Environmental Pollution, Endocrine Disruptors, and Metabolic Status: Impact on Female Fertility—A Narrative Review
Abstract
1. Introduction
2. Materials and Methods
3. Results
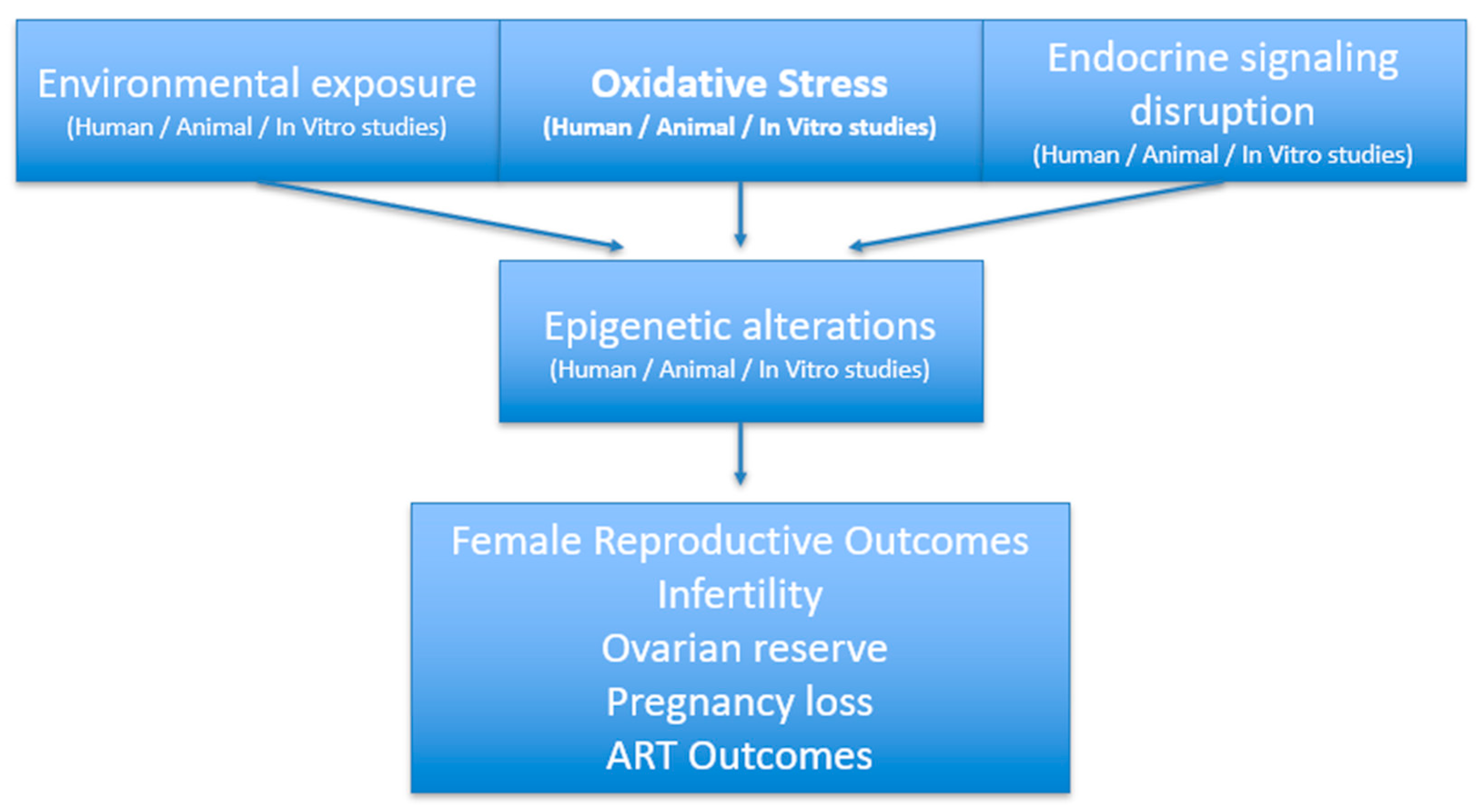
3.1. Interplay of Metabolic and Environmental Factors in Female Reproductive Health
3.1.1. Infertility in Obese Women: Pathophysiologic Mechanisms
3.1.2. Bariatric Surgery, Rapid Weight Loss and Fertility (Text About Bariatric Surgery Only to Illustrate Metabolic Status as a Potential Modifier of Environmental Exposure)
3.1.3. Environmental Factors Relevant to Fertility (Table 2)
3.1.4. Presence of Microplastics in Follicular Fluid
3.1.5. Microplastics, Endocrine Disruptors and Oocyte Quality
3.1.6. Rapid Weight Loss Improves Fertility but Introduces Oxidative and Toxicant Vulnerability
3.1.7. PFAS Compounds: Links to Reduced Fecundability and Time to Pregnancy
3.1.8. Ambient Air Pollution Impairs Ovarian Reserve and ART Outcomes
3.1.9. Synergy of Oxidative Stress and Micronutrient Deficiency
3.1.10. Postoperative PFAS Redistribution into Circulation
3.1.11. Microplastics Induce Ovarian Oxidative Injury In Vivo
| Exposure | Outcome | Direction of Effect | Design/N | Key Confounders | Certainty | |
|---|---|---|---|---|---|---|
| Obesity | Infertility, ovulatory dysfunction, ↓ ovarian reserve | Negative | Human cohorts (n > 500) | Age, PCOS, lifestyle | High | Practice Committee of the American Society for Reproductive Medicine. Electronic address: asrm@asrm.org; Practice Committee of the American Society for Reproductive Medicine. Obesity and reproduction: a committee opinion. Fertil Steril. 2021 Nov;116(5):1266–1285. doi: 10.1016/j.fertnstert.2021.08.018. Epub 2021 Sep 25. PMID: 34583840 [18]. |
| Bariatric surgery or Rapid weight loss | ↑ Ovulation, ↑ spontaneous conception, ↑ ART outcomes; transient toxicant exposure | Positive | Human clinical meta-analysis (n = 231–444) | Age, BMI, nutrition | High (fertility), Moderate (toxicant) | Choromańska B, Myśliwiec P, Łuba M, Wojskowicz P, Dadan J, Myśliwiec H, Choromańska K, Zalewska A, Maciejczyk M. A Longitudinal Study of the Antioxidant Barrier and Oxidative Stress in Morbidly Obese Patients after Bariatric Surgery. Does the Metabolic Syndrome Affect the Redox Homeostasis of Obese People? J Clin Med. 2020 Apr 1;9(4):976. doi: 10.3390/jcm9040976. PMID: 32244612; PMCID: PMC7230760 [32]. |
| PFAS (PFOA, PFOS, PFNA, PFDA) | ↓ Fecundability, ↓ live birth, impaired oocyte quality | Negative | Prospective and cross-sectional human cohorts | Age, BMI, parity, lifestyle, metabolic status | Moderate–High | Rickard BP, Rizvi I, Fenton SE. Per- and poly-fluoroalkyl substances (PFAS) and female reproductive outcomes: PFAS elimination, endocrine-mediated effects, and disease. Toxicology. 2022 Jan 15;465:153031. doi: 10.1016/j.tox.2021.153031. Epub 2021 Nov 10. PMID: 34774661; PMCID: PMC8743032 [55]. |
| Air pollutants (PM1, PM2.5, PM10, NO2, SO2, O3) | ↓ AMH, ↓ AFC, ↓ ART success | Negative | Human IVF studies (n = 194–16,290) | Age, BMI, geography | High | Huang K, Hu M, Zhang Z, Li Z, Hu C, Bai S, Li R, Wu LM, Zhang XJ, Xu B. Associations of ambient air pollutants with pregnancy outcomes in women undergoing assisted reproductive technology and the mediating role of ovarian reserve: A longitudinal study in eastern China. Sci Total Environ. 2025 Jan 1;958:177919. doi: 10.1016/j.scitotenv.2024.177919. Epub 2024 Dec 9. PMID: 39657336 [63]. |
| Endocrine-disrupting chemicals (BPA, phthalates, PFAS) | ↓ AMH, ↓ AFC, impaired oocyte quality | Negative | Human clinical cohorts | Age, BMI, lifestyle | Moderate–High | Björvang RD, Damdimopoulou P. Persistent environmental endocrine-disrupting chemicals in ovarian follicular fluid and in vitro fertilization treatment outcome in women. Ups J Med Sci. 2020 May;125(2):85–94. doi: 10.1080/03009734.2020.1727073. Epub 2020 Feb 25. PMID: 32093529; PMCID: PMC7721012 [50]. |
| Heavy metals (Cd, Pb, Hg) | ↓ Ovarian reserve, ↓ fertilization | Negative | Human case–control and cohorts | Age, BMI, occupation, smoking | Moderate | Génard-Walton M, Warembourg C, Duros S, Ropert-Bouchet M, Lefebvre T, Guivarc’h-Levêque A, Le Martelot MT, Jacquemin B, Cordier S, Costet N, Multigner L, Garlantézec R. Heavy metals and diminished ovarian reserve: single-exposure and mixture analyses amongst women consulting in French fertility centers. Reprod Biomed Online. 2023 Sep;47(3):103241. doi: 10.1016/j.rbmo.2023.05.013. Epub 2023 Jun 2. PMID: 37451971 [38]. |
| Microplastics | Detected in follicular fluid, impaired oocyte maturation | Negative | Human (n = 18), animal, in vitro | Age, BMI, exposures | Low–Moderate (human), High (mechanistic) | Jeong J, Thi Quynh Mai N, Moon BS, Choi JK. Impact of polystyrene microplastics (PS-MPs) on the entire female mouse reproductive cycle: Assessing reproductive toxicity of microplastics through in vitro follicle culture. Ecotoxicol Environ Saf. 2025 Jun 1;297:118228. doi: 10.1016/j.ecoenv.2025.118228. Epub 2025 May 1. PMID: 40315747 [70]. |
| Tobacco smoke | ↓ Ovarian reserve, ↑ miscarriage, impaired fertility | Negative | Human, in vitro studies | Age, BMI, lifestyle, comorbidities | High | Lyngsø J, Kesmodel US, Bay B, Ingerslev HJ, Pisinger CH, Ramlau-Hansen CH. Female cigarette smoking and successful fertility treatment: A Danish cohort study. Acta Obstet Gynecol Scand. 2021 Jan;100(1):58–66. doi: 10.1111/aogs.13979. Epub 2020 Sep 18. PMID: 32865819 [43]. |
| Rapid weight loss, micronutrient deficiencies | ↑ Oxidative stress in ovaries, mobilization of stored toxicants | Negative | Human clinical and prospective (n = 51–67) | Baseline BMI, micronutrients, lifestyle | Moderate | Brown RH, Ng DK, Steele K, Schweitzer M, Groopman JD. Mobilization of Environmental Toxicants Following Bariatric Surgery. Obesity (Silver Spring).27(11):1865–1873. doi: 10.1002/oby.22618. PMID: 31689012 [51]. |
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AMH | Anti-Müllerian Hormone |
| AFC | Antral Follicle Count |
| ART | Assisted Reproductive Technology |
| BPA | Bisphenol A |
| EDC | Endocrine-Disrupting Chemical |
| PFAS | Per- and Polyfluoroalkyl Substances |
| PCBs | Polychlorinated Biphenyls |
| PM | Particulate Matter |
| PM1/PM2.5/PM10 | Particulate Matter ≤ 1 μm/≤2.5 μm/≤10 μm |
| NO2 | Nitrogen Dioxide |
| O3 | Ozone |
| PFDA | Perfluorodecanoic Acid |
| PFNA | Perfluorononanoic Acid |
| PFOA | Perfluorooctanoic Acid |
| PFOS | Perfluorooctanesulfonic Acid |
| PFHxS | Perfluorohexanesulfonic Acid |
| ROS | Reactive Oxygen Species |
| GLP-1 | Glucagon-Like Peptide 1 |
| SHBG | Sex Hormone-Binding Globulin |
| GnRH | Gonadotropin-Releasing Hormone |
| FSH | Follicle-Stimulating Hormone |
| LH | Luteinizing Hormone |
| PCOS | Polycystic Ovary Syndrome |
| IVF | In Vitro Fertilization |
| AOPP | Advanced Oxidation Protein Products |
| PMC | PubMed Central |
References
- Almutairi, H.; Aldhalea, M.S.; Almaaz, M.A.; Aljuhani, S.A.; Aloraini, R.I.; Alamoudi, A.A.; Alkhalifah, W.F.; Alrushaid, L.A.; Alanzy, H.W.; Alzuwayyid, M.; et al. The Effectiveness of Bariatric Surgery on Treating Infertility in Women-A Systematic Review and Meta-Analysis. J. Clin. Med. 2024, 13, 5569. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Nilsson-Condori, E.; Mattsson, K.; Thurin-Kjellberg, A.; Hedenbro, J.L.; Friberg, B. Outcomes of in-vitro fertilization after bariatric surgery: A national register-based case-control study. Hum. Reprod. 2022, 37, 2474–2481. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Montano, L.; Raimondo, S.; Piscopo, M.; Ricciardi, M.; Guglielmino, A.; Chamayou, S.; Gentile, R.; Gentile, M.; Rapisarda, P.; Oliveri Conti, G.; et al. First evidence of microplastics in human ovarian follicular fluid: An emerging threat to female fertility. Ecotoxicol. Environ. Saf. 2025, 291, 117868. [Google Scholar] [CrossRef] [PubMed]
- Samarasinghe, S.N.S.; Leca, B.; Alabdulkader, S.; Dimitriadis, G.K.; Davasgaium, A.; Thadani, P.; Parry, K.; Luli, M.; O’Donnell, K.; Johnson, B.; et al. Bariatric surgery for spontaneous ovulation in women living with polycystic ovary syndrome: The BAMBINI multicentre, open-label, randomised controlled trial. Lancet 2024, 403, 2489–2503. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Kan, H. Very Early Pregnancy Loss: The Role of PM2.5 Exposure in IVF-ET Outcomes. Environ. Health 2024, 2, 854–855. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Li, F.; Duan, X.; Li, M.; Gao, Y.; Kang, Y.; Zheng, W.; Guo, X.; Chen, Y. Environmental pollution and human fertility: Investigating the relationship between PM2.5 exposure and assisted reproductive technology outcomes. BMC Public Health 2025, 25, 1357. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pasquali, R.; Pelusi, C.; Genghini, S.; Cacciari, M.; Gambineri, A. Obesity and reproductive disorders in women. Hum. Reprod. Update 2003, 9, 359–372. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Bao, Y.; Zhou, X.; Zheng, L. Polycystic ovary syndrome and mitochondrial dysfunction. Reprod. Biol. Endocrinol. 2019, 17, 67. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Robker, R.L. Evidence that obesity alters the quality of oocytes and embryos. Pathophysiology 2008, 15, 115–121. [Google Scholar] [CrossRef] [PubMed]
- Pourghazi, F.; Eslami, M.; Mohammadi, S.; Ghoreshi, R.; Ejtahed, H.S.; Qorbani, M. Association between childhood obesity and infertility in later life: A systematic review of cohort studies. BMC Endocr. Disord. 2023, 23, 235. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Merhi, Z.; Bazzi, A.A.; Bonney, E.A.; Buyuk, E. Role of adiponectin in ovarian follicular development and ovarian reserve. Biomed. Rep. 2019, 10, 337–342. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sasaki, H.; Hamatani, T.; Kamijo, S.; Iwai, M.; Kobanawa, M.; Ogawa, S.; Miyado, K.; Tanaka, M. Impact of Oxidative Stress on Age-Associated Decline in Oocyte Developmental Competence. Front. Endocrinol. 2019, 10, 811. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cena, H.; Chiovato, L.; Nappi, R.E. Obesity, Polycystic Ovary Syndrome, and Infertility: A New Avenue for GLP-1 Receptor Agonists. J. Clin. Endocrinol. Metab. 2020, 105, e2695–e2709. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Schneider, E.; Hamer, O.; Smith, C.; Hill, J. Weight loss interventions for improving fertility: A synthesis of current evidence. Pract. Midwife 2024, 27, 34–39. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lu, F.F.; Wang, Z.; Yang, Q.Q.; Yan, F.S.; Xu, C.; Wang, M.T.; Xu, Z.J.; Cai, S.Y.; Guan, R. Investigating the metabolomic pathways in female reproductive endocrine disorders: A Mendelian randomization study. Front. Endocrinol. 2024, 15, 1438079. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Marinelli, S.; Napoletano, G.; Straccamore, M.; Basile, G. Female obesity and infertility: Outcomes and regulatory guidance. Acta Biomed. 2022, 93, e2022278. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhang, H.; Zhang, J.; Chen, W.; Liu, H.; Chen, J.; Chen, J. Association between bedtime and female infertility: A secondary analysis from a cross-sectional study. Front. Endocrinol. 2024, 15, 1340131. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Practice Committee of the American Society for Reproductive Medicine. Electronic address: Asrm@asrm.org; Practice Committee of the American Society for Reproductive Medicine. Obesity and reproduction: A committee opinion. Fertil. Steril. 2021, 116, 1266–1285. [Google Scholar] [CrossRef] [PubMed]
- Butterworth, J.; Deguara, J.; Borg, C.M. Bariatric Surgery, Polycystic Ovary Syndrome, and Infertility. J. Obes. 2016, 2016, 1871594. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Makhsosi, B.R.; Ghobadi, P.; Otaghi, M.; Tardeh, Z. Impact of bariatric surgery on infertility in obese women: A systematic review and meta-analysis. Ann. Med. Surg. 2024, 86, 7042–7048. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Herrera-Martínez, A.D.; Junquera-Bañares, S.; Turrión-Merino, L.; Arrieta-Blanco, F.; Botella-Carretero, J.; Vázquez-Martínez, C.; Calañas-Continente, A. Case Report: Extensive Dermatitis Secondary to Severe Malnutrition, Zinc and Vitamin Deficiencies After Malabsorptive Bariatric Surgery. Front. Endocrinol. 2021, 12, 623543. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- ACOG Committee. ACOG Committee Opinion No. 804: Physical Activity and Exercise During Pregnancy and the Postpartum Period: Correction. Obstet. Gynecol. 2021, 138, 683. [Google Scholar] [CrossRef] [PubMed]
- Grzegorczyk-Martin, V.; Fréour, T.; De Bantel Finet, A.; Bonnet, E.; Merzouk, M.; Roset, J.; Roger, V.; Cédrin-Durnerin, I.; Wainer, R.; Avril, C.; et al. IVF outcomes in patients with a history of bariatric surgery: A multicenter retrospective cohort study. Hum. Reprod. 2020, 35, 2755–2762. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Alibhai, K.M.; Churchill, I.F.; Vause, T.; Lochnan, H.A. The Impact of Bariatric Surgery on Assisted Reproductive Technology Outcomes: A Systematic Review. J. Obstet. Gynaecol. Can. 2022, 44, 915–923. [Google Scholar] [CrossRef] [PubMed]
- İlyas Öner, R.; Özdaş, S.; Sarıaydın, M.; Aslan, S. The impact of bariatric surgery on obesity-related infertility. Eur. Rev. Med. Pharmacol. Sci. 2023, 27, 2865–2870. [Google Scholar] [CrossRef] [PubMed]
- Liang, M.; Wu, X.; Xie, Y.; Wang, Y.; Luo, B.; Xue, H.; Li, Z. The relationship between dietary inflammation potential, dietary oxidative balance score, and female reproductive function: A mediation analysis of obesity indicators. Front. Endocrinol. 2025, 16, 1517318. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kamal, F.A.; Fernet, L.Y.; Rodriguez, M.; Kamal, F.; Da Silva, N.K.; Kamal, O.A.; Ayala Aguilar, A.; Arruarana, V.S.; Martinez Ramirez, M. Nutritional Deficiencies Before and After Bariatric Surgery in Low- and High-Income Countries: Prevention and Treatment. Cureus 2024, 16, e55062. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mechanick, J.I.; Apovian, C.; Brethauer, S.; Garvey, W.T.; Joffe, A.M.; Kim, J.; Kushner, R.F.; Lindquist, R.; Pessah-Pollack, R.; Seger, J.; et al. Clinical practice guidelines for the perioperative nutrition, metabolic, and nonsurgical support of patients undergoing bariatric procedures—2019 update: Cosponsored by American Association of Clinical Endocrinologists/American College of Endocrinology, The Obesity Society, American Society for Metabolic & Bariatric Surgery, Obesity Medicine Association, and American Society of Anesthesiologists. Surg. Obes. Relat. Dis. 2020, 16, 175–247. [Google Scholar] [CrossRef] [PubMed]
- Pg Baharuddin, D.M.; Payus, A.O.; Abdel Malek Fahmy, E.H.; Sawatan, W.; Than, W.W.; Abdelhafez, M.M.; Oo Leik, N.K.; Ag Daud, D.M.; Mohd Daud, M.N.; Ahmad, Z.N.S. Bariatric surgery and its impact on fertility, pregnancy and its outcome: A narrative review. Ann. Med. Surg. 2021, 72, 103038. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Voros, C.; Varthaliti, A.; Bananis, K.; Mavrogianni, D.; Athanasiou, D.; Athanasiou, A.; Athanasiou, A.; Papahliou, A.M.; Zografos, C.G.; Kondili, P.; et al. The Relationship Between Obesity, Bariatric Surgery, and Infertility: A Systematic Review. Life 2025, 15, 758. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Nori, W.; Akram, W.; Amer Ali, E. Fertility outcomes following bariatric surgery. World J. Exp. Med. 2023, 13, 1–3. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Choromańska, B.; Myśliwiec, P.; Łuba, M.; Wojskowicz, P.; Dadan, J.; Myśliwiec, H.; Choromańska, K.; Zalewska, A.; Maciejczyk, M. A Longitudinal Study of the Antioxidant Barrier and Oxidative Stress in Morbidly Obese Patients After Bariatric Surgery. Does the Metabolic Syndrome Affect the Redox Homeostasis of Obese People? J. Clin. Med. 2020, 9, 976. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Berardi, G.; Vitiello, A.; Abu-Abeid, A.; Schiavone, V.; Franzese, A.; Velotti, N.; Musella, M. Micronutrients Deficiencies in Candidates of Bariatric Surgery: Results from a Single Institution over a 1-Year Period. Obes. Surg. 2023, 33, 212–218. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Jain, M.; Carlson, K.; Singh, M. Environmental Toxins and Infertility. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar] [PubMed]
- González-Comadran, M.; Jacquemin, B.; Cirach, M.; Lafuente, R.; Cole-Hunter, T.; Nieuwenhuijsen, M.; Brassesco, M.; Coroleu, B.; Checa, M.A. The effect of short term exposure to outdoor air pollution on fertility. Reprod. Biol. Endocrinol. 2021, 19, 151. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mínguez-Alarcón, L.; Messerlian, C.; Bellavia, A.; Gaskins, A.J.; Chiu, Y.H.; Ford, J.B.; Azevedo, A.R.; Petrozza, J.C.; Calafat, A.M.; Hauser, R.; et al. Urinary concentrations of bisphenol A, parabens and phthalate metabolite mixtures in relation to reproductive success among women undergoing in vitro fertilization. Environ. Int. 2019, 126, 355–362. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Clark, K.L.; George, J.W.; Davis, J.S. Adolescent exposure to a mixture of per- and polyfluoroalkyl substances (PFAS) depletes the ovarian reserve, increases ovarian fibrosis, and alters the Hippo pathway in adult female mice. Toxicol. Sci. 2024, 202, 36–49. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Génard-Walton, M.; Warembourg, C.; Duros, S.; Ropert-Bouchet, M.; Lefebvre, T.; Guivarc’h-Levêque, A.; Le Martelot, M.T.; Jacquemin, B.; Cordier, S.; Costet, N.; et al. Heavy metals and diminished ovarian reserve: Single-exposure and mixture analyses amongst women consulting in French fertility centres. Reprod. Biomed. Online 2023, 47, 103241. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Zhuan, Q.; Zhang, L.; Meng, L.; Fu, X.; Hou, Y. Polystyrene microplastics induced female reproductive toxicity in mice. J. Hazard. Mater. 2022, 424, 127629. [Google Scholar] [CrossRef] [PubMed]
- Dhage, V.D.; Nagtode, N.; Kumar, D.; Bhagat, A.K. A Narrative Review on the Impact of Smoking on Female Fertility. Cureus 2024, 16, e58389. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Oladipupo, I.; Ali, T.; Hein, D.W.; Pagidas, K.; Bohler, H.; Doll, M.A.; Mann, M.L.; Gentry, A.; Chiang, J.L.; Pierson, R.C.; et al. Association between cigarette smoking and ovarian reserve among women seeking fertility care. PLoS ONE 2022, 17, e0278998. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mueller, L.; Ciervo, C.A. Smoking in women. J. Osteopath. Med. 2023, 98, s7–s10. [Google Scholar] [CrossRef] [PubMed]
- Lyngsø, J.; Kesmodel, U.S.; Bay, B.; Ingerslev, H.J.; Pisinger, C.H.; Ramlau-Hansen, C.H. Female cigarette smoking and successful fertility treatment: A Danish cohort study. Acta Obstet. Gynecol. Scand. 2021, 100, 58–66. [Google Scholar] [CrossRef] [PubMed]
- Montjean, D.; Godin Pagé, M.H.; Bélanger, M.C.; Benkhalifa, M.; Miron, P. An Overview of E-Cigarette Impact on Reproductive Health. Life 2023, 13, 827. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zurub, R.E.; Cariaco, Y.; Wade, M.G.; Bainbridge, S.A. Microplastics exposure: Implications for human fertility, pregnancy and child health. Front. Endocrinol. 2024, 14, 1330396. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Balali, H.; Morabbi, A.; Karimian, M. Concerning influences of micro/nano plastics on female reproductive health: Focusing on cellular and molecular pathways from animal models to human studies. Reprod. Biol. Endocrinol. 2024, 22, 141. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ferraboschi, I.; Canzolino, F.; Ferrari, E.; Sissa, C.; Masino, M.; Rizzi, M.; Bussolati, S.; Basini, G.; Bertini, S.; Grolli, S.; et al. Detection of microplastics in the feline placenta and fetus. PLoS ONE 2025, 20, e0320694. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wang, Q.; Chi, F.; Liu, Y.; Chang, Q.; Chen, S.; Kong, P.; Yang, W.; Liu, W.; Teng, X.; Zhao, Y.; et al. Polyethylene microplastic exposure adversely affects oocyte quality in human and mouse. Environ. Int. 2025, 195, 109236. [Google Scholar] [CrossRef] [PubMed]
- Ni, D.; Yu, K.; Yan, N.; Chen, X.; Xie, Q.; Yang, Y.; Jiang, W.; Yang, Y.; Zhang, J.; Ling, X. Characterization of microplastics in human follicular fluid and assessment of their potential impact on mouse oocyte maturation in vitro. Ecotoxicol. Environ. Saf. 2025, 291, 117796. [Google Scholar] [CrossRef] [PubMed]
- Björvang, R.D.; Damdimopoulou, P. Persistent environmental endocrine-disrupting chemicals in ovarian follicular fluid and in vitro fertilization treatment outcome in women. Ups. J. Med. Sci. 2020, 125, 85–94. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Brown, R.H.; Ng, D.K.; Steele, K.; Schweitzer, M.; Groopman, J.D. Mobilization of Environmental Toxicants Following Bariatric Surgery. Obesity 2019, 27, 1865–1873. [Google Scholar] [CrossRef] [PubMed]
- Snoek, K.M.; Steegers-Theunissen, R.P.M.; Hazebroek, E.J.; Willemsen, S.P.; Galjaard, S.; Laven, J.S.E.; Schoenmakers, S. The effects of bariatric surgery on periconception maternal health: A systematic review and meta-analysis. Hum. Reprod. Update 2021, 27, 1030–1055. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Soykan, Y.; Bayhan, H.; Akogul, S.; Bedirli, A. The Influence of Bariatric Surgery on Reproductive Hormones and Ovarian Morphology and Clinical Findings in Women: A Prospective Study. Obes. Surg. 2025. Epub ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Cohen, N.J.; Yao, M.; Midya, V.; India-Aldana, S.; Mouzica, T.; Andra, S.S.; Narasimhan, S.; Meher, A.K.; Arora, M.; Chan, J.K.Y.; et al. Exposure to perfluoroalkyl substances and women’s fertility outcomes in a Singaporean population-based preconception cohort. Sci. Total Environ. 2023, 873, 162267. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rickard, B.P.; Rizvi, I.; Fenton, S.E. Per- and poly-fluoroalkyl substances (PFAS) and female reproductive outcomes: PFAS elimination, endocrine-mediated effects, and disease. Toxicology 2022, 465, 153031. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lockington, C.; Favetta, L.A. How Per- and Poly-Fluoroalkyl Substances Affect Gamete Viability and Fertilization Capability: Insights from the Literature. J. Xenobiotics 2024, 14, 651–678. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Huang, G.; Li, J.; Zhou, L.; Duan, T.; Deng, L.; Yang, P.; Gong, Y. Perfluoroalkyl and Polyfluoroalkyl Substances in Relation to the Participant-Reported Total Pregnancy and Live Birth Numbers Among Reproductive-Aged Women in the United States. Toxics 2024, 12, 613. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wang, X.; Zhang, S.; Yan, H.; Ma, Z.; Zhang, Y.; Luo, H.; Yang, X. Association of exposure to ozone and fine particulate matter with ovarian reserve among women with infertility. Environ. Pollut. 2024, 340 Pt 1, 122845. [Google Scholar] [CrossRef] [PubMed]
- Leathersich, S.J.; Roche, C.S.; Walls, M.; Nathan, E.; Hart, R.J. Particulate air pollution at the time of oocyte retrieval is independently associated with reduced odds of live birth in subsequent frozen embryo transfers. Hum. Reprod. 2025, 40, 110–118. [Google Scholar] [CrossRef] [PubMed]
- Pang, L.; Yu, W.; Lv, J.; Dou, Y.; Zhao, H.; Li, S.; Guo, Y.; Chen, G.; Cui, L.; Hu, J.; et al. Air pollution exposure and ovarian reserve impairment in Shandong province, China: The effects of particulate matter size and exposure window. Environ. Res. 2023, 218, 115056. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Zhao, J.; Ye, X.; Fu, M.; Zhang, K.; Wang, H.; Zou, Y.; Yu, K. Fine particulate matter and its constituent on ovarian reserve: Identifying susceptible windows of exposure. Sci. Total Environ. 2023, 904, 166744. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Cai, J.; Liu, L.; Jiang, X.; Li, P.; Sha, A.; Ren, J. Association between outdoor air pollution during in vitro culture and the outcomes of frozen-thawed embryo transfer. Hum. Reprod. 2019, 34, 441–451. [Google Scholar] [CrossRef] [PubMed]
- Huang, K.; Hu, M.; Zhang, Z.; Li, Z.; Hu, C.; Bai, S.; Li, R.; Wu, L.M.; Zhang, X.J.; Xu, B. Associations of ambient air pollutants with pregnancy outcomes in women undergoing assisted reproductive technology and the mediating role of ovarian reserve: A longitudinal study in eastern China. Sci. Total Environ. 2025, 958, 177919. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Soto-Moreno, E.J.; Prakash, A.; Balboula, A.Z.; Qiao, H. Adverse PFAS effects on mouse oocyte in vitro maturation are associated with carbon-chain length and inclusion of a sulfonate group. Cell Prolif. 2023, 56, e13353. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Li, S.; Li, L.; Zhang, C.; Fu, H.; Yu, S.; Zhou, M.; Guo, J.; Fang, Z.; Li, A.; Zhao, M.; et al. PM2.5 leads to adverse pregnancy outcomes by inducing trophoblast oxidative stress and mitochondrial apoptosis via KLF9/CYP1A1 transcriptional axis. Elife 2023, 12, e85944. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Taibl, K.R.; Schantz, S.; Aung, M.T.; Padula, A.; Geiger, S.; Smith, S.; Park, J.S.; Milne, G.L.; Robinson, J.F.; Woodruff, T.J.; et al. Associations of per- and polyfluoroalkyl substances (PFAS) and their mixture with oxidative stress biomarkers during pregnancy. Environ. Int. 2022, 169, 107541. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Baumert, B.O.; Eckel, S.P.; Goodrich, J.A.; Li, Z.; Stratakis, N.; Walker, D.I.; Zhao, Y.; Fischer, F.C.; Bartell, S.; Valvi, D.; et al. Changes in plasma concentrations of per- and Polyfluoroalkyl substances after bariatric surgery in adolescents from the Teen-Longitudinal Assessment of Bariatric Surgery (Teen-LABS) study. Sci. Total Environ. 2024, 930, 172840. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- An, R.; Wang, X.; Yang, L.; Zhang, J.; Wang, N.; Xu, F.; Hou, Y.; Zhang, H.; Zhang, L. Polystyrene microplastics cause granulosa cells apoptosis and fibrosis in ovary through oxidative stress in rats. Toxicology 2021, 449, 152665, Erratum in Toxicology 2022, 478, 153291. [Google Scholar] [CrossRef] [PubMed]
- Hou, J.; Lei, Z.; Cui, L.; Hou, Y.; Yang, L.; An, R.; Wang, Q.; Li, S.; Zhang, H.; Zhang, L. Polystyrene microplastics lead to pyroptosis and apoptosis of ovarian granulosa cells via NLRP3/Caspase-1 signaling pathway in rats. Ecotoxicol. Environ. Saf. 2021, 212, 112012. [Google Scholar] [CrossRef] [PubMed]
- Jeong, J.; Thi Quynh Mai, N.; Moon, B.S.; Choi, J.K. Impact of polystyrene microplastics (PS-MPs) on the entire female mouse reproductive cycle: Assessing reproductive toxicity of microplastics through in vitro follicle culture. Ecotoxicol. Environ. Saf. 2025, 297, 118228. [Google Scholar] [CrossRef] [PubMed]
- Afreen, V.; Hashmi, K.; Nasir, R.; Saleem, A.; Khan, M.I.; Akhtar, M.F. Adverse health effects and mechanisms of microplastics on female reproductive system: A descriptive review. Environ. Sci. Pollut. Res. Int. 2023, 30, 76283–76296. [Google Scholar] [CrossRef] [PubMed]
| (a) | ||
|---|---|---|
| Pathophysiological Mechanism | Impact in Obese Women | Reference |
| Hypothalamic–Pituitary–Ovarian Axis | Disrupted GnRH pulsatility, altered LH/FSH secretion | Practice Committee of the American Society for Reproductive Medicine. Electronic address: asrm@asrm.org; Practice Committee of the American Society for Reproductive Medicine. Obesity and reproduction: a committee opinion. Fertil Steril. 2021 Nov;116(5):1266–1285. doi: 10.1016/j.fertnstert.2021.08.018. Epub 2021 Sep 25. PMID: 34583840 [18]. |
| Hyperinsulinemia and Insulin Resistance | Increased ovarian androgens, decreased SHBG, hyperandrogenism | Cena H, Chiovato L, Nappi RE. Obesity, Polycystic Ovary Syndrome, and Infertility: A New Avenue for GLP-1 Receptor Agonists. J Clin Endocrinol Metab. 2020 Aug 1;105(8):e2695–709. doi: 10.1210/clinem/dgaa285. PMID: 32442310; PMCID: PMC7457958 [13]. |
| Adipokine imbalance | Elevated leptin resistance, low adiponectin → impaired folliculogenesis and oocyte quality | Merhi Z, Bazzi AA, Bonney EA, Buyuk E. Role of adiponectin in ovarian follicular development and ovarian reserve. 1(1):1–5. doi: 10.3892/br.2019.1213. PMID: 31258901; PMCID: PMC6566571 [11]. |
| Oxidative stress and inflammation | Mitochondrial dysfunction, ROS accumulation → granulosa cell apoptosis, poor oocyte quality | Sasaki H, Hamatani T, Kamijo S, Iwai M, Kobanawa M, Ogawa S, Miyado K, Tanaka M. Impact of Oxidative Stress on Age-Associated Decline in Oocyte Developmental Competence. Front Endocrinol (Lausanne). doi: 10.3389/fendo.2019.00811. PMID: 31824426; PMCID: PMC6882737 [12]. |
| Anovulation and menstrual irregularity | Chronic anovulation, PCOS-like symptoms | Cena H, Chiovato L, Nappi RE. Obesity, Polycystic Ovary Syndrome, and Infertility: A New Avenue for GLP-1 Receptor Agonists. J Clin Endocrinol Metab. 2020 Aug 1;105(8):e2695–709. doi: 10.1210/clinem/dgaa285. PMID: 32442310; PMCID: PMC7457958 [13]. |
| Altered hormone profiles | Lower AMH, altered kisspeptin, estrogen/progesterone imbalance | Lu FF, Wang Z, Yang QQ, Yan FS, Xu C, Wang MT, Xu ZJ, Cai SY, Guan R. Investigating the metabolomic pathways in female reproductive endocrine disorders: a Mendelian randomization study. Front Endocrinol (Lausanne). 2024 Oct 31;15:1438079. doi: 10.3389/fendo.2024.1438079. PMID: 39544240; PMCID: PMC11560792 [15]. |
| Impaired ART outcomes | Lower oocyte yield, poor embryo quality, reduced implantation and live birth rates | Nilsson-Condori E, Mattsson K, Thurin-Kjellberg A, Hedenbro JL, Friberg B. Outcomes of in vitro fertilization after bariatric surgery: a national register-based case–control study. Hum Reprod. 2022 Sep 30;37(10):2474–2481. doi: 10.1093/humrep/deac164. PMID: 35904469; PMCID: PMC9527453 [2]. |
| Endometrial dysfunction | Impaired receptivity due to inflammation and leptin signaling | BLu FF, Wang Z et al. Front Endocrinol (Lausanne). 2024;15:1438079. PMID: 39544240ellver J et al., Fertil Steril. 2021;115(6):1453–1463. doi:10.1016/j.fertnstert.2021.03.003 [15] |
| Pregnancy outcomes | Increased risk of infertility, miscarriage, preeclampsia, cesarean delivery | Almutairi H, Aldhalea MS, Almaaz MA, Aljuhani SA, Aloraini RI, Alamoudi AA, Alkhalifah WF, Alrushaid LA, Alanzy HW, Alzuwayyid M, Alrumaih FA, Al-Harbi MM, Al-Aboudi AA, Alqadi FS, Alshammari RS. The Effectiveness of Bariatric Surgery on Treating Infertility in Women-A Systematic Review and Meta-Analysis. J Clin Med. 2024 Sep 19;13(18):5569. doi: 10.3390/jcm13185569. PMID: 39337056; PMCID: PMC11433424 [1]. |
| (b) | ||
| Pathophysiological Mechanism | Effect After Bariatric Surgery or Rapid Weight Loss | Reference |
| Hypothalamic–Pituitary–Ovarian axis | Restoration of GnRH pulsatility and hormonal balance; resumption of ovulatory cycles | Samarasinghe SNS, Leca B, Alabdulkader S, Dimitriadis GK, Davasgaium A, Thadani P, Parry K, Luli M, O’Donnell K, Johnson B, Abbara A, Seyfried F, Morman R, Ahmed AR, Hakky S, Tsironis C, Purkayastha S, le Roux CW, Franks S, Menon V, Randeva H, Miras AD. Bariatric surgery for spontaneous ovulation in women living with polycystic ovary syndrome: the BAMBINI multicentre, open-label, randomized controlled trial. Lancet. 2024 Jun 8;403(10443):2489–2503. doi: 10.1016/S0140-6736(24)00538-5. Epub 2024 May 20. PMID: 38782004 [4]. |
| Hyperinsulinemia and insulin resistance | Improved insulin sensitivity, decreased androgen excess, normalized SHBG | Cena H, Chiovato L, Nappi RE. Obesity, Polycystic Ovary Syndrome, and Infertility: A New Avenue for GLP-1 Receptor Agonists. J Clin Endocrinol Metab. 2020 Aug 1;105(8):e2695–709. doi: 10.1210/clinem/dgaa285. PMID: 32442310; PMCID: PMC7457958 [13]. |
| Adipokine imbalance | Decreased leptin levels, increased adiponectin → improved folliculogenesis | Merhi Z, Bazzi AA, Bonney EA, Buyuk E. Role of adiponectin in ovarian follicular development and ovarian reserve. Biomed Rep. 1(1):1–5. PMID: 31258901 [11] |
| Oxidative stress and inflammation | Reduced systemic inflammation and oxidative stress → improved oocyte quality and endometrial receptivity | Choromańska B, Myśliwiec P, Łuba M, Wojskowicz P, Dadan J, Myśliwiec H, Choromańska K, Zalewska A, Maciejczyk M. A Longitudinal Study of the Antioxidant Barrier and Oxidative Stress in Morbidly Obese Patients after Bariatric Surgery. Does the Metabolic Syndrome Affect the Redox Homeostasis of Obese People? J Clin Med. 2020 Apr 1;9(4):976. doi: 10.3390/jcm9040976. PMID: 32244612; PMCID: PMC7230760 [32]. |
| Anovulation and menstrual irregularity | Regularized menstrual cycles and restored spontaneous ovulation | Samarasinghe SNS et al. Lancet. 2024;403(10443):2489–2503. PMID: 38782004 [4] |
| Altered hormone profiles | Improved AMH levels and hormonal regulation | Lu FF, Wang Z, Yang QQ, Yan FS, Xu C, Wang MT, Xu ZJ, Cai SY, Guan R. Investigating the metabolomic pathways in female reproductive endocrine disorders: a Mendelian randomization study. Front Endocrinol (Lausanne). 2024 Oct 31;15:1438079. doi: 10.3389/fendo.2024.1438079. PMID: 39544240; PMCID: PMC11560792 [15]. |
| ART outcomes | Enhanced ART success rates comparable to BMI-matched controls | Nilsson-Condori E, Mattsson K, Thurin-Kjellberg A, Hedenbro JL, Friberg B. Outcomes of in vitro fertilization after bariatric surgery: a national register-based case–control study. Hum Reprod. 2022 Sep 30;37(10):2474–2481. doi: 10.1093/humrep/deac164. PMID: 35904469; PMCID: PMC9527453 [2]. |
| Endometrial function | Improved receptivity; enhanced embryo implantation | Lu FF, Wang Z et al. Front Endocrinol (Lausanne). 2024;15:1438079. PMID: 39544240 [15] |
| Micronutrient status | Risk of iron, B12, folate, zinc, vitamin D deficiencies → requires close follow-up | Berardi G, Vitiello A, Abu-Abeid A, Schiavone V, Franzese A, Velotti N, Musella M. Micronutrients Deficiencies in Candidates of Bariatric Surgery: Results from a Single Institution over a 1-Year Period. Obes Surg. 2023 Jan;33(1):212–218. doi: 10.1007/s11695-022-06355-8. Epub 2022 Nov 4. PMID: 36331725; PMCID: PMC9834098 [33]. |
| Pregnancy outcomes | Lower preeclampsia rates post-surgery; small-for-gestational-age risk if conception occurs too early | Almutairi H et al. The Effectiveness of Bariatric Surgery on Treating Infertility in Women. J Clin Med. 2024;13(18):5569. PMID: 39337056 [1] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Popescu, C.-D.; Sima, R.M.; Poenaru, M.-O.; Constantin, A.-A.; Gorecki, G.-P.; Diaconescu, A.-S.; Mihai, M.; Toma, C.-V.; Pleș, L. Environmental Pollution, Endocrine Disruptors, and Metabolic Status: Impact on Female Fertility—A Narrative Review. Reprod. Med. 2025, 6, 37. https://doi.org/10.3390/reprodmed6040037
Popescu C-D, Sima RM, Poenaru M-O, Constantin A-A, Gorecki G-P, Diaconescu A-S, Mihai M, Toma C-V, Pleș L. Environmental Pollution, Endocrine Disruptors, and Metabolic Status: Impact on Female Fertility—A Narrative Review. Reproductive Medicine. 2025; 6(4):37. https://doi.org/10.3390/reprodmed6040037
Chicago/Turabian StylePopescu, Cristina-Diana, Romina Marina Sima, Mircea-Octavian Poenaru, Ancuta-Alina Constantin, Gabriel-Petre Gorecki, Andrei-Sebastian Diaconescu, Mara Mihai, Cristian-Valentin Toma, and Liana Pleș. 2025. "Environmental Pollution, Endocrine Disruptors, and Metabolic Status: Impact on Female Fertility—A Narrative Review" Reproductive Medicine 6, no. 4: 37. https://doi.org/10.3390/reprodmed6040037
APA StylePopescu, C.-D., Sima, R. M., Poenaru, M.-O., Constantin, A.-A., Gorecki, G.-P., Diaconescu, A.-S., Mihai, M., Toma, C.-V., & Pleș, L. (2025). Environmental Pollution, Endocrine Disruptors, and Metabolic Status: Impact on Female Fertility—A Narrative Review. Reproductive Medicine, 6(4), 37. https://doi.org/10.3390/reprodmed6040037