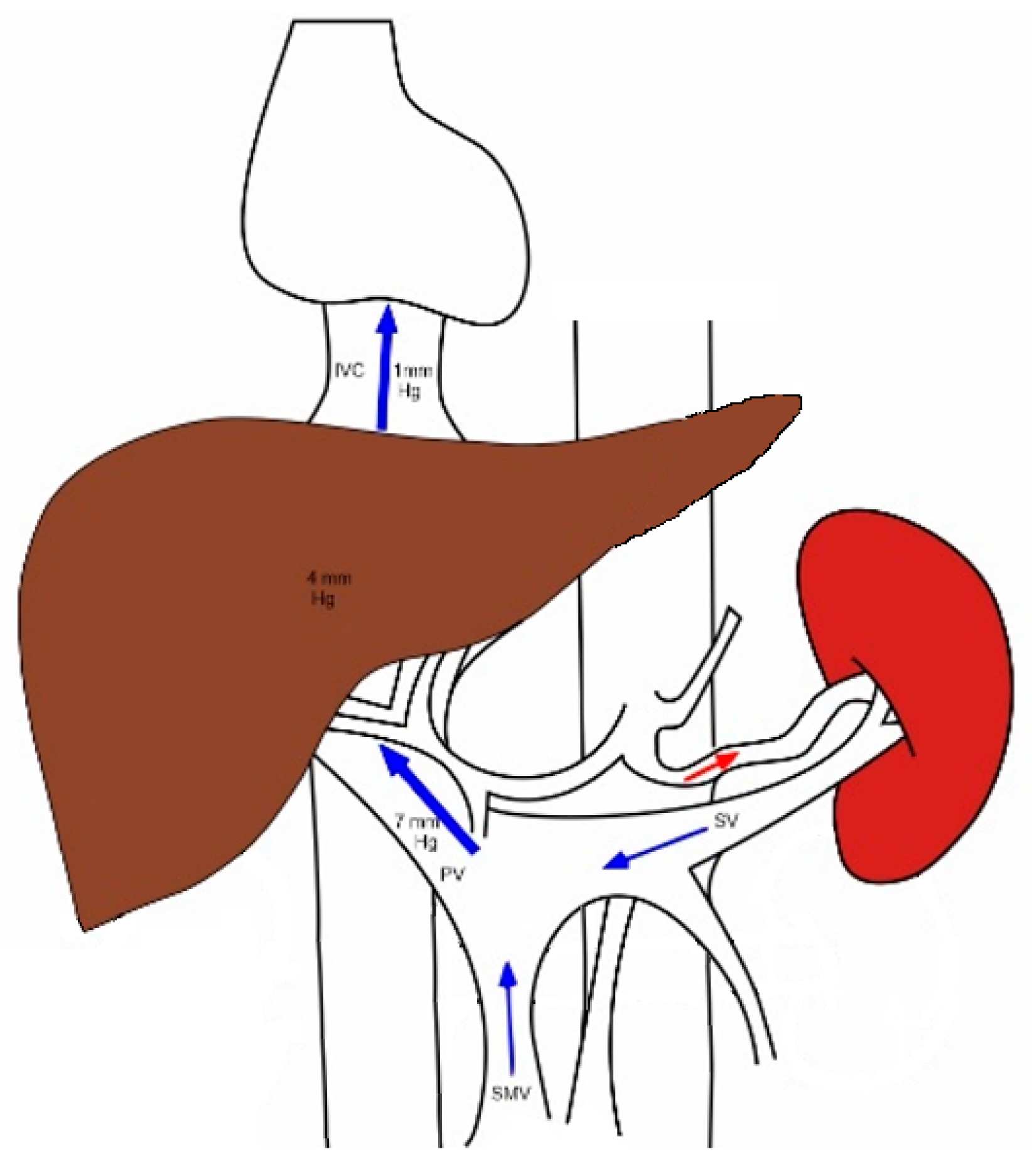
2. Normal Splanchnic Hemodynamics
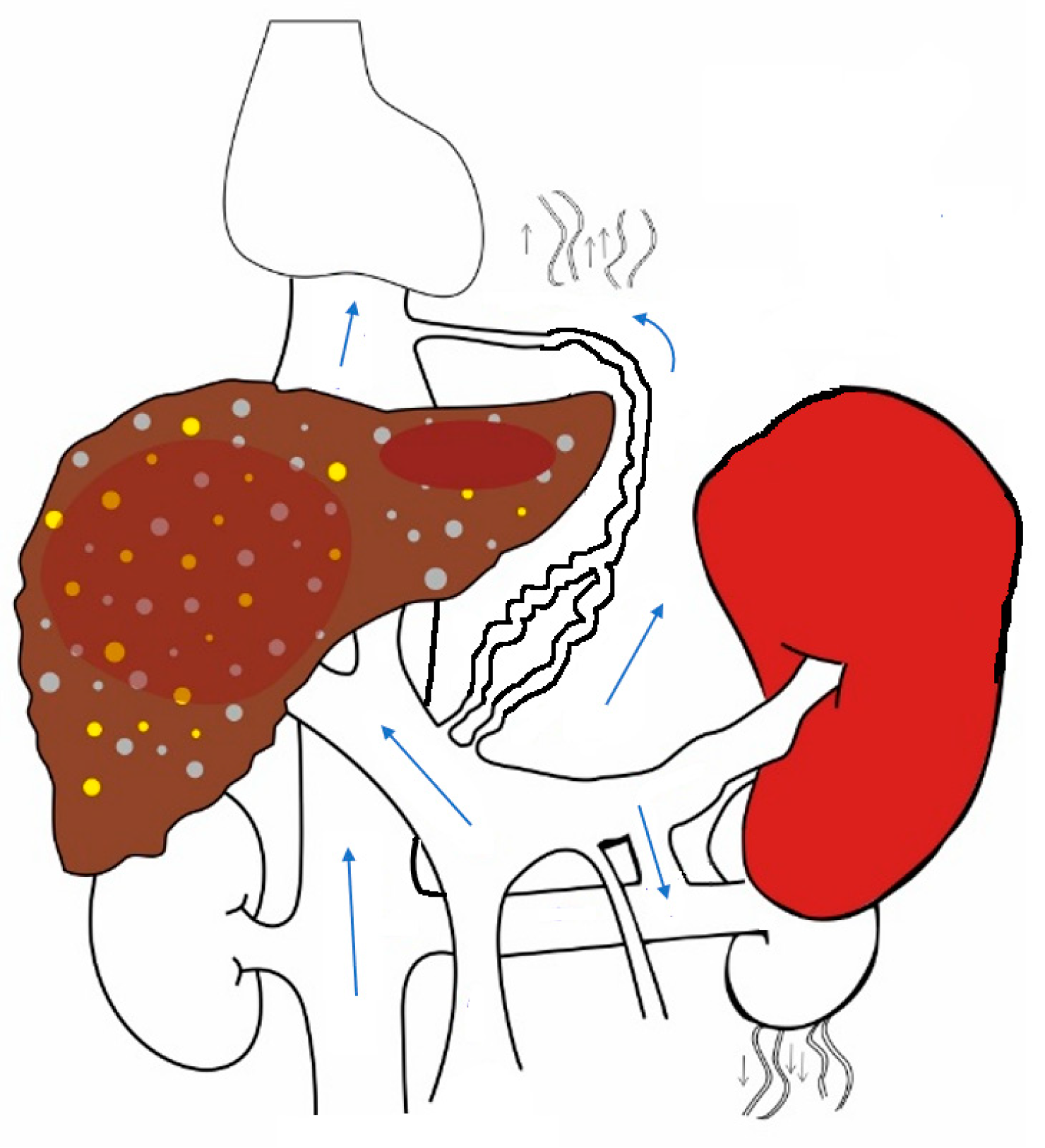
3. Changes That Occur in Splanchnic and Systemic Circulation in Chronic Liver Disease with Portal Hypertension
4. The Hepatic-Artery-Buffer Response
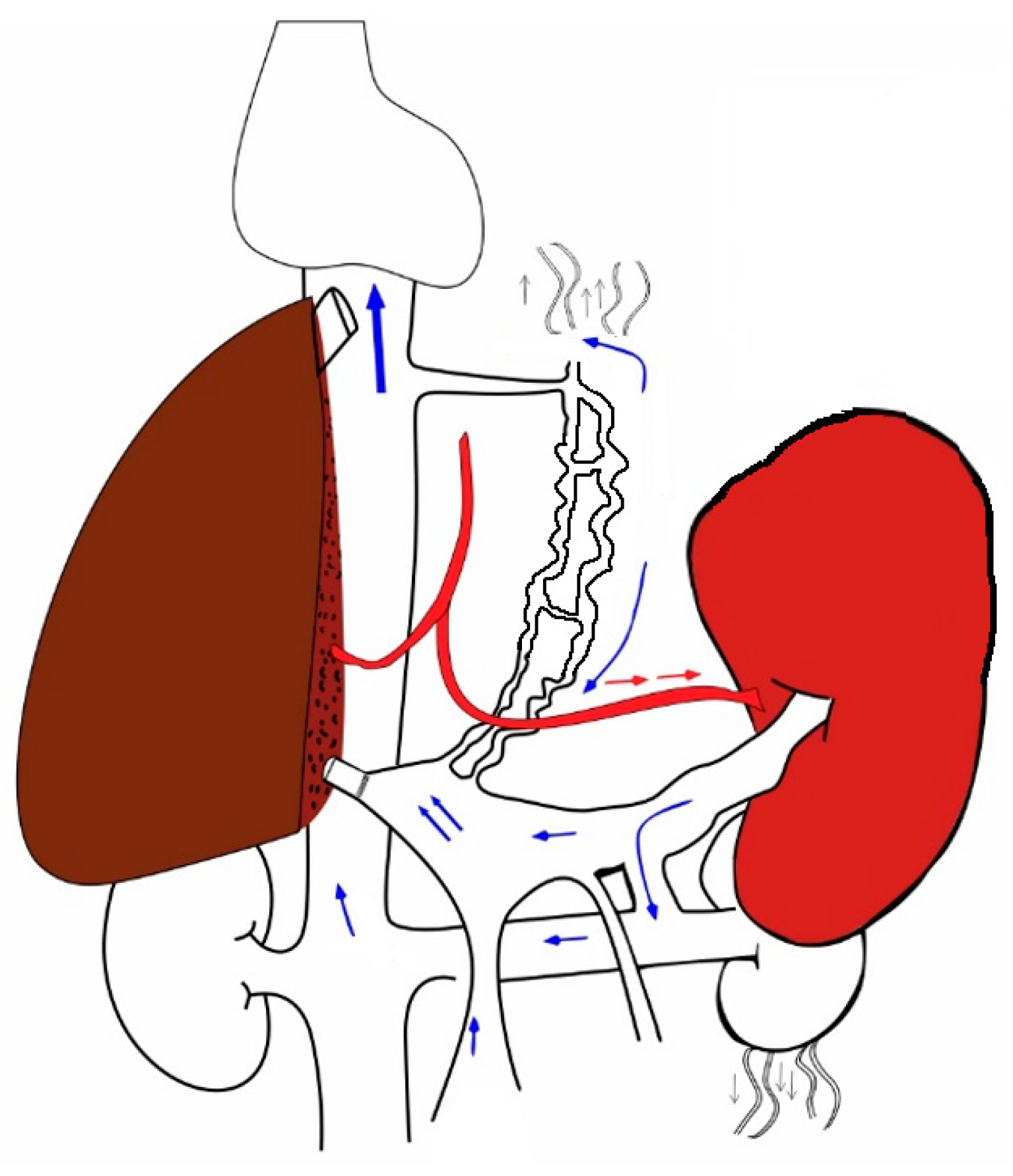
5. Changes That Occur When a New Liver Is Transplanted into the Hyperdynamic Circuit
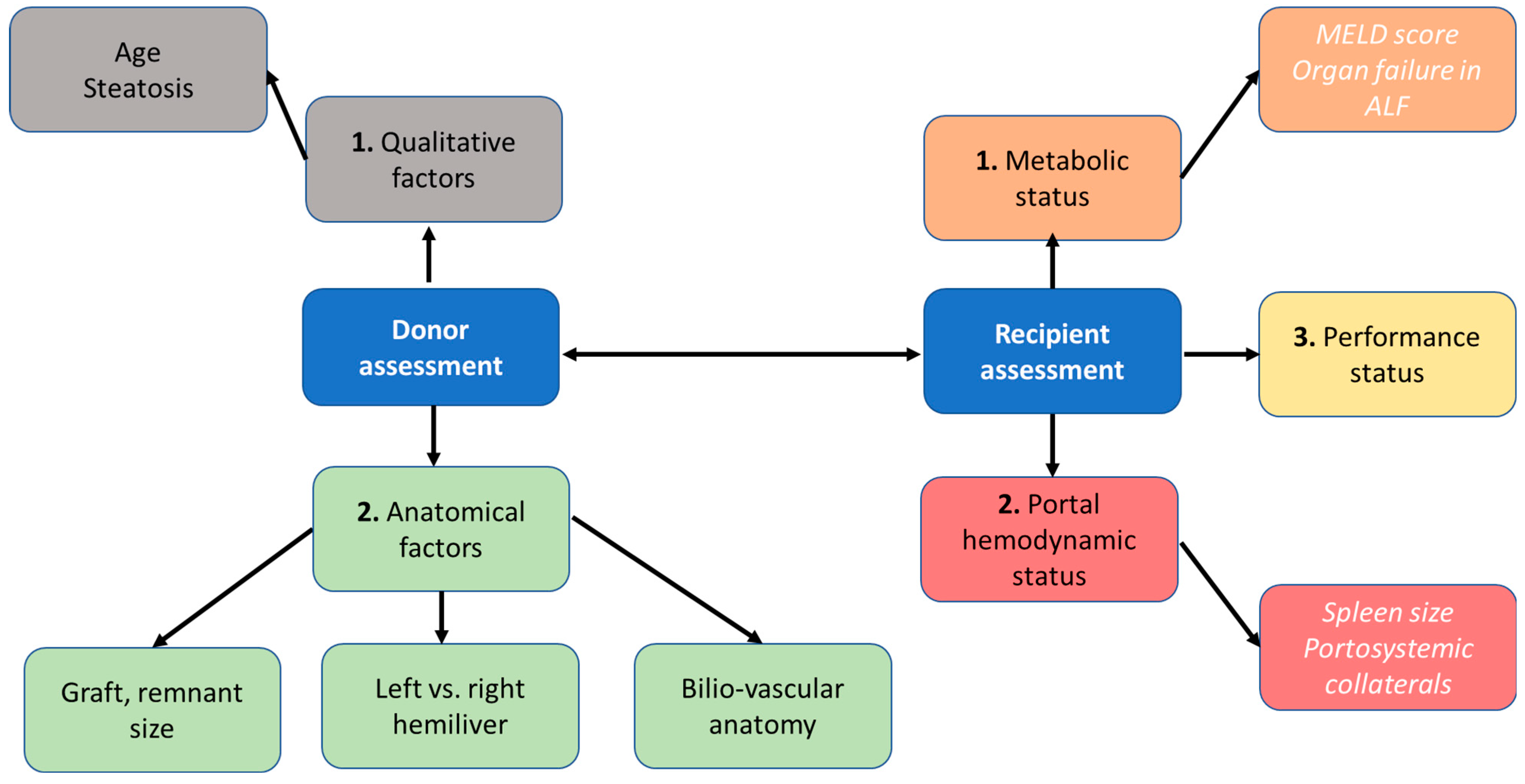
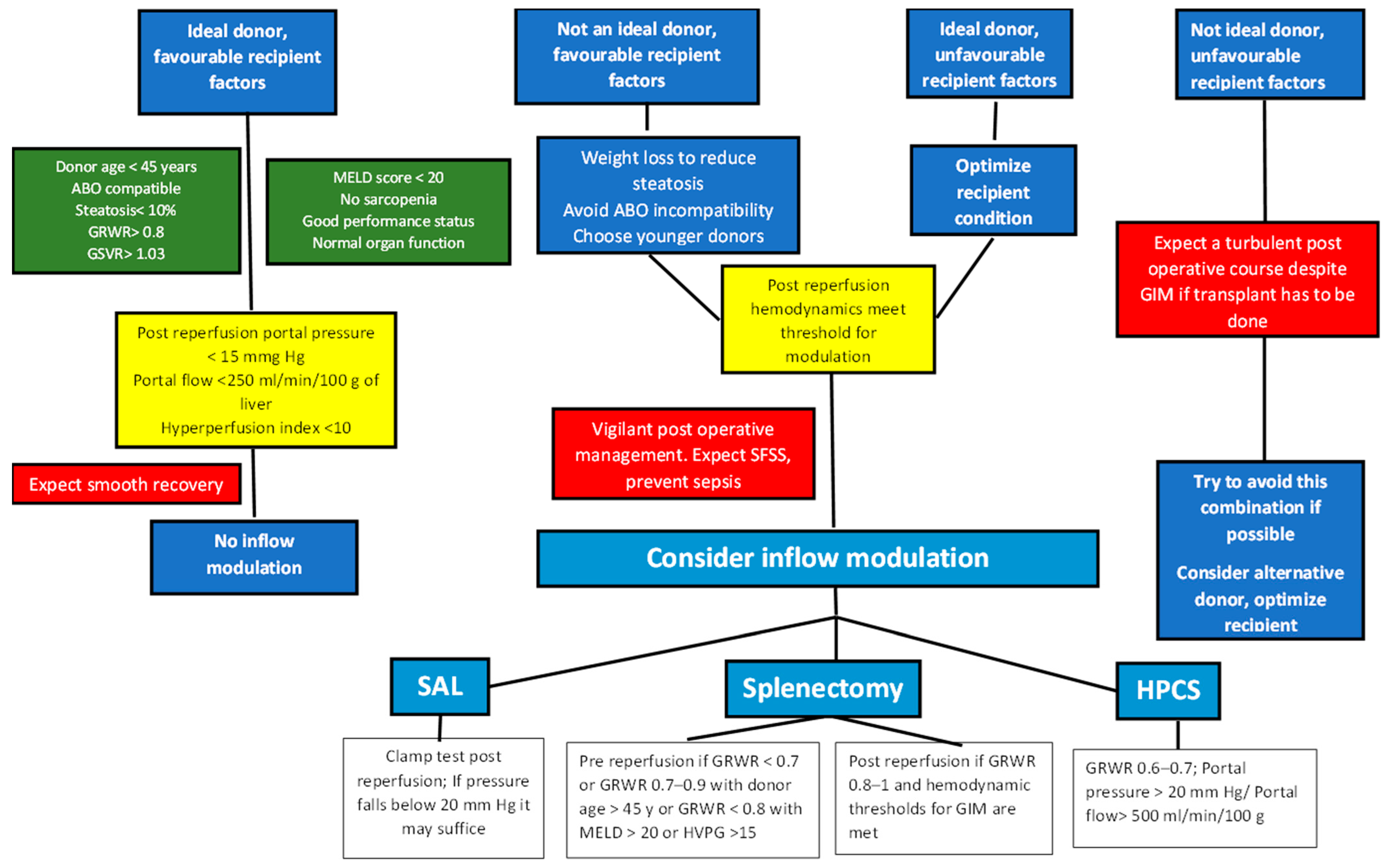
6. Donor Selection
7. Preoperative Recipient Evaluation
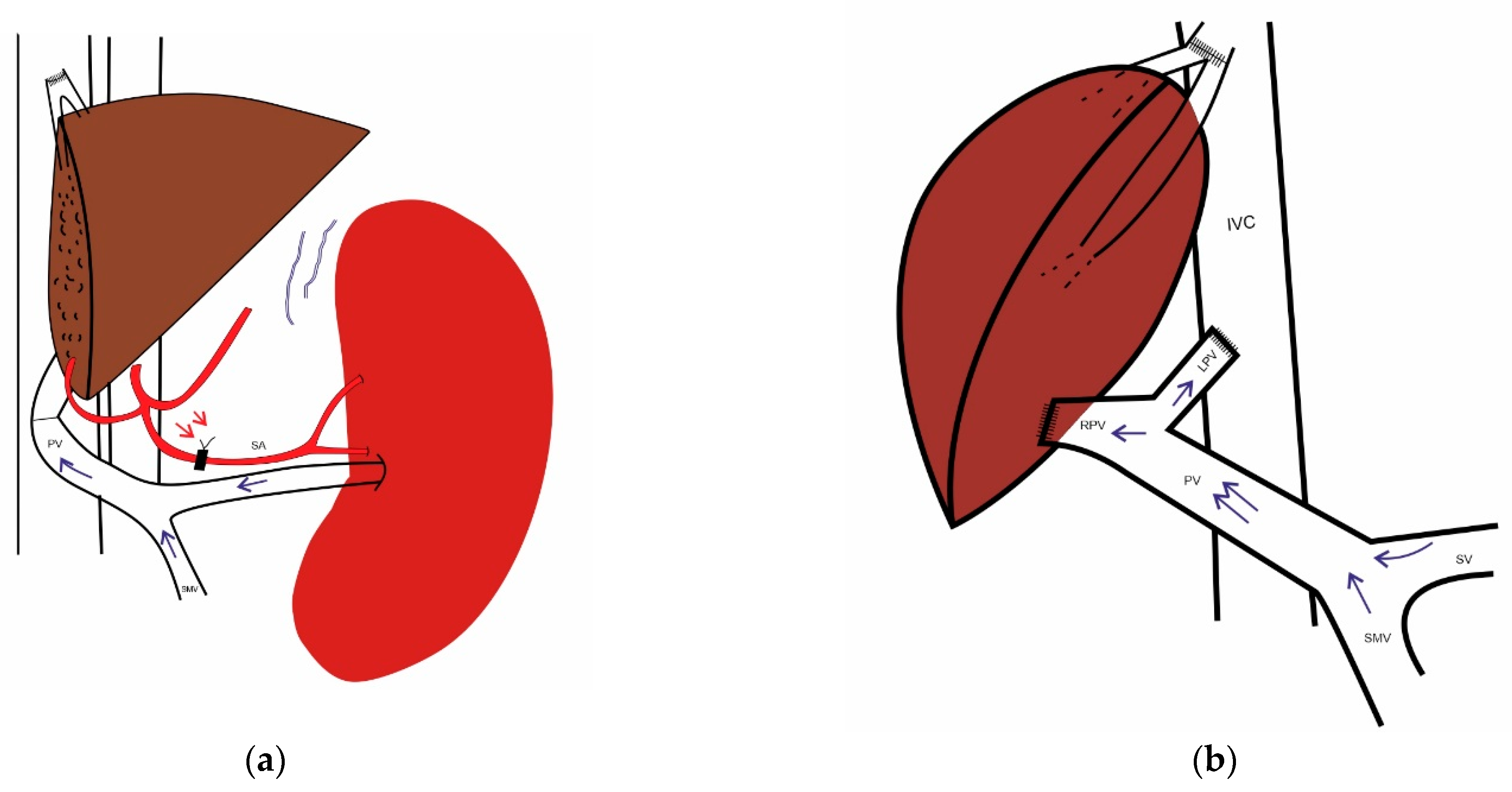
8. Outflow Reconstruction
9. The Concept of Small for Size, Small for Flow
10. Recipient Portal Hemodynamic Status
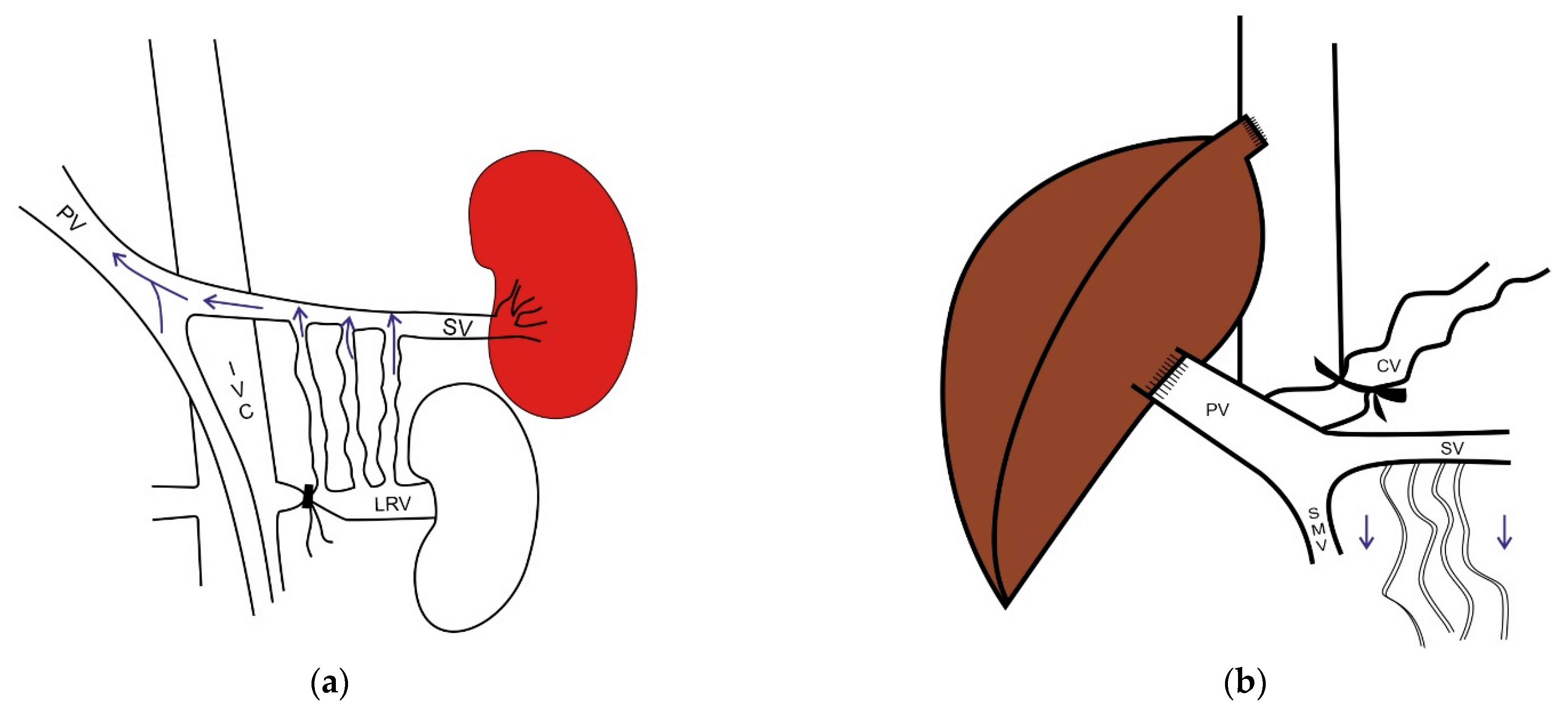
10.1. The Role of the Spleen
10.2. Portosystemic Collaterals
11. Intraoperative Measurement of Portal Hemodynamics
12. Inflow Modulation
12.1. Splenic-Artery Ligation (SAL)
12.2. Splenectomy
12.3. Portocaval Shunt
12.4. Other Strategies
13. Type and Timing of Inflow Modulation
14. Pharmacological Measures
15. Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bradley, S.E. Variations in Hepatic Blood Flow in Man during Health and Disease. N. Engl. J. Med. 1949, 240, 456–461. [Google Scholar] [CrossRef]
- Greenway, C.V.; Stark, R.D. Hepatic vascular bed. Physiol. Rev. 1971, 51, 23–65. [Google Scholar] [CrossRef]
- Vollmar, B.; Menger, M.D. The hepatic microcirculation: Mechanistic contributions and therapeutic targets in liver injury and repair. Physiol. Rev. 2009, 89, 1269–1339. [Google Scholar] [CrossRef]
- Feng, A.-C.; Fan, H.-L.; Chen, T.-W.; Hsieh, C.-B. Hepatic hemodynamic changes during liver transplantation: A review. World J. Gastroenterol. 2014, 20, 11131–11141. [Google Scholar] [CrossRef]
- Davis, W.D.; Batson, H.M.; Reichman, S.; Gorlin, R.; Storaasli, J.P. Clinical Applications of Intrasplenic Technique of Portal Pressure and Hepatic Blood Flow Determinations. Gastroenterology 1958, 34, 52–64. [Google Scholar] [CrossRef]
- Eipel, C.; Abshagen, K.; Vollmar, B. Regulation of hepatic blood flow: The hepatic arterial buffer response revisited. World J. Gastroenterol. 2010, 16, 6046–6057. [Google Scholar] [CrossRef]
- Garcia-Tsao, G.; Groszmann, R.J.; Fisher, R.L.; Conn, H.O.; Atterbury, C.E.; Glickman, M. Portal pressure, presence of gastroesophageal varices and variceal bleeding. Hepatology 1985, 5, 419–424. [Google Scholar] [CrossRef]
- Nagula, S.; Jain, D.; Groszmann, R.J.; Garcia-Tsao, G. Histological-hemodynamic correlation in cirrhosis—A histological classification of the severity of cirrhosis. J. Hepatol. 2006, 44, 111–117. [Google Scholar] [CrossRef]
- Gressner, A.M. Hepatic fibrogenesis: The puzzle of interacting cells, fibrogenic cytokines, regulatory loops, and extracellular matrix molecules. Z. Gastroenterol. 1992, 30 (Suppl. S1), 5–16. [Google Scholar]
- Schaffner, F.; Popper, H. Capillarization of Hepatic Sinusoids in Man. Gastroenterology 1963, 44, 239–242. [Google Scholar] [CrossRef]
- Iwakiri, Y. Pathophysiology of portal hypertension. Clin. Liver Dis. 2014, 18, 281–291. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H. Effects of portal hyperperfusion on partial liver grafts in the presence of hyperdynamic splanchnic circulation: Hepatic regeneration versus portal hyperperfusion injury. Anesth. Pain Med. 2016, 11, 117–129. [Google Scholar] [CrossRef]
- Lautt, W.W. (Ed.) Role and control of hepatic artery. In Hepatic Circulation in Health and Disease; Raven Press: New York, NY, USA, 1982; pp. 203–226. [Google Scholar]
- Sainz-Barriga, M.; Scudeller, L.; Costa, M.G.; de Hemptinne, B.; Troisi, R.I. Lack of a correlation between portal vein flow and pressure: Toward a shared interpretation of hemodynamic stress governing inflow modulation in liver transplantation. Liver Transplant. 2011, 17, 836–848. [Google Scholar] [CrossRef] [PubMed]
- Morsiani, E.; Aleotti, A.; Ricci, D. Haemodynamic and ultrastructural observations on the rat liver after two-thirds partial hepatectomy. J. Anat. 1998, 192, 507–515. [Google Scholar] [CrossRef] [PubMed]
- Soejima, Y.; Shimada, M.; Suehiro, T.; Hiroshige, S.; Ninomiya, M.; Shiotani, S.; Harada, N.; Hideki, I.; Yonemura, Y.; Maehara, Y. Outcome analysis in adult-to-adult living donor liver transplantation using the left lobe. Liver Transplant. 2003, 9, 581–586. [Google Scholar] [CrossRef]
- Soejima, Y.; Taketomi, A.; Yoshizumi, T.; Uchiyama, H.; Harada, N.; Ijichi, H.; Yonemura, Y.; Shimada, M.; Maehara, Y. Feasibility of Left Lobe Living Donor Liver Transplantation between Adults: An 8-Year, Single-Center Experience of 107 Cases. Am. J. Transplant. 2006, 6 Pt 1, 1004–1011. [Google Scholar] [CrossRef]
- Dahm, F.; Georgiev, P.; Clavien, P. Small-for-Size Syndrome after Partial Liver Transplantation: Definition, Mechanisms of Disease and Clinical Implications. Am. J. Transplant. 2005, 5, 2605–2610. [Google Scholar] [CrossRef]
- Olthoff, K.M.; Emond, J.C.; Shearon, T.H.; Everson, G.; Baker, T.B.; Fisher, R.A.; Freise, C.E.; Gillespie, B.W.; Everhart, J.E. Liver regeneration after living donor transplantation: Adult-to-adult living donor liver transplantation cohort study. Liver Transplant. 2015, 21, 79–88. [Google Scholar] [CrossRef]
- Hernandez-Alejandro, R.; Sharma, H. Small-for-size syndrome in liver transplantation: New horizons to cover with a good launchpad. Liver Transplant. 2016, 22, 33–36. [Google Scholar] [CrossRef]
- Iesari, S.; Núñez, M.E.I.; Juri, J.M.R.; Ciccarelli, O.; Bonaccorsi-Riani, E.; Coubeau, L.; Laterre, P.-F.; Goffette, P.; De Reyck, C.; Lengelé, B.; et al. Adult-to-adult living-donor liver transplantation: The experience of the Université catholique de Louvain. Hepatobiliary Pancreat. Dis. Int. 2019, 18, 132–142. [Google Scholar] [CrossRef]
- Wu, T.-J.; Dahiya, D.; Lee, C.-S.; Lee, C.-F.; Chou, H.-S.; Chan, K.-M.; Lee, W.-C. Impact of portal venous hemodynamics on indices of liver function and graft regeneration after right lobe living donor liver transplantation. Liver Transplant. 2011, 17, 1035–1045. [Google Scholar] [CrossRef] [PubMed]
- Byun, S.H.; Yang, H.S.; Kim, J.H. Liver graft hyperperfusion in the early postoperative period promotes hepatic regeneration 2 weeks after living donor liver transplantation: A prospective observational cohort study. Medicine 2016, 95, e5404. [Google Scholar] [CrossRef] [PubMed]
- Hou, C.-T.; Chen, Y.-L.; Lin, C.-C.; Chou, C.-T.; Lin, K.-H.; Lin, P.-Y.; Hsu, Y.-L.; Chen, C.-B.; Lin, H.-C.; Ko, C.-J.; et al. Portal venous velocity affects liver regeneration after right lobe living donor hepatectomy. PLoS ONE 2018, 13, e0204163. [Google Scholar] [CrossRef] [PubMed]
- García-Valdecasas, J.C.; Fuster, J.; Charco, R.; Bombuy, E.; Fondevila, C.; Ferrer, J.; Ayuso, C.; Taura, P. Changes in portal vein flow after adult living-donor liver transplantation: Does it influence postoperative liver function? Liver Transplant. 2003, 9, 564–569. [Google Scholar] [CrossRef] [PubMed]
- Piscaglia, F.; Zironi, G.; Gaiani, S.; Mazziotti, A.; Cavallari, A.; Gramantieri, L.; Valgimigli, M.; Bolondi, L. Systemic and splanchnic hemodynamic changes after liver transplantation for cirrhosis: A long-term prospective study. Hepatology 1999, 30, 58–64. [Google Scholar] [CrossRef]
- Hwang, S.; Lee, S.G.; Lee, Y.J.; Sung, K.B.; Park, K.M.; Kim, K.H.; Ahn, C.S.; Moon, D.B.; Hwang, G.S.; Kim, K.M.; et al. Lessons learned from 1,000 living donor liver transplantations in a single center: How to make living donations safe. Liver Transplant. 2006, 12, 920–927. [Google Scholar] [CrossRef]
- Lee, S.-G. Techniques of reconstruction of hepatic veins in living-donor liver transplantation, especially for right hepatic vein and major short hepatic veins of right-lobe graft. J. Hepato-Biliary-Pancreat. Surg. 2006, 13, 131–138. [Google Scholar] [CrossRef]
- Fujiki, M.; Hashimoto, K.; Quintini, C.; Aucejo, F.; Kwon, C.H.D.; Matsushima, H.; Sasaki, K.; Campos, L.; Eghtesad, B.; Diago, T.; et al. Living Donor Liver Transplantation with Augmented Venous Outflow and Splenectomy: A Promised Land for Small Left Lobe Grafts. Ann. Surg. 2022, 276, 838–845. [Google Scholar] [CrossRef]
- Lee, S.D.; Kim, S.H.; Kim, Y.-K.; Lee, S.-A.; Park, S.-J. Graft-to-recipient weight ratio lower to 0.7% is safe without portal pressure modulation in right-lobe living donor liver transplantation with favorable conditions. Hepatobiliary Pancreat. Dis. Int. 2014, 13, 18–24. [Google Scholar] [CrossRef]
- Asencio, J.M.; Vaquero, J.; Olmedilla, L.; Sabrido, J.G. “Small-for-flow” syndrome: Shifting the “size” paradigm. Med. Hypotheses 2013, 80, 573–577. [Google Scholar] [CrossRef]
- Kaido, T.; Mori, A.; Ogura, Y.; Hata, K.; Yoshizawa, A.; Iida, T.; Yagi, S.; Uemoto, S. Lower Limit of the Graft-to-Recipient Weight Ratio Can Be Safely Reduced to 0.6% in Adult-to-Adult Living Donor Liver Transplantation in Combination with Portal Pressure Control. Transplant. Proc. 2011, 43, 2391–2393. [Google Scholar] [CrossRef] [PubMed]
- Yao, S.; Kaido, T.; Uozumi, R.; Yagi, S.; Miyachi, Y.; Fukumitsu, K.; Anazawa, T.; Kamo, N.; Taura, K.; Okajima, H.; et al. Is Portal Venous Pressure Modulation Still Indicated for All Recipients in Living Donor Liver Transplantation? Liver Transplant. 2018, 24, 1578–1588. [Google Scholar] [CrossRef] [PubMed]
- Yoshizumi, T.; Itoh, S.; Shimokawa, M.; Inokuchi, S.; Harada, N.; Takeishi, K.; Mano, Y.; Yoshiya, S.; Kurihara, T.; Nagao, Y.; et al. Simultaneous splenectomy improves outcomes after adult living donor liver transplantation. J. Hepatol. 2021, 74, 372–379. [Google Scholar] [CrossRef]
- Gyoten, K.; Mizuno, S.; Kato, H.; Murata, Y.; Tanemura, A.; Azumi, Y.; Kuriyama, N.; Kishiwada, M.; Usui, M.; Sakurai, H.; et al. A Novel Predictor of Posttransplant Portal Hypertension in Adult-to-Adult Living Donor Liver Transplantation: Increased Estimated Spleen/Graft Volume Ratio. Transplantation 2016, 100, 2138–2145. [Google Scholar] [CrossRef]
- Xiao, F.; Wei, L.; Qu, W.; Zeng, Z.-G.; Sun, L.-Y.; Liu, Y.; Zhang, H.-M.; Tan, Y.-L.; Wang, J.; Zhu, Z.-J. Liver Graft-to-Spleen Volume Ratio as a Useful Predictive Factor of the Outcomes in Living Donor Liver Transplantation: A Retrospective Study. Front. Surg. 2022, 9, 855695. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Singhal, S.; Venuthurimilli, A.; Pareek, S.; Maung, P.M.; Aung, T.H.; Garg, H.K.; Vohra, S.; Sahni, R.; Goyal, N. HPi: A Novel Parameter to Predict Graft-related Outcome in Adult Living Donor Liver Transplant. Transplantation 2022, 106, 767–780. [Google Scholar] [CrossRef] [PubMed]
- Jang, Y.-J.; Kim, K.W.; Jeong, W.K.; Shin, Y.M.; Song, G.-W.; Hwang, S.; Lee, S.-G. Influence of Preoperative Portal Hypertension and Graft Size on Portal Blood Flow Velocity in Recipient after Living Donor Liver Transplantation with Right-Lobe Graft. Am. J. Roentgenol. 2010, 194, W165–W170. [Google Scholar] [CrossRef]
- Gomez Gavara, C.; Bhangui, P.; Salloum, C.; Osseis, M.; Esposito, F.; Moussallem, T.; Lahat, E.; Fuentes, L.; Compagnon, P.; Ngongang, N.; et al. Ligation versus no ligation of spontaneous portosystemic shunts during liver transplantation: Audit of a prospective series of 66 consecutive patients. Liver Transplant. 2018, 24, 505–515. [Google Scholar] [CrossRef]
- Ito, T.; Kiuchi, T.; Yamamoto, H.; Oike, F.; Ogura, Y.; Fujimoto, Y.; Hirohashi, K.; Tanaka, K. Changes in portal venous pressure in the early phase after living donor liver transplantation: Pathogenesis and clinical implications. Transplantation 2003, 75, 1313–1317. [Google Scholar] [CrossRef]
- Shimamura, T. Excessive portal venous inflow as a cause of allograft dysfunction in small-for-size living donor liver transplantation. In Transplantation Proceedings; Elsevier Science Publishing Company, Inc.: Amsterdam, The Netherlands, 2001. [Google Scholar]
- Troisi, R.; de Hemptinne, B. Clinical relevance of adapting portal vein flow in living donor liver transplantation in adult patients. Liver Transplant. 2003, 9, S36–S41. [Google Scholar] [CrossRef]
- Vasavada, B.; Chen, C.L.; Zakaria, M. Using low graft/recipient’s body weight ratio graft with portal flow modulation an effective way to prevent small-for-size syndrome in living-donor liver transplant: A retrospective analysis. Exp. Clin. Transplant. 2014, 12, 437–442. [Google Scholar] [PubMed]
- Matsushima, H.; Sasaki, K.; Fujiki, M.; Uso, T.D.; Aucejo, F.; Kwon, C.H.D.; Eghtesad, B.; Miller, C.; Quintini, C.; Hashimoto, K. Too Much, Too Little, or Just Right? The Importance of Allograft Portal Flow in Deceased Donor Liver Transplantation. Transplantation 2020, 104, 770–778. [Google Scholar] [CrossRef] [PubMed]
- Yagi, S.; Iida, T.; Hori, T.; Taniguchi, K.; Yamamoto, C.; Yamagiwa, K.; Uemoto, S. Optimal portal venous circulation for liver graft function after living-donor liver transplantation. Transplantation 2006, 81, 373–378. [Google Scholar] [CrossRef] [PubMed]
- Miyagi, S.; Shono, Y.; Tokodai, K.; Nakanishi, W.; Nishimura, R.; Fujio, A.; Sasaki, K.; Miyazaki, Y.; Kakizaki, Y.; Sasajima, H.; et al. Risks of Living Donor Liver Transplantation Using Small-for-Size Grafts. Transplant. Proc. 2020, 52, 1825–1828. [Google Scholar] [CrossRef] [PubMed]
- Rammohan, A.; Rela, M.; Kim, D.; Soejima, Y.; Kasahara, M.; Ikegami, T.; Spiro, M.; Aristotle Raptis, D.; Humar, A. Does modification of portal pressure and flow enhance recovery of the recipient after living donor liver transplantation? A systematic review of literature and expert panel recommendations. Clin. Transplant. 2022, 36, e14657. [Google Scholar] [CrossRef] [PubMed]
- Gavriilidis, P.; Azoulay, D. Graft Inflow Modulation in Living Donor Liver Transplantation with a Small-for-Size Graft: A Systematic Review and Meta-Analysis. Chirurgia 2022, 117, 245. [Google Scholar] [CrossRef]
- Troisi, R.; Cammu, G.; Militerno, G.; de Baerdemaeker, L.; Decruyenaere, J.; Hoste, E.; Smeets, P.; Colle, I.; Van Vlierberghe, H.; Petrovic, M.; et al. Modulation of Portal Graft Inflow: A Necessity in Adult Living-Donor Liver Transplantation? Ann. Surg. 2003, 237, 429. [Google Scholar] [CrossRef]
- Su, C.-M.; Chou, T.-C.; Yang, T.-H.; Lin, Y.-J. Graft Inflow Modulation in Living-Donor Liver Transplantation: Hepatic Hemodynamic Changes in Splenic Artery Ligation and Splenectomy. Ann. Transplant. 2022, 27, e936609-1. [Google Scholar] [CrossRef]
- Ogura, Y.; Hori, T.; El Moghazy, W.M.; Yoshizawa, A.; Oike, F.; Mori, A.; Kaido, T.; Takada, Y.; Uemoto, S. Portal pressure <15 mmHg is a key for successful adult living donor liver transplantation utilizing smaller grafts than before. Liver Transplant. 2010, 16, 718–728. [Google Scholar]
- Uemura, T.; Wada, S.; Kaido, T.; Mori, A.; Ogura, Y.; Yagi, S.; Fujimoto, Y.; Ogawa, K.; Hata, K.; Yoshizawa, A.; et al. How far can we lower graft-to-recipient weight ratio for living donor liver transplantation under modulation of portal venous pressure? Surgery 2016, 159, 1623–1630. [Google Scholar] [CrossRef]
- Ikegami, T.; Yoshizumi, T.; Sakata, K.; Uchiyama, H.; Harimoto, N.; Harada, N.; Itoh, S.; Nagatsu, A.; Soejima, Y.; Maehara, Y. Left lobe living donor liver transplantation in adults: What is the safety limit? Liver Transplant. 2016, 22, 1666–1675. [Google Scholar] [CrossRef] [PubMed]
- Moon, D.; Lee, S.; Hwang, S.; Ahn, C.; Kim, K.; Ha, T.; Song, G.; Jung, D.; Park, G.; Yoon, Y.; et al. Splenic devascularization can replace splenectomy during adult living donor liver transplantation—A historical cohort study. Transpl. Int. 2019, 32, 535–545. [Google Scholar] [CrossRef] [PubMed]
- Kokai, H.; Sato, Y.; Yamamoto, S.; Oya, H.; Nakatsuka, H.; Watanabe, T.; Takizawa, K.; Hatakeyama, K. Successful Super-Small-for-Size Graft Liver Transplantation by Decompression of Portal Hypertension via Splenectomy and Construction of a Mesocaval Shunt: A Case Report. Transplant. Proc. 2008, 40, 2825–2827. [Google Scholar] [CrossRef] [PubMed]
- Sato, Y.; Yamamoto, S.; Takeishi, T.; Kato, T.; Nakatsuka, H.; Kobayashi, T.; Oya, H.; Watanabe, T.; Kokai, H.; Hatakeyama, K. Inferior mesenteric venous left renal vein shunting for decompression of excessive portal hypertension in adult living related liver transplantation. Transplant. Proc. 2004, 36, 2234–2236. [Google Scholar] [CrossRef]
- Soin, A.S.; Yadav, S.K.; Saha, S.K.; Rastogi, A.; Bhangui, P.; Srinivasan, T.; Saraf, N.; Choudhary, N.S.; Saigal, S.; Vohra, V. Is Portal Inflow Modulation always Necessary for Successful Utilization of Small Volume Living Donor Liver Grafts? Liver Transplant. 2019, 25, 1811–1821. [Google Scholar] [CrossRef]
- Yamada, T.; Tanaka, K.; Uryuhara, K.; Ito, K.; Takada, Y.; Uemoto, S. Selective hemi-portocaval shunt based on portal vein pressure for small-for-size graft in adult living donor liver transplantation. Am. J. Transplant. 2008, 8, 847–853. [Google Scholar] [CrossRef]
- Reddy, M.S.; Rela, M. Portosystemic collaterals in living donor liver transplantation: What is all the fuss about? Liver Transplant. 2017, 23, 537–544. [Google Scholar] [CrossRef]
- Lee, C.Y.; Lim, W.X.; Chen, C.L.; Yong, C.C.; Yu, C.Y.; Tsang, L.L.C.; Hsu, H.W.; Cheng, Y.F.; Ou, H.Y. Efficacy and safety of splenic artery embolization for intractable ascites using Amplatzer vascular plug versus coil after living donor liver transplantation. Diagn. Interv. Radiol. 2022, 28, 478–485. [Google Scholar] [CrossRef]
- Umeda, Y.; Yagi, T.; Sadamori, H.; Matsukawa, H.; Matsuda, H.; Shinoura, S.; Iwamoto, T.; Satoh, D.; Iwagaki, H.; Tanaka, N. Preoperative proximal splenic artery embolization: A safe and efficacious portal decompression technique that improves the outcome of live donor liver transplantation. Transpl. Int. 2007, 20, 947–955. [Google Scholar] [CrossRef]
- Troisi, R.; Ricciardi, S.; Smeets, P.; Petrovic, M.; van Maele, G.; Colle, I.; Van Vlierberghe, H.; De Hemptinne, B. Effects of Hemi-Portocaval Shunts for Inflow Modulation on the Outcome of Small-for-Size Grafts in Living Donor Liver Transplantation. Am. J. Transplant. 2005, 5, 1397–1404. [Google Scholar] [CrossRef]
- Lauro, A.; Diago Uso, T.; Quintini, C.; di Benedetto, F.; Dazzi, A.; de Ruvo, N.; Masetti, M.; Cautero, N.; Risaliti, A.; Zanfi, C.; et al. Adult-to-Adult Living Donor Liver Transplantation Using Left Lobes: The Importance of Surgical Modulations on Portal Graft Inflow. Transplant. Proc. 2007, 39, 1874–1876. [Google Scholar] [CrossRef] [PubMed]
- Yoshizumi, T.; Taketomi, A.; Soejima, Y.; Ikegami, T.; Uchiyama, H.; Kayashima, H.; Harada, N.; Yamashita, Y.-I.; Kawanaka, H.; Nishizak, T.; et al. The beneficial role of simultaneous splenectomy in living donor liver transplantation in patients with small-for-size graft. Transpl. Int. 2008, 21, 833–842. [Google Scholar] [CrossRef]
- Ou, H.-Y.; Huang, T.-L.; Chen, T.-Y.; Tsang, L.-C.; Chen, C.-L.; Cheng, Y.-F. Early Modulation of Portal Graft Inflow in Adult Living Donor Liver Transplant Recipients with High Portal Inflow Detected by Intraoperative Color Doppler Ultrasound. Transplant. Proc. 2010, 42, 876–878. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Ikegami, T.; Harada, N.; Yoshizumi, T.; Soejima, Y.; Uchiyama, H.; Yamashita, Y.I.; Itoh, S.; Harimoto, N.; Kawanaka, H. Optimal changes in portal hemodynamics induced by splenectomy during living donor liver transplantation. Surg. Today 2015, 45, 979–985. [Google Scholar] [CrossRef]
- Emond, J.C.; Goodrich, N.P.; Pomposelli, J.J.; Baker, T.B.; Humar, A.; Grant, D.R.; Abt, P.; Friese, C.E.; Fisher, R.A.; Kam, I.; et al. Hepatic Hemodynamics and Portal Flow Modulation. Transplantation 2017, 101, 2375–2384. [Google Scholar] [CrossRef]
- Ito, K.; Akamatsu, N.; Ichida, A.; Ito, D.; Kaneko, J.; Arita, J.; Sakamoto, Y.; Hasegawa, K.; Kokudo, N. Splenectomy is not indicated in living donor liver transplantation. Liver Transplant. 2016, 22, 1526–1535. [Google Scholar] [CrossRef] [PubMed]
- Wong, T.C.-L.; Fung, J.Y.Y.; Cui, T.Y.S.; Sin, S.L.; Ma, K.W.; She, B.W.H.; Chan, A.C.Y.; Chok, K.S.H.; Dai, J.W.C.; Cheung, T.-T.; et al. The Risk of Going Small. Ann. Surg. 2021, 274, e1260–e1268. [Google Scholar] [CrossRef]
- Jo, H.-S.; Yu, Y.-D.; Choi, Y.J.; Kim, D.-S. Left liver graft in adult-to-adult living donor liver transplantation with an optimal portal flow modulation strategy to overcome the small-for-size syndrome—A retrospective cohort study. Int. J. Surg. 2022, 106, 106953. [Google Scholar] [CrossRef]
- Osman, A.M.A.; Hosny, A.A.; El-Shazli, M.A.; Uemoto, S.; Abdelaziz, O.; Helmy, A.S. A Portal Pressure Cut-off of 15 versus a Cut-off of 20 for Prevention of Small-For-Size Syndrome in Liver Transplantation: A Comparative Study. Hepatol. Res. 2017, 47, 293–302. [Google Scholar] [CrossRef]
- Groszmann, R.J.; Garcia-Tsao, G.; Bosch, J.; Grace, N.D.; Burroughs, A.K.; Planas, R.; Escorsell, A.; Garcia-Pagan, J.C.; Patch, D.; Matloff, D.S.; et al. Beta-Blockers to Prevent Gastroesophageal Varices in Patients with Cirrhosis. N. Engl. J. Med. 2005, 353, 2254–2261. [Google Scholar] [CrossRef]
- Mehrabi, A.; Golling, M.; Kashfi, A.; Boucsein, T.; Schemmer, P.; Gutt, C.N.; Schmidt, J.; Büchler, M.W.; Kraus, T.W. Negative impact of systemic catecholamine administration on hepatic blood perfusion after porcine liver transplantation. Liver Transplant. 2005, 11, 174–187. [Google Scholar] [CrossRef] [PubMed]
- Schmeisch, A.P.; de Oliveira, D.S.; Ide, L.T.; Suzuki-Kemmelmeier, F.; Bracht, A. Zonation of the metabolic action of vasopressin in the bivascularly perfused rat liver. Regul. Pept. 2005, 129, 233–243. [Google Scholar] [CrossRef] [PubMed]
- Wagener, G.; Gubitosa, G.; Renz, J.; Kinkhabwala, M.; Brentjens, T.; Guarrera, J.V.; Emond, J.; Lee, H.T.; Landry, D. Vasopressin decreases portal vein pressure and flow in the native liver during liver transplantation. Liver Transplant. 2008, 14, 1664–1670. [Google Scholar] [CrossRef] [PubMed]
- Reddym, M.S.; Kaliamoorthy, I.; Rajakumar, A.; Malleeshwaran, S.; Appuswamy, E.; Lakshmi, S.; Varghese, J.; Rela, M. Double-blind randomized controlled trial of the routine perioperative use of terlipressin in adult living donor liver transplantation. Liver Transplant. 2017, 23, 1007–1014. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Man, K.; Zheng, S.S.; Liang, T.B.; Lee, T.K.; Ng, K.T.; Fan, S.T.; Lo, C.M. Attenuation of acute phase shear stress by somatostatin improves small-for-size liver graft survival. Liver Transplant. 2006, 12, 621–627. [Google Scholar] [CrossRef] [PubMed]
- Ji, Y.; Dahmen, U.; Madrahimov, N.; Madrahimova, F.; Xing, W.; Dirsch, O. G-CSF Administration in a Small-for-Size Liver Model. J. Investig. Surg. 2009, 22, 167–177. [Google Scholar] [CrossRef]
- Ijichi, H.; Taketomi, A.; Yoshizumi, T.; Uchiyama, H.; Yonemura, Y.; Soejima, Y.; Shimada, M.; Maehara, Y. Hyperbaric oxygen induces vascular endothelial growth factor and reduces liver injury in regenerating rat liver after partial hepatectomy. J. Hepatol. 2006, 45, 28–34. [Google Scholar] [CrossRef]
- Suehiro, T.; Shimada, M.; Kishikawa, K.; Shimura, T.; Soejima, Y.; Yoshizumi, T.; Hashimoto, K.; Mochida, Y.; Hashimoto, S.; Maehara, Y.; et al. Effect of intraportal infusion to improve small for size graft injury in living donor adult liver transplantation. Transpl. Int. 2005, 18, 923–928. [Google Scholar] [CrossRef]
- Yu, Y.-D.; Kim, D.-S.; Byun, G.-Y.; Seo, S.-O. Can propranolol be a viable option for the treatment of small-for-size syndrome? Liver Transplant. 2012, 18, 747–748. [Google Scholar] [CrossRef]
- Ozden, I.; Kara, M.; Pinarbasi, B.; Salmaslioglu, A.; Yavru, A.; Kaymakoglu, S.; Emre, A.; Bilge, O.; Alper, A. Somatostatin and propranolol to treat small-for-size syndrome that occurred despite splenic artery ligation. Exp. Clin. Transplant. 2007, 5, 686–689. [Google Scholar]
- Troisi, R.I.; Vanlander, A.; Giglio, M.C.; van Limmen, J.; Scudeller, L.; Heyse, B.; De Baerdemaeker, L.; Croo, A.; Voet, D.; Praet, M.; et al. Somatostatin as Inflow Modulator in Liver-transplant Recipients with Severe Portal Hypertension. Ann. Surg. 2019, 269, 1025–1033. [Google Scholar] [CrossRef] [PubMed]
- Kelly, D.M.; Zhu, X.; Shiba, H.; Irefin, S.; Trenti, L.; Cocieru, A.; Diago, T.; Wang, L.F.; Quintini, C.; Chen, Z.; et al. Adenosine restores the hepatic artery buffer response and improves survival in a porcine model of small-for-size syndrome. Liver Transplant. 2009, 15, 1448–1457. [Google Scholar] [CrossRef] [PubMed]
- Orue-Echebarria, M.I.; Lozano, P.; Olmedilla, L.; García Sabrido, J.L.; Asencio, J.M. “Small-for-Flow” Syndrome: Concept Evolution. J. Gastrointest. Surg. 2020, 24, 1386–1391. [Google Scholar] [CrossRef] [PubMed]
| Author | Year | Terminology | Criteria |
|---|---|---|---|
| Soejima Y et al. [16] | 2003 | SFSS | Total Bilirubin > 5 mg/dL (on POD 14), ascites > 1 L (on POD 14) or ascites > 0.5 L (on POD 28) |
| Soejima Y et al. [17] | 2006 | SFSS | Total Bilirubin > 10 mg/dL (on POD 14), ascites > 1 L (on POD14) or ascites > 0.5 L (on POD 28) |
| Dahm F et al. [18] | 2006 | SFSD | Total Bilirubin > 100 µmol/L, INR > 2, Encephalopathy grade III-IV |
| SFNF | Retransplantation or death within the first postoperative week | ||
| Olthoff KM et al. [19] | 2014 | EAD | Total Bilirubin > 10 mg/dL (on POD 7) or INR > 1.6 (on POD 7) |
| Hernandez-Alejandro R et al. [20] | 2019 | SFSS |
INR > 2 (lasts for 3 consecutive days within the first postoperative week) Ascites > 1 L (lasts for 3 consecutive days within the first postoperative week or on POD 14) or ascites > 0.5 L (POD 28) Encephalopathy grade III–IV
|
| Iesari S et al. [21] | 2019 | SFSS | Total Bilirubin > 20 mg/dL (lasts for 7 consecutive days after POD 7) INR > 2 (lasts for 3 consecutive days within the first postoperative week) Ascites > 1 L (lasts for 3 consecutive days within the first postoperative week or on POD 14) or ascites > 0.5 L (POD 28) Encephalopathy grade III–IV |
| Author/Year (Type of Study) | Number of Patients Type of Graft GRWR | Type of Modulation | Threshold for Modulation Pressure/Flow | Outcome | Comments |
|---|---|---|---|---|---|
| Ito T et al., 2003 [40] (Prospective observational study) | 79 75 right hemiliver two left hemiliver 0.73–2.02% (median, 1.06%) | SAL (7 patients) | Small-for-size graft less than 1.0% of GRWR or PVP ≥ 20 mmHg | Cumulative graft survival at 6 months was 83.5%, 86.1% and 38.5% for the SAL, non-SAL low-PVP and non-SAL high-PVP groups, respectively | High postreperfusion portal pressure is associated with poorer survival |
| Trosi R et al., 2003 [42] (Prospective observational study) | 24 23 right hemiliver Mean GRWR 1.12 in patient without GIM, 1.13 in patients with GIM | SAL in 13 patients, one needed additional HPCS | GRWR < 0.8 with PVF > 250 mL/min per 100 g of liver | 3 patients (27%) who did not receive GIM developed SFSS, no patients who received GIM had SFSS 1 YOS was 62% and 93%, respectively, for patients without and with GIM, respectively | GIM modulation prevents SFSS; mean GRWR was >1 in this population |
| Trosi R et al., 2005 [62] (Prospective observational study) | 13 Group 1 without GIM; n = 5 (4 right and 1 left hemiliver) GRWR 0.73 (0.58–0.80) Group 2 with GIM; n = 8 (equal right, left) GRWR 0.71 (0.56–0.80) | HPCS | GRWR < 0.8 | SFSS 80% without GIM, none with GIM 1-year graft survival was 20% in patients without GIM, 75% with GIM 1-year patient survival was 40% in patients without GIM, 87.5% with GIM | GIM prevented SFSS and improved graft and patient survival |
| Lauro et al., 2007 [63] (Retrospective) | 8 All left hemiliver Two patients had GRWR of 0.4, others had between 0.7 and 0.8 | Splenectomy in 2, splenorenal shunt in 2, splenectomy and portocaval shunt in one | PVP-CVP various thresholds used by authors | SFSS 50% 2 died, 2 retransplanted | Early experience of using left-sided grafts with low GRWR, GIM |
| Yagi S et al., 2008 [45] (Retrospective) | 28 Left-hemiliver graft with caudate in 7, modified right-hemiliver graft in 12 GRWR 0.67–1.60 (median, 1.06%) | Splenectomy (n = 4) or splenorenal shunt (n = 1) | PVP > 20 | SFSS in 2 patients 1-year graft and patient survival were 92.3% | Early experience of splenectomy as a form of GIM |
| Yoshizumi T et al., 2008 [64] (Retrospective, comparative) | 113 Left hemiliver with caudate (n = 63), modified right-hemiliver graft (n = 46) GRWR 0.88 ± 0.20 in patients who did not undergo splenectomy and 0.77 ± 0.18 in patients who underwent splenectomy | Splenectomy in 44 patients | Portal pressure after portal reperfusion > 20 mmHg | SFSS in 27.4% 4-year patient survival rate in all patients was 85.8%, while that of the without-splenectomy and with-splenectomy groups were 84.4% and 92.1%, respectively | Patients underwent splenectomy for reasons other than GIM as well |
| Ou HY et al., 2010 [65] (Retrospective) | 138 GRWR 1.14 (0.73–1.71) | 6 patients had SAL and one patient also had splenectomy | PVF > 250 mL/min/100 g | 3 out of 8 patients who had PVF > 250 mL/min/100 g developed SFSS; only one with GIM developed SFSS. One patient died | Small number underwent GIM; median GRWR in this study was >1; PVF was the trigger for GIM |
| Ogura et al., 2010 [51] (Retrospective comparative) | 566 502 right, 64 left hemiliver 1.15 in era without GIM, 0.92 during the era of GIM | Splenectomy 84 SAL 1 Portosystemic shunt (IMV-LRV) in addition to splenectomy | GRWR < 0.8 | 12.9% (4 of 31 SFS grafts with a GRWR < 0.8%) developed SFSS Overall, 1-, 3- and 5-year survival rates after LDLT in period I were 76.2%, 71.1% and 68.8%, respectively 1- and 2-year survival rates in period II were 87.9% and 81.6%, respectively | GIM helps in selection of grafts with lower GRWR with similar outcomes as larger grafts without GIM |
| Wang et al., 2014 [66] (Retrospective comparative) | 276 Left-hemiliver grafts (n = 168, 60.9%) Right-hemiliver grafts (n = 108, 39.1%) Mean GV/SLV was 41.8 ± 8.5 | Splenectomy 154 | PVP ≥ 20 mmHg | Incidence of primary graft dysfunction was 9.7% in the splenectomy group and 19.7% (p = 0.018) in the non-splenectomy group. 30 patients had early graft loss in 6 months | Splenectomy with PVP > 20 mmHg resulted in better outcomes |
| Osman A et al., 2016 [71] (Retrospective) | 76 1.06 ± 0.22 in patients with PVP less than 15 and 1.00 ± 0.17 in patients with PVP 15–19 | Splenectomy | PVP > 20 mmHg | 6 patients had SFSS when PVP was 15–19 (16.2%) compared to 1 patient (2.6%) in group with PVP < 15 mmHg 9 patients died in group with PVP between 15 and 19 (24.3%), 4 of whom died of SFSS, compared to 3 in the group where PVP was <15 mmHg (7.7%) | The authors proposed 15 mmHg as a cut-off for GIM |
| Uemura T et al., 2016 [52] (Retrospective comparative) | 221 LL 106, RL 115 Average GRWR 0.620 ± 0.0465 (for small), 0.744 ± 0.027 (for medium), 1.010 ± 0.178 (for large) | Splenectomy | Portal pressure > 15 mmHg | 28% SFSS For patients with GRWR < 0.7, 80% of grafts survived at 5 years 49 patients died by 1 year, out of which 14 died of SFSS Satisfactory outcomes in LDLT with GRWR as low as 0.6% using PVP modulation | Authors attributed mortality to nutritional depletion and sarcopenia in low-GRWR patients |
| Emond J et al., 2017 [67] (Multicentric prospective observational study) | 274 233 (85.0%) right hemiliver, 40 (14.6%) left hemiliver and 1 (0.5%) left lateral section GRWR 1.030 (no GIM) GRWR 0.828 (GIM used) | Portocaval shunt 26.9% Splenectomy 8 (15.3%) SAL 34 (65.4%) | Elevated portal pressure reported in 56% of cases, elevated portal flow in 42%. Portal gradient (21%), graft size (15%) and decreased arterial flow (8%) | Graft dysfunction was most common in the SAL patients (42%), two patients of portocaval shunt (17%) and occurred in a single splenectomy patient (13%). Survival at 2 years posttransplant was 90% for the modulated subjects and 81% for the unmodulated subjects | A higher percentage of the modulated (sicker) patients experienced graft dysfunction compared to unmodulated subjects (31% vs. 18%, p = 0.03) A2ALL study: variability in practice among participating centers |
| Ito et al., 2016 [68] (Prospective comparative study) | 395 241RL, 154 LL | Splenectomy in 169 | Threshold for modulation was not specified | 5% SFSS The 1-, 3- and 5-year graft survival rates with splenectomy were 88.7%, 85.2% and 81.3%, respectively, and 92.9%, 88.4% and 86.0%, respectively, without splenectomy | Splenectomy in majority of the patients was performed for indications other than GIM |
| Yao S et al., 2018 [33] (Retrospective comparative) | 319 184 RL, 135 LL GRWR < 0.8% in 98 patients (30.7%) | Splenectomy in 59.9% patients, SAL 1, HPCS 1 | Portal pressure > 15 mmHg | Cumulative graft survival was 84.1% in patients needing modulation at 1 year and 75.6% at 5 years. In-hospital mortality was 17.2% | GIM modulation was recommended with GRWR < 0.8 when donors were >45 y/ABO–incompatible |
| Gyoten K et al., 2016 [35] (Retrospective) | 73 Left hemiliver 27 Right hemiliver 45 Estimated GRWR 0.882 (0.464–1.291) in 55 patients where data were available | Splenectomy | PVP > 20 mmHg | 2 patients had SFSS 1–, 3- and 5-year cumulative survival rates were 79.6%, 73.3% and 71.2%, respectively, in the 54 recipients with PVP < 20 mmHg and 89.5%, 77.5% and 69.8% in the 19 with PVP > 20 mmHg followed by splenectomy | Splenectomy with PVP > 20 mmHg results in similar survival to patients with lower pressures, and seems to mitigate effects of low GRWR |
| Soin A et al., 2019 [57] (Retrospective comparative) | 1321, out of which 287 had GRWR < 0.8 13 LL 1308 RL GRWR 0.54–0.69 in 79 (5.9%) GRWR 0.70 to 0.74 in 81 (6.1%) GRWR 0.75 to 0.79 in 134 (10.1%) | 109-HPCS, 14-SAL | GRWR < 0.8 No GIM if PVP < 16 PVP 16–18 SAL PVP > 18 HPCS | 2.8% SFSS 0.5% 30-day mortality | Excellent results are a reflection of good selection policy for GIM in an experienced center |
| Miyagi S et al., 2020 [46] (Retrospective) | 188 (83 adults; 105 pediatric) GRWR < 0.8 (n = 22) GRWR 0.8–3.5 (n = 154) GRWR > 3.5 (n = 12) | Splenectomy in 7 patients | GRWR < 0.8 (PVP > 17 mmHg in the later part of the study) | 11.7% SFSS 5 YSR in SFSG without GIM 52.8% 5YSR in SFSG with splenectomy 80.0% | Splenectomy for GIM resulted in similar survival with smaller grafts as with larger grafts without GIM |
| Wong TC et al., 2021 [69] (Retrospective Comparative) | 545 GRWR < 0.6 (n = 39; LL 33.3%) GRWR 0.6–0.8 (n = 159; LL 10.7%) GRWR > 0.8 (n = 347; LL 2.9%) | GRWR < 0.6 | 4.7% SFSS 2 in-hospital mortalities | Good results with GIM despite the use of smaller grafts | |
| Hye-Sung Jo et al., 2022 [70] (Retrospective comparative) | 118 93 RL, 25 LL | SAL | Portal flow >300 mL/min/100 g and HVPG > 10 mmHg | SFSS 16% in LL and 3.2% in RL No SFSS-related mortalities | SAL based on intraoperative portal flow and HVPG results in satisfactory outcomes |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bharathy, K.G.; Shenvi, S. Portal Hemodynamics after Living-Donor Liver Transplantation: Management for Optimal Graft and Patient Outcomes—A Narrative Review. Transplantology 2023, 4, 38-58. https://doi.org/10.3390/transplantology4020006
Bharathy KG, Shenvi S. Portal Hemodynamics after Living-Donor Liver Transplantation: Management for Optimal Graft and Patient Outcomes—A Narrative Review. Transplantology. 2023; 4(2):38-58. https://doi.org/10.3390/transplantology4020006
Chicago/Turabian StyleBharathy, Kishore GS, and Sunil Shenvi. 2023. "Portal Hemodynamics after Living-Donor Liver Transplantation: Management for Optimal Graft and Patient Outcomes—A Narrative Review" Transplantology 4, no. 2: 38-58. https://doi.org/10.3390/transplantology4020006
APA StyleBharathy, K. G., & Shenvi, S. (2023). Portal Hemodynamics after Living-Donor Liver Transplantation: Management for Optimal Graft and Patient Outcomes—A Narrative Review. Transplantology, 4(2), 38-58. https://doi.org/10.3390/transplantology4020006







