Advances in Hypothermic and Normothermic Perfusion in Kidney Transplantation
Abstract
:1. Introduction
- -
- Improvement of transplant outcomes through delivery of therapeutic agents to repair and regenerate kidneys.
- -
- Reduction in the number of discarded kidneys by developing robust techniques of organ assessment.
- -
- Reduction in ischaemic injury during the preservation interval to improve the ‘shelf life’ of donated kidneys and increase the number available for transplant.
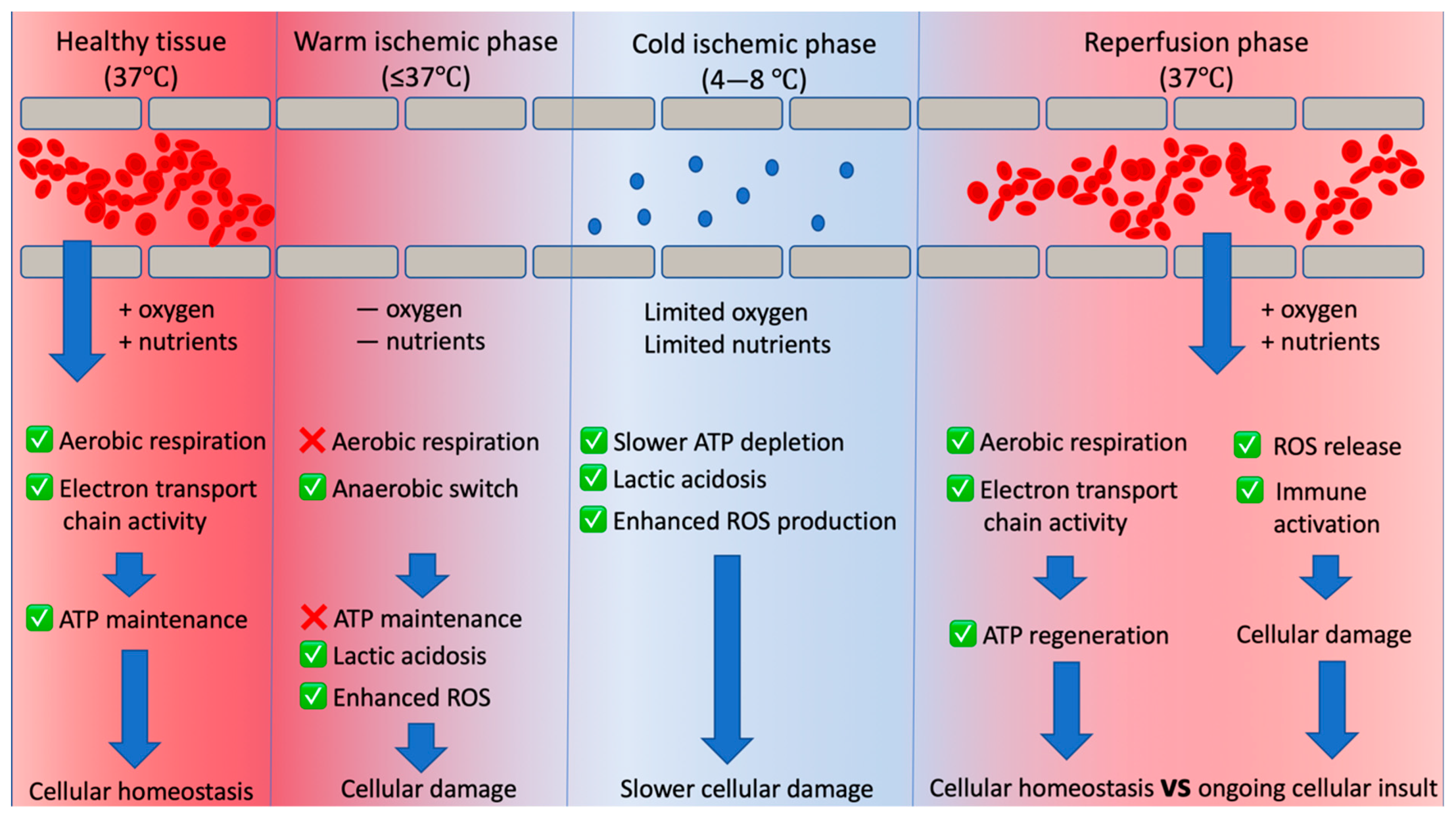
2. Why Do We Need Organ Preservation? What Are the Factors Diminishing Kidney Quality?
3. Warm Ischaemia
4. Cold Ischaemia
5. Current Kidney Preservation Methods, Their Advantages and Limitations
5.1. Static Cold Storage
5.2. Hypothermic Machine Perfusion
5.3. Normothermic Machine Perfusion
6. What Are the Advances in HMP?
6.1. Active Perfusate Oxygenation under HMP
6.2. Modification of Perfusate under HMP
6.3. Measures of Graft Quality during HMP
7. What Are the Advances in NMP?
7.1. Modification of the Normothermic Perfusion Conditions
7.2. Regenerative Therapies
7.3. Therapeutic Agents
7.4. Modifying the Duration of NMP
7.5. Assessment of Graft Quality during NMP
8. Summary
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ATP | adenosine triphosphate |
| CIT | cold ischaemic time |
| CKD | chronic kidney disease |
| COR | controlled oxygenated rewarming |
| DBD | donation after brain death |
| DCD | donation after circulatory death |
| DGF | delayed graft function |
| ECD | extended criteria donor |
| eGFR | estimated glomerular filtration rate |
| ESRD | end-stage renal disease |
| GFR | glomerular filtration rate |
| HBOC | haemoglobin-based oxygen carrier |
| HLA | human leucocyte antigen |
| HMP | hypothermic machine perfusion |
| IRI | ischaemia reperfusion injury |
| KPS-1 | kidney perfusion solution 1 |
| MAPCs | multipotent adult progenitor cells |
| MHC | major histocompatibility complex |
| MRI | magnetic resonance imaging |
| MSCs | mesenchymal stem cells |
| NEVKP | normothermic ex vivo kidney perfusion |
| NFκB | nuclear factor kappa B |
| NGAL | neutrophil gelatinase-associated lipocalin |
| NMP | normothermic machine perfusion |
| NMR | nuclear magnetic resonance |
| NRP | normothermic regional perfusion |
| PNF | primary non-function |
| ROS | reactive oxygen species |
| SCS | static cold storage |
| TNF-α | tumour necrosis factor α |
| UK | United Kingdom |
| US | United States |
| WIT | warm ischaemic time |
References
- NHSBT. Fact Sheet 7: Cost-Effectiveness of Transplantation. 2009. Available online: https://nhsbtmediaservices.blob.core.windows.net/organ-donation-assets/pdfs/Organ_Donation_Registry_Fact_Sheet_7_21337.pdf (accessed on 26 September 2021).
- Kaballo, M.A.; Canney, M.; O’Kelly, P.; Williams, Y.; O’Seaghdha, C.M.; Conlon, P.J. A comparative analysis of survival of patients on dialysis and after kidney transplantation. Clin. Kidney J. 2018, 11, 389–393. [Google Scholar] [CrossRef]
- Santos, A.H., Jr.; Casey, M.J.; Wen, X.; Zendejas, I.; Rehman, S.; Womer, K.L.; Andreoni, K.A. Survival with Dialysis Versus Kidney Transplantation in Adult Hemolytic Uremic Syndrome Patients. Transplantation 2015, 99, 2608–2616. [Google Scholar] [CrossRef]
- Wolfe, R.A.; Ashby, V.B.; Milford, E.L.; Ojo, A.O.; Ettenger, R.E.; Agodoa, L.Y.; Held, P.J.; Port, F.K. Comparison of Mortality in All Patients on Dialysis, Patients on Dialysis Awaiting Transplantation, and Recipients of a First Cadaveric Transplant. N. Engl. J. Med. 1999, 341, 1725–1730. [Google Scholar] [CrossRef] [Green Version]
- Yoo, K.D.; Kim, C.T.; Kim, M.H.; Noh, J.; Kim, G.; Kim, H.; An, J.N.; Park, J.Y.; Cho, H.; Kim, K.H.; et al. Superior outcomes of kidney transplantation compared with dialysis: An optimal matched analysis of a national population-based cohort study between 2005 and 2008 in Korea. Medicine 2016, 95, e4352. [Google Scholar] [CrossRef]
- Caskey, F.; Dawnay, C.C.; Farrington, A.; Fogarty, K.; Kumwenda, F.S.; Macphee, M.; Md, S.; Steenkamp, R.; Aj, W. UK Renal Registry UK Renal Registry 18th Annual Report of the Renal Association. Nephron 2016, 132, 9–40. [Google Scholar]
- Zhong, Z.; Hu, Q.; Fu, Z.; Wang, R.; Xiong, Y.; Zhang, Y.; Liu, Z.; Wang, Y.; Ye, Q. Increased Expression of Aldehyde Dehydrogenase 2 Reduces Renal Cell Apoptosis During Ischaemia/Reperfusion Injury After Hypothermic Machine Perfusion. Artif. Organs 2016, 40, 596–603. [Google Scholar] [CrossRef]
- Yang, Z.; Zhong, Z.; Li, M.; Xiong, Y.; Wang, Y.; Peng, G.; Ye, Q. Hypothermic machine perfusion increases A20 expression which protects renal cells against Ischaemia/reperfusion injury by suppressing inflammation, apoptosis and necroptosis. Int. J. Mol. Med. 2016, 38, 161–171. [Google Scholar] [CrossRef] [Green Version]
- Chatauret, N.; Coudroy, R.; Delpech, P.O.; Vandebrouck, C.; Hosni, S.; Scepi, M.; Hauet, T. Mechanistic analysis of nonoxygenated hypothermic machine perfusion’s protection on warm ischemic kidney uncovers greater enos phosphorylation and vasodilation. Am. J. Transpl. 2014, 14, 2500–2514. [Google Scholar] [CrossRef] [PubMed]
- Moers, C.; Smits, J.M.; Maathuis, M.H.J.; Treckmann, J.; van Gelder, F.; Napieralski, B.P.; van Kasterop-Kutz, M.; van der Heide, H.; Squifflet, J.-P.; van Heurn, E.; et al. Machine perfusion or cold storage in deceased-donor kidney transplantation. N. Engl. J. Med. 2009, 360, 7–19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jochmans, I.; Moers, C.; Smits, J.M.; Leuvenink, H.G.; Treckmann, J.; Paul, A.; Rahmel, A.; Squifflet, J.-P.; van Heurn, E.; Monbaliu, D.; et al. Machine Perfusion Versus Cold Storage for the Preservation of Kidneys Donated After Cardiac Death. Ann. Surg. 2010, 252, 756–764. [Google Scholar] [CrossRef] [PubMed]
- Moers, C.; Pirenne, J.; Paul, A.; Ploeg, R.J. Machine Preservation Trial Study Group. Machine perfusion or cold storage in deceased-donor kidney transplantation. N. Engl. J. Med. 2012, 366, 770–771. [Google Scholar] [CrossRef] [Green Version]
- Bradley, J.A.; Pettigrew, G.J.; Watson, C.J. Time to death after withdrawal of treatment in donation after circulatory death (DCD) donors. Curr. Opin. Organ Transpl. 2013, 18, 133–139. [Google Scholar] [CrossRef] [PubMed]
- Van Erp, A.C.; Rebolledo, R.A.; Hoeksma, D.; Jespersen, N.R.; Ottens, P.J.; Nørregaard, R.; Pedersen, M.; Laustsen, C.; Burgerhof, J.G.M.; Wolters, J.C.; et al. Organ-specific responses during brain death: Increased aerobic metabolism in the liver and anaerobic metabolism with decreased perfusion in the kidneys. Sci. Rep. 2018, 8, 4405. [Google Scholar] [CrossRef] [Green Version]
- Schiffer, T.A.; Gustafsson, H.; Palm, F. Kidney outer medulla mitochondria are more efficient compared with cortex mitochondria as a strategy to sustain ATP production in a suboptimal environment. Am. J. Physiol. Physiol. 2018, 315, F677–F681. [Google Scholar] [CrossRef] [PubMed]
- Peris, A.; Fulceri, G.E.; Lazzeri, C.; Bonizzoli, M.; Li Marzi, V.; Serni, S.; Cirami, L.; Migliaccio, M.L. Delayed graft function and perfusion parameters of kidneys from uncontrolled donors after circulatory death. Perfusion 2021, 36, 299–304. [Google Scholar] [CrossRef] [PubMed]
- Kaminski, J.; Delpech, P.O.; Kaaki-Hosni, S.; Promeyrat, X.; Hauet, T.; Hannaert, P. Oxygen Consumption by Warm Ischaemia-Injured Porcine Kidneys in Hypothermic Static and Machine Preservation. J. Surg. Res. 2019, 242, 78–86. [Google Scholar] [CrossRef]
- Belzer, F.O.; Southard, J.H. Principles of Solid-Organ Preservation by Cold Storage. Transplantation 1988, 45, 673–676. [Google Scholar] [CrossRef]
- Hochachka, P. Defense strategies against hypoxia and hypothermia. Science 1986, 231, 234–241. [Google Scholar] [CrossRef]
- Bienholz, A.; Walter, B.; Pless-Petig, G.; Guberina, H.; Kribben, A.; Witzke, O.; Rauen, U. Characterization of injury in isolated rat proximal tubules during cold incubation and rewarming. PLoS ONE 2017, 12, e0180553. [Google Scholar] [CrossRef] [Green Version]
- Kellerman, P.S. Exogenous adenosine triphosphate (ATP) preserves proximal tubule microfilament structure and function in vivo in a maleic acid model of ATP depletion. J. Clin. Investig. 1993, 92, 1940–1949. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lieberthal, W.; Menza, S.A.; Levine, J.S. Graded ATP depletion can cause necrosis or apoptosis of cultured mouse proximal tubular cells. Am. J. Physiol. Physiol. 1998, 274, F315–F327. [Google Scholar] [CrossRef] [PubMed]
- Pefanis, A.; Ierino, F.L.; Murphy, J.M.; Cowan, P.J. Regulated necrosis in kidney Ischaemia-reperfusion injury. Kidney Int. 2019, 96, 291–301. [Google Scholar] [CrossRef]
- Kox, J.; Moers, C.; Monbaliu, D.; Strelniece, A.; Treckmann, J.; Jochmans, I.; Leuvenink, H.; Van Heurn, E.; Pirenne, J.; Paul, A.; et al. The Benefits of Hypothermic Machine Preservation and Short Cold Ischaemia Times in Deceased Donor Kidneys. Transplantation 2018, 102, 1344–1350. [Google Scholar] [CrossRef]
- Hendriks, K.D.W.; Brüggenwirth, I.M.A.; Maassen, H.; Gerding, A.; Bakker, B.; Porte, R.J.; Henning, R.H.; Leuvenink, H.G.D. Renal temperature reduction progressively favors mitochondrial ROS production over respiration in hypothermic kidney preservation. J. Transl. Med. 2019, 17, 1–10. [Google Scholar] [CrossRef]
- Stubenitsky, B.M.; Ametani, M.; Danielewicz, R.; Southard, J.H.; Belzer, F.O. Regeneration of ATP in kidney slices after warm Ischaemia and hypothermic preservation. Transpl. Int. 1995, 8, 293–297. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Shi, J.; Xia, T.C.; Xu, R.; He, X.; Xia, Y. Preservation Solutions for Kidney Transplantation: History, Advances and Mechanisms. Cell Transpl. 2019, 28, 1472. [Google Scholar] [CrossRef] [PubMed]
- Hosgood, S.A.; Saeb-Parsy, K.; Wilson, C.; Callaghan, C.; Collett, D.; Nicholson, M.L. Protocol of a randomised controlled, open-label trial of ex vivo normothermic perfusion versus static cold storage in donation after circulatory death renal transplantation. BMJ Open 2017, 7, e012237. [Google Scholar] [CrossRef] [Green Version]
- Minor, T.; Horn, C.; Gallinat, A.; Kaths, M.; Kribben, A.; Treckmann, J.; Paul, A. First-in-man controlled rewarming and normothermic perfusion with cell-free solution of a kidney prior to transplantation. Am. J. Transpl. 2020, 20, 1192–1195. [Google Scholar] [CrossRef] [Green Version]
- Schlegel, A.; Muller, X.; Mueller, M.; Stepanova, A.; Kron, P.; de Rougemont, O.; Muiesan, P.; Clavien, P.A.; Galkin, A.; Meierhofer, D.; et al. Hypothermic oxygenated perfusion protects from mitochondrial injury before liver transplantation. EBioMedicine 2020, 60, 103014. [Google Scholar] [CrossRef]
- Nath, J.; Smith, T.B.; Patel, K.; Ebbs, S.R.; Hollis, A.; Tennant, D.A.; Ludwig, C.; Ready, A.R. Metabolic differences between cold stored and machine perfused porcine kidneys: A 1H NMR based study. Cryobiology 2017, 74, 115–120. [Google Scholar] [CrossRef] [PubMed]
- Res, E.S.; Houtzager, J.H.E.; David Hemelrijk, S.; Mirza, I.C.J.H.; Idu, M.; Bemelman, F.J.; Van Gulik, T.M.; Helene, J.; Surgery, E.H. The Use of the Oxygenated AirdriveTM Machine Perfusion System in Kidney Graft Preservation: A Clinical Pilot Study. Eur. Surg. Res. 2020, 61, 153–162. [Google Scholar] [CrossRef]
- Ravaioli, M.; Baldassarre, M.; Vasuri, F.; Pasquinelli, G.; Laggetta, M.; Valente, S.; De Pace, V.; Neri, F.; Siniscalchi, A.; Zanfi, C.; et al. Strategies to Restore Adenosine Triphosphate (ATP) Level After More than 20 Hours of Cold Ischaemia Time in Human Marginal Kidney Grafts. Ann. Transpl. 2018, 23, 34–44. [Google Scholar] [CrossRef] [PubMed]
- Ravaioli, M.; De Pace, V.; Angeletti, A.; Comai, G.; Vasuri, F.; Baldassarre, M.; Maroni, L.; Odaldi, F.; Fallani, G.; Caraceni, P.; et al. Hypothermic Oxygenated New Machine Perfusion System in Liver and Kidney Transplantation of Extended Criteria Donors:First Italian Clinical Trial. Sci. Rep. 2020, 10, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Patel, K.; Nath, J.; Hodson, J.; Inston, N.; Ready, A. Outcomes of Donation after Circulatory Death kidneys undergoing Hypothermic Machine Perfusion following Static Cold Storage: A UK population-based cohort study. Am. J. Transpl. 2018, 18, 1408–1414. [Google Scholar] [CrossRef] [Green Version]
- Bellini, M.I.; Charalampidis, S.; Herbert, P.E.; Bonatsos, V.; Crane, J.; Muthusamy, A.; Dor, F.J.M.F.; Papalois, V. Cold pulsatile machine perfusion versus static cold storage in kidney transplantation: A single centre experience. BioMed Res. Int. 2019, 2019, 7435248. [Google Scholar] [CrossRef] [Green Version]
- Savoye, E.; Macher, M.A.; Videcoq, M.; Gatault, P.; Hazzan, M.; Abboud, I.; Thierry, A.; Bertrand, D.; Drouin, S.; Sayegh, J.; et al. Evaluation of outcomes in renal transplantation with hypothermic machine perfusion for the preservation of kidneys from expanded criteria donors. Clin. Transpl. 2019, 33, e13536. [Google Scholar] [CrossRef] [PubMed]
- Kruszyna, T.; Richter, P. Hypothermic Machine Perfusion of Kidneys Compensates for Extended Storage Time: A Single Intervention With a Significant Impact. Transpl. Proc. 2021, 53, 1085–1090. [Google Scholar] [CrossRef]
- Tedesco Silva, H.; Evans, R.W.; Gavaghan, M.B.; Vazquez, V.C. A Cost-Effectiveness Analysis of Organ Preservation Methods for Deceased Donor Kidneys at High Risk for Delayed Graft Function in Brazil. Transpl. Proc. 2018, 50, 3121–3127. [Google Scholar] [CrossRef]
- Martínez Arcos, L.; Fabuel Alcañiz, J.J.; Gómez Dos Santos, V.; Burgos Revilla, F.J. Functional Results of Renal Preservation in Hypothermic Pulsatile Machine Perfusion Versus Cold Preservation: Systematic Review and Meta-Analysis of Clinical Trials. Transpl. Proc. 2018, 50, 24–32. [Google Scholar] [CrossRef]
- Ruiz-Hernández, M.; Gómez-Dos Santos, V.; Díaz-Pérez, D.; Fernández-Alcalde, Á.; Hevia-Palacios, V.; Álvarez-Rodríguez, S.; Díez-Nicolás, V.; Elías-Triviño, S.; Burgos-Revilla, F.J. Experience with Hypothermic Machine Perfusion in Expanded Criteria Donors: Functional Outcomes. Transpl. Proc. 2019, 51, 303–306. [Google Scholar] [CrossRef] [PubMed]
- Sandal, S.; Luo, X.; Massie, A.B.; Paraskevas, S.; Cantarovich, M.; Segev, D.L. Machine perfusion and long-term kidney transplant recipient outcomes across allograft risk strata. Nephrol. Dial. Transpl. 2018, 33, 1251–1259. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rijkse, E.; IJzermans, J.N.; Minnee, R.C. Machine perfusion in abdominal organ transplantation: Current use in the Netherlands. World J. Transpl. 2020, 10, 15–28. [Google Scholar] [CrossRef]
- Wijk, J.; Slooff, M.J.; Rijkmans, B.G.; Kootstra, G. Successful 96- and 144-hour experimental kidney preservation: A combination of standard machine preservation and newly developed normothermic ex vivo perfusion. Cryobiology 1980, 17, 473–477. [Google Scholar] [CrossRef]
- Hosgood, S.A.; Nicholson, M.L. First in man renal transplantation after ex vivo normothermic perfusion. Transplantation 2011, 92, 735–738. [Google Scholar] [CrossRef] [PubMed]
- Hosgood, S.A.; Thompson, E.; Moore, T.; Wilson, C.H.; Nicholson, M.L. Normothermic machine perfusion for the assessment and transplantation of declined human kidneys from donation after circulatory death donors. Br. J. Surg. 2018, 105, 388–394. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hosgood, S.A.; Saeb-Parsy, K.; Hamed, M.O.; Nicholson, M.L. Successful Transplantation of Human Kidneys Deemed Untransplantable but Resuscitated by Ex Vivo Normothermic Machine Perfusion. Am. J. Transpl. 2016, 16, 3282. [Google Scholar] [CrossRef] [Green Version]
- Schopp, I.; Reissberg, E.; Lüer, B.; Efferz, P.; Minor, T. Controlled Rewarming after Hypothermia: Adding a New Principle to Renal Preservation. Clin. Transl. Sci. 2015, 8, 475. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- von Horn, C.; Zlatev, H.; Kaths, M.; Andreas, P.; Minor, T. Controlled oxygenated rewarming compensates for cold storage-induced dysfunction in kidney grafts. Transplantation 2021. published online ahead of print. [Google Scholar] [CrossRef]
- Elliott, T.R.; Nicholson, M.L.; Hosgood, S.A. Normothermic kidney perfusion: An overview of protocols and strategies. Am. J. Transpl. 2021, 21, 1382–1390. [Google Scholar] [CrossRef]
- Ferdinand, J.R.; Hosgood, S.A.; Moore, T.; Ferro, A.; Ward, C.J.; Castro-Dopico, T.; Nicholson, M.L.; Clatworthy, M.R. Cytokine absorption during human kidney perfusion reduces delayed graft function–associated inflammatory gene signature. Am. J. Transpl. 2021, 21, 2188–2199. [Google Scholar] [CrossRef]
- Hameed, A.M.; Miraziz, R.; Lu, D.B.; Warwick, N.; El-Ayoubi, A.; Burns, H.; Chew, Y.V.; Matthews, R.; O’Grady, G.; Yuen, L.; et al. Extra-corporeal normothermic machine perfusion of the porcine kidney: Working towards future utilization in Australasia. ANZ J. Surg. 2018, 88, E429–E434. [Google Scholar] [CrossRef] [PubMed]
- Bellini, M.I.; Yiu, J.; Nozdrin, M.; Papalois, V. The Effect of Preservation Temperature on Liver, Kidney, and Pancreas Tissue ATP in Animal and Preclinical Human Models. J. Clin. Med. 2019, 8, 1421. [Google Scholar] [CrossRef] [Green Version]
- Tejchman, K.; Sierocka, A.; Kotowski, M.; Zair, L.; Pilichowska, E.; Ostrowski, M.; Sieńko, J. Acid-Base Balance Disorders During Kidney Preservation in Cold Ischemia. Transpl. Proc. 2020, 52, 2036–2042. [Google Scholar] [CrossRef] [PubMed]
- Bunegin, L.; Tolstykh, G.P.; Gelineau, J.F.; Cosimi, A.B.; Anderson, L.M. Oxygen Consumption During Oxygenated Hypothermic Perfusion as a Measure of Donor Organ Viability. ASAIO J. 2013, 59, 427–432. [Google Scholar] [CrossRef] [PubMed]
- Hoyer, D.P.; Gallinat, A.; Swoboda, S.; Wohlschlaeger, J.; Rauen, U.; Paul, A.; Minor, T. Influence of oxygen concentration during hypothermic machine perfusion on porcine kidneys from donation after circulatory death. Transplantation 2014, 98, 944–950. [Google Scholar] [CrossRef]
- Jochmans, I.; Brat, A.; Davies, L.; Hofker, H.S.; van de Leemkolk, F.E.M.; Leuvenink, H.G.D.; Knight, S.R.; Pirenne, J.; Ploeg, R.J. Oxygenated versus standard cold perfusion preservation in kidney transplantation (COMPARE): A randomised, double-blind, paired, phase 3 trial. Lancet 2020, 396, 1653–1662. [Google Scholar] [CrossRef]
- Patel, K.; Smith, T.B.; Neil, D.A.H.; Thakker, A.; Tsuchiya, Y.; Higgs, E.B.; Hodges, N.J.; Ready, A.R.; Nath, J.; Ludwig, C. The Effects of Oxygenation on Ex Vivo Kidneys Undergoing Hypothermic Machine Perfusion. Transplantation 2019, 103, 314–322. [Google Scholar] [CrossRef]
- Venema, L.H.; Brat, A.; Moers, C.; Hart, N.A.; Ploeg, R.J.; Hannaert, P.; Minor, T.; Leuvenink, A.H.G.D. Effects of Oxygen During Long-term Hypothermic Machine Perfusion in a Porcine Model of Kidney Donation After Circulatory Death. Transplantation 2019, 103, 2057–2064. [Google Scholar] [CrossRef] [Green Version]
- Fuller, B.J.; Lee, C.Y. Hypothermic perfusion preservation: The future of organ preservation revisited? Cryobiology 2007, 54, 129–145. [Google Scholar] [CrossRef] [PubMed]
- Darius, T.; Gianello, P.; Vergauwen, M.; Mourad, N.; Buemi, A.; De Meyer, M.; Mourad, M. The effect on early renal function of various dynamic preservation strategies in a preclinical pig Ischaemia–reperfusion autotransplant model. Am. J. Transpl. 2019, 19, 752–762. [Google Scholar] [CrossRef]
- Darius, T.; Vergauwen, M.; Smith, T.B.; Patel, K.; Craps, J.; Joris, V.; Aydin, S.; Ury, B.; Buemi, A.; De Meyer, M.; et al. Influence of Different Partial Pressures of Oxygen during Continuous Hypothermic Machine Perfusion in a Pig Kidney Ischaemia-reperfusion Autotransplant Model. Transplantation 2020, 104, 731–743. [Google Scholar] [CrossRef] [PubMed]
- Darius, T.; Vergauwen, M.; Smith, T.; Gerin, I.; Joris, V.; Mueller, M.; Aydin, S.; Muller, X.; Schlegel, A.; Nath, J.; et al. Brief O2 uploading during continuous hypothermic machine perfusion is simple yet effective oxygenation method to improve initial kidney function in a porcine autotransplant model. Am. J. Transpl. 2020, 20, 2030–2043. [Google Scholar] [CrossRef]
- Darius, T.; Vergauwen, M.; Mueller, M.; Aydin, S.; Dutkowski, P.; Gianello, P.; Mourad, M. Brief Bubble and Intermittent Surface Oxygenation Is a Simple and Effective Alternative for Membrane Oxygenation during Hypothermic Machine Perfusion in Kidneys. Transpl. Direct 2020, 6, e571. [Google Scholar] [CrossRef]
- Meister, F.A.; Czigany, Z.; Rietzler, K.; Miller, H.; Reichelt, S.; Liu, W.J.; Boecker, J.; Moeller, M.J.; Tolba, R.H.; Hamesch, K.; et al. Decrease of renal resistance during hypothermic oxygenated machine perfusion is associated with early allograft function in extended criteria donation kidney transplantation. Sci. Rep. 2020, 10, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Meister, F.A.; Czigany, Z.; Bednarsch, J.; Boecker, J.; Wiltberger, G.; Rohlfs, W.; Neumann, U.P.; Lurje, G. Hypothermic oxygenated machine perfusion—Preliminary experience with end-ischemic reconditioning of marginal kidney allografts. Clin. Transpl. 2019, 33, e13673. [Google Scholar] [CrossRef] [PubMed]
- Kasil, A.; Giraud, S.; Couturier, P.; Amiri, A.; Danion, J.; Donatini, G.; Matillon, X.; Hauet, T.; Badet, L. Individual and Combined Impact of Oxygen and Oxygen Transporter Supplementation during Kidney Machine Preservation in a Porcine Preclinical Kidney Transplantation Model. Int. J. Mol. Sci. 2019, 20, 1992. [Google Scholar] [CrossRef] [Green Version]
- Le Meur, Y.; Badet, L.; Essig, M.; Thierry, A.; Büchler, M.; Drouin, S.; Deruelle, C.; Morelon, E.; Pesteil, F.; Delpech, P.; et al. First-in-human use of a marine oxygen carrier (M101) for organ preservation: A safety and proof-of-principle study. Am. J. Transpl. 2020, 20, 1729–1738. [Google Scholar] [CrossRef]
- Soussi, D.; Danion, J.; Baulier, E.; Favreau, F.; Sauvageon, Y.; Bossard, V.; Matillon, X.; Turpin, F.; Belgsir, E.M.; Thuillier, R.; et al. Vectisol formulation enhances solubility of resveratrol and brings its benefits to kidney transplantation in a preclinical porcine model. Int. J. Mol. Sci. 2019, 20, 2268. [Google Scholar] [CrossRef] [Green Version]
- Sedigh, A.; Larsson, R.; Brännström, J.; Magnusson, P.; Larsson, E.; Tufveson, G.; Lorant, T. Modifying the vessel walls in porcine kidneys during machine perfusion. J. Surg. Res. 2014, 191, 455–462. [Google Scholar] [CrossRef] [PubMed]
- Sedigh, A.; Nordling, S.; Carlsson, F.; Larsson, E.; Norlin, B.; Lübenow, N.; Lennmyr, F.; Tufveson, G.; Magnusson, P.U.; Lorant, T. Perfusion of Porcine Kidneys with Macromolecular Heparin Reduces Early Ischaemia Reperfusion Injury. Transplantation 2019, 103, 420–427. [Google Scholar] [CrossRef]
- Tierie, E.L.; Roodnat, J.I.; Dor, F.J.M.F. Systematic Surgical Assessment of Deceased-Donor Kidneys as a Predictor of Short-Term Transplant Outcomes. Eur. Surg. Res. 2019, 60, 97–105. [Google Scholar] [CrossRef] [PubMed]
- Bissolati, M.; Gazzetta, P.G.; Caldara, R.; Guarneri, G.; Adamenko, O.; Giannone, F.; Mazza, M.; Maggi, G.; Tomanin, D.; Rosati, R.; et al. Renal Resistance Trend During Hypothermic Machine Perfusion Is More Predictive of Postoperative Outcome Than Biopsy Score: Preliminary Experience in 35 Consecutive Kidney Transplantations. Artif. Organs 2018, 42, 714–722. [Google Scholar] [CrossRef]
- Chen, G.; Wang, C.; Zhao, Y.; Qiu, L.; Yuan, X.; Qiu, J.; Wang, C.; He, X.; Chen, L. Evaluation of quality of kidneys from donation after circulatory death/expanded criteria donors by parameters of machine perfusion. Nephrology 2018, 23, 103–106. [Google Scholar] [CrossRef]
- Tai, Q.; Xue, W.; Ding, X.; Tian, P.; Xiang, H.; Feng, X.; Yan, H.; Hou, J. Perfusion Parameters of Donation After Cardiac Death Kidneys Predict Early Transplant Outcomes Based on Expanded Criteria Donor Designation. Transpl. Proc. 2018, 50, 79–84. [Google Scholar] [CrossRef]
- Sandal, S.; Paraskevas, S.; Cantarovich, M.; Baran, D.; Chaudhury, P.; Tchervenkov, J.I.; Sapir-Pichhadze, R. Renal resistance thresholds during hypothermic machine perfusion and transplantation outcomes—A retrospective cohort study. Transpl. Int. 2018, 31, 658–669. [Google Scholar] [CrossRef] [Green Version]
- Wszola, M.; Domagala, P.; Serwanska-Swietek, M.; Ostaszewska, A.; Perkowska-Ptasinska, A.; Piatek, T.; Gozdowska, J.; Durlik, M.; Chmura, A.; Kwiatkowski, A. Should Immunosuppression After Kidney Transplant Be Adjusted Based on Renal Resistance during Pretransplant Hypothermic Machine Perfusion? Transpl. Proc. 2019, 51, 2676–2682. [Google Scholar] [CrossRef]
- Jochmans, I.; Moers, C.; Smits, J.M.; Leuvenink, H.D.; Treckman, J.; Paul, A.; Rahmel, A.; Squifflet, J.P.; Heurn, E.; Monbaliu, D.; et al. The prognostic value of renal resistance during hypothermic machine perfusion of deceased donor kidneys. Am. J. Transpl. 2011, 11, 2214–2220. [Google Scholar] [CrossRef]
- Longchamp, A.; Klauser, A.; Songeon, J.; Agius, T.; Nastasi, A.; Ruttiman, R.; Moll, S.; Meier, R.P.H.; Buhler, L.; Corpataux, J.M.; et al. Ex Vivo Analysis of Kidney Graft Viability Using 31P Magnetic Resonance Imaging Spectroscopy. Transplantation 2020, 104, 1825–1831. [Google Scholar] [CrossRef] [PubMed]
- Konkel, B.; Lavin, C.; Wu, T.T.; Anderson, E.; Iwamoto, A.; Rashid, H.; Gaitian, B.; Boone, J.; Cooper, M.; Abrams, P.; et al. Fully automated analysis of OCT imaging of human kidneys for prediction of post-transplant function. Biomed. Opt. Express 2019, 10, 1794. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bon, D.; Claire, B.; Thuillier, R.; Hebrard, W.; Boildieu, N.; Celhay, O.; Irani, J.; Seguin, F.; Hauet, T. Analysis of Perfusates during Hypothermic Machine Perfusion by NMR Spectroscopy. Transplantation 2014, 97, 810–816. [Google Scholar] [CrossRef]
- Gowers, S.A.N.; Hamaoui, K.; Vallant, N.; Hanna, G.B.; Darzi, A.; Casanova, D.; Papalois, V.; Boutelle, M.G. An improved rapid sampling microdialysis system for human and porcine organ monitoring in a hospital setting. Anal. Methods 2018, 10, 5273–5281. [Google Scholar] [CrossRef] [Green Version]
- Patel, K.; Smith, T.; Thakker, A.; Inston, N.; Ready, A.; Ludwig, C.; Nath, J. Abstracts of the 19th Congress of the European Society for Organ Transplantation, 15–18 September 2019, Copenhagen, Denmark. Transpl. Int. 2019, 32, 5–433. [Google Scholar]
- Moers, C.; Varnav, O.C.; Van Heurn, E.; Jochmans, I.; Kirste, G.R.; Rahmel, A.; Leuvenink, H.G.D.; Squifflet, J.P.; Paul, A.; Pirenne, J.; et al. The value of machine perfusion perfusate biomarkers for predicting kidney transplant outcome. Transplantation 2010, 90, 966–973. [Google Scholar] [CrossRef] [Green Version]
- Parikh, C.R.; Hall, I.E.; Bhangoo, R.S.; Ficek, J.; Abt, P.L.; Thissen-Philbrook, H.; Lin, H.; Bimali, M.; Murray, P.T.; Rao, V.; et al. Associations of Perfusate Biomarkers and Pump Parameters with Delayed Graft Function and Deceased Donor Kidney Allograft Function. Am. J. Transpl. 2016, 16, 1526–1539. [Google Scholar] [CrossRef] [PubMed]
- Guzzi, F.; Knight, S.R.; Ploeg, R.J.; Hunter, J.P. A systematic review to identify whether perfusate biomarkers produced during hypothermic machine perfusion can predict graft outcomes in kidney transplantation. Transpl. Int. 2020, 33, 590–602. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Chen, G.; Zhu, Z.; Zhang, Z.; Yuan, X.; Han, M.; Zhao, Q.; Zheng, Y.; Tang, Y.; Huang, S.; et al. The First Case of Ischaemia-Free Kidney Transplantation in Humans. Front. Med. 2019, 6, 276. [Google Scholar] [CrossRef] [Green Version]
- Antoine, C.; Savoye, E.; Gaudez, F.; Cheisson, G.; Badet, L.; Videcoq, M.; Legeai, C.; Bastien, O.; Barrou, B. Kidney transplant from uncontrolled donation after circulatory death: Contribution of normothermic regional perfusion. Transplantation 2019, 104, 130–136. [Google Scholar] [CrossRef] [PubMed]
- Kerforne, T.; Allain, G.; Giraud, S.; Bon, D.; Ameteau, V.; Couturier, P.; Hebrard, W.; Danion, J.; Goujon, J.M.; Thuillier, R.; et al. Defining the optimal duration for normothermic regional perfusion in the kidney donor: A porcine preclinical study. Am. J. Transpl. 2019, 19, 737–751. [Google Scholar] [CrossRef] [PubMed]
- Aburawi, M.M.; Fontan, F.M.; Karimian, N.; Eymard, C.; Cronin, S.; Pendexter, C.; Nagpal, S.; Banik, P.; Ozer, S.; Mahboub, P.; et al. Synthetic hemoglobin-based oxygen carriers are an acceptable alternative for packed red blood cells in normothermic kidney perfusion. Am. J. Transpl. 2019, 19, 2814–2824. [Google Scholar] [CrossRef]
- Minor, T.; von Horn, C.; Paul, A. Role of erythrocytes in short-term rewarming kidney perfusion after cold storage. Artif. Organs 2019, 43, 584–592. [Google Scholar] [CrossRef] [PubMed]
- Maassen, H.; Hendriks, K.D.W.; Venema, L.H.; Henning, R.H.; Hofker, S.H.; van Goor, H.; Leuvenink, H.G.D.; Coester, A.M. Hydrogen sulphide-induced hypometabolism in human-sized porcine kidneys. PLoS ONE 2019, 14, e0225152. [Google Scholar] [CrossRef]
- Lohmann, S.; Pool, M.; Rozenberg, K.; Keller, A.; Moers, C.; Møldrup, U.; Møller, B.; Lignell, S.; Krag, S.; Sierra-Parraga, J.; et al. Mesenchymal stromal cell treatment of donor kidneys during ex-vivo normothermic machine perfusion: A porcine renal autotransplantation study. Am. J. Transpl. 2021, 21, 2348–2359. [Google Scholar] [CrossRef]
- Pool, M.B.F.; Vos, J.; Eijken, M.; Van Pel, M.; Reinders, M.E.J.; Ploeg, R.J.; Hoogduijn, M.J.; Jespersen, B.; Leuvenink, H.G.D.; Moers, C. Treating Ischemically Damaged Porcine Kidneys with Human Bone Marrow—And Adipose Tissue-Derived Mesenchymal Stromal Cells during Ex Vivo Normothermic Machine Perfusion. Stem Cells Dev. 2020, 29, 1320–1330. [Google Scholar] [CrossRef]
- Thompson, E.R.; Bates, L.; Ibrahim, I.K.; Sewpaul, A.; Stenberg, B.; McNeill, A.; Figueiredo, R.; Girdlestone, T.; Wilkins, G.C.; Wang, L.; et al. Novel delivery of cellular therapy to reduce Ischaemia reperfusion injury in kidney transplantation. Am. J. Transpl. 2021, 21, 1402–1414. [Google Scholar] [CrossRef]
- Brasile, L.; Henry, N.; Orlando, G.; Stubenitsky, B. Potentiating Renal Regeneration using Mesenchymal Stem Cells. Transplantation 2019, 103, 307. [Google Scholar] [CrossRef] [PubMed]
- Hameed, A.M.; Lu, D.B.; Burns, H.; Byrne, N.; Chew, Y.V.; Julovi, S.; Ghimire, K.; Zanjani, N.T.; P’ng, C.H.; Meijles, D.; et al. Pharmacologic targeting of renal Ischaemia-reperfusion injury using a normothermic machine perfusion platform. Sci. Rep. 2020, 10, 1–17. [Google Scholar] [CrossRef]
- DiRito, J.R.; Hosgood, S.A.; Reschke, M.; Albert, C.; Bracaglia, L.G.; Ferdinand, J.R.; Stewart, B.J.; Edwards, C.M.; Vaish, A.G.; Thiru, S.; et al. Lysis of cold-storage-induced microvascular obstructions for ex vivo revitalization of marginal human kidneys. Am. J. Transpl. 2021, 21, 161–173. [Google Scholar] [CrossRef] [PubMed]
- Yuzefovych, Y.; Valdivia, E.; Rong, S.; Hack, F.; Rother, T.; Schmitz, J.; Bräsen, J.H.; Wedekind, D.; Moers, C.; Wenzel, N.; et al. Genetic Engineering of the Kidney to Permanently Silence MHC Transcripts During ex vivo Organ Perfusion. Front. Immunol. 2020, 11, 265. [Google Scholar] [CrossRef] [Green Version]
- Rijkse, E.; De Jonge, J.; Kimenai, H.J.A.N.; Hoogduijn, M.J.; De Bruin, R.W.F.; Van Den Hoogen, M.W.F.; Ijzermans, J.N.M.; Minnee, R.C. Safety and feasibility of 2 h of normothermic machine perfusion of donor kidneys in the Eurotransplant Senior Program. BJS Open 2021, 5, zraa024. [Google Scholar] [CrossRef] [PubMed]
- Weissenbacher, A.; Lo Faro, L.; Boubriak, O.; Soares, M.F.; Roberts, I.S.; Hunter, J.P.; Voyce, D.; Mikov, N.; Cook, A.; Ploeg, R.J.; et al. Twenty-four–hour normothermic perfusion of discarded human kidneys with urine recirculation. Am. J. Transpl. 2019, 19, 178–192. [Google Scholar] [CrossRef]
- Hosgood, S.A.; Barlow, A.D.; Hunter, J.P.; Nicholson, M.L. Ex vivo normothermic perfusion for quality assessment of marginal donor kidney transplants. Br. J. Surg. 2015, 102, 1433–1440. [Google Scholar] [CrossRef] [PubMed]
- Yu, B.; Liang, H.; Zhou, S.; Ye, Q.; Wang, Y. A novel genomic model for predicting the likelihood of delayed graft function in DCD kidney transplantation. Transl. Androl. Urol. 2021, 10, 1637–1646. [Google Scholar] [CrossRef] [PubMed]
- Gelpi, R.; Paredes, D.; Rodríguez-Villar, C.; Roque, R.; Ruiz, A.; Adalia, R.; Peri-Cusí, L.; Sole, M.; Oppenheimer, F.; Diekmann, F. The development of a predictive model of graft function in uncontrolled donors after circulatory death: Validity of a pulsatile renal preservation machine cut-off value for kidney acceptance. Nephrol. Dial. Transpl. 2019, 34, 531–538. [Google Scholar] [CrossRef]
- Hosgood, S.A.; Nicholson, M.L. A Short Period of Normothermic Machine Perfusion May Not Be Able to Predict Primary Nonfunction in Uncontrolled Circulatory Death Kidneys. Transplantation 2021, 105, E11–E12. [Google Scholar] [CrossRef] [PubMed]
| SCS Fluids | HMP Fluids | NMP Fluids | |||
|---|---|---|---|---|---|
| University of Wisconsin (UW) solution | Custodial-N solution | UW Machine perfusion solution (UWPS) | Hosgood protocol [28] | Minor protocol [29] | |
| Base fluid | Water | Water | Water | Ringer’s solution | Steen solution Ringer’s solution |
| Volume expanders/osmotic agents | Hydroxyethyl starch Raffinose pentahydrate | Mannitol | Hydroxyethyl starch Mannitol (USP) Magnesium gluconate Sodium gluconate | Mannitol | Calcium gluconate |
| Oxygen carriers | - | - | - | 1 unit red blood cells (group O) | - |
| Drugs | Allopurinol Magnesium sulphate heptahydrate Lactobionic acid | Deferoxamine | Dexamethasone Heparin Prostacyclin Insulin | Ampicillin | |
| Antioxidants | Glutathione | Tryptophan | Glutathione | ||
| Metabolic support | Adenosine | Potassium hydrogen 2-ketoglutarate Sucrose Aspartate Arginine Alanine Glycine | Glucose, beta D (+) Ribose | Glucose, beta D (+) Synthamin 17 Cernevit multivitamins | - |
| Individual electrolyte additives | - | Magnesium chloride Calcium chloride Potassium chloride Sodium chloride | Calcium chloride | - | - |
| Buffering agents | Potassium dihydrogen phosphate | Histidine Histidine · HCI | HEPES (free acid) Potassium phosphate (monobasic) | Sodium bicarbonate | Sodium bicarbonate |
| pH adjustment | Sodium hydroxide/hydrochloric acid Potassium hydroxide | - | Sodium hydroxide | - | - |
| NMP Clinical Trials | ||||
|---|---|---|---|---|
| NCT Number | Title | Primary Outcome Measure | Start Date | Completion Date |
| NCT05031052 | Normothermic machine perfusion (NMP) vs Static Cold Storage (SCS) in Human Kidney transplantation | Kidney function at 6 months post-transplant (eGFR) | August 2021 | December 2025 |
| NCT04882254 | Normothermic Machine Perfusion: An Additional Value for Kidney Transplant Outcomes? | Number of patients with immediate graft function within three months post-transplant | May 2021 | February 2023 |
| NCT03136848 | The Feasibility and Safety of Normothermic ex Vivo Kidney Perfusion |
| December 2016 | April 2019 |
| NCT04693325 | PROlonged Ex-vivo Normothermic Machine PERfusion for Kidney Regeneration | Glomerular filtration rate (GFR) at: 6 months post-transplantation | February 2021 | July 2022 |
| NCT02525510 | Deceased Organ Donor Interventions to Protect Kidney Graft Function | Delayed Graft Function incidence within 1 week of transplantation | August 2017 | March 2022 |
| ISRCTN15821205 | Ex Vivo Normothermic machine perfusion Trial | Delayed Graft Function incidence within 1 week of transplantation | January 2017 | - |
| HMP Clinical trials | ||||
| NCT Number | Title | Primary outcome measure | Start date | Completion Date |
| NCT04619732 | Real-time Monitoring of Kidney Grafts on Hypothermic Machine Perfusion | Post-operative recovery of kidney function within: 30 days of transplant | June 2021 | December 2021 |
| NCT03378817 | Hypothermic Oxygenated Machine Perfusion of Extended Criteria Kidney Allografts from Brain Death Donors | Delayed Graft Function incidence within 1 week of transplantation | December 2017 | March 2020 |
| NCT03031067 | Hypothermic Oxygenated Perfusion Versus Static Cold Storage for Marginal Graft | Graft function at 3 months post-transplantation | October 2016 | February 2018 |
| NCT04359173 | Propensity Score Matched Comparison of HMP vs. SCS in Kidney Transplantation | Delayed Graft Function incidence within 1 week of transplantation | August 2015 | March 2020 |
| NCT02055950 | Pulsed Perfusion for Marginal Kidneys |
| July 2013 | August 2018 |
| NCT03837197 | Clinical Trial of New Hypothermic Oxygenated Perfusion System Versus Static Cold Storage | Delayed Graft Function incidence within 0–30 days of transplantation | December 2018 | December 2021 |
| NCT02876692 | Prediction and Management of Delayed Graft Function Based on Donor Criteria and LifePort Platform |
| January 2016 | December 2019 |
| NCT02652520 | Evaluation of a Marine OXYgen Carrier: HEMO2Life for hypOthermic Kidney Graft Preservation, Before Transplantation (OXYOP) | Charting within three months of transplant:
| March 2016 | February 2018 |
| NCT03773211 | Renaparin in Kidney Transplantation | Adverse events within 30 days | February 2019 | 1 April 2020 |
| NCT03024229 | Metabolomics in Assessing the Quality of Kidney Transplants Retained on a LifePort Perfusion Machine | Immediate graft function (IGF) ( i.e. the absence of a requirement for dialysis) within 7 days post-transplant | March 2017 | January 2020 |
| NCT01848249 | Deceased Donor Biomarkers and Recipient Outcomes |
| May 2010 | March 2020 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Smith, T.B.; Nicholson, M.L.; Hosgood, S.A. Advances in Hypothermic and Normothermic Perfusion in Kidney Transplantation. Transplantology 2021, 2, 460-477. https://doi.org/10.3390/transplantology2040044
Smith TB, Nicholson ML, Hosgood SA. Advances in Hypothermic and Normothermic Perfusion in Kidney Transplantation. Transplantology. 2021; 2(4):460-477. https://doi.org/10.3390/transplantology2040044
Chicago/Turabian StyleSmith, Thomas B., Michael L. Nicholson, and Sarah A. Hosgood. 2021. "Advances in Hypothermic and Normothermic Perfusion in Kidney Transplantation" Transplantology 2, no. 4: 460-477. https://doi.org/10.3390/transplantology2040044
APA StyleSmith, T. B., Nicholson, M. L., & Hosgood, S. A. (2021). Advances in Hypothermic and Normothermic Perfusion in Kidney Transplantation. Transplantology, 2(4), 460-477. https://doi.org/10.3390/transplantology2040044





