Hemodynamic Comparison of Inferior Vena Cava Collapsibility Index in Patients with Preeclampsia vs. Controls: A Pilot Study
Abstract
1. Introduction
2. Materials and Methods
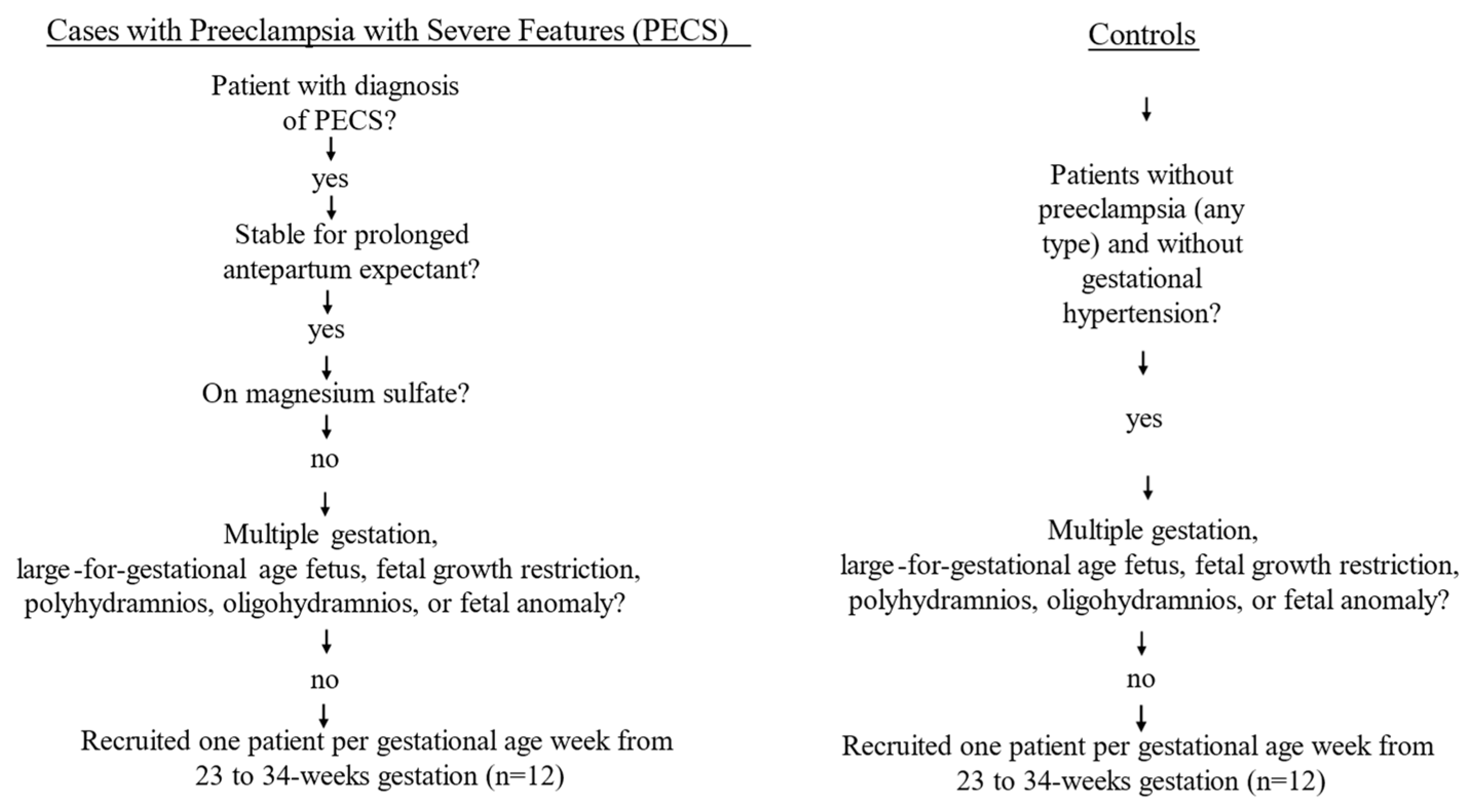
2.1. Patient Selection
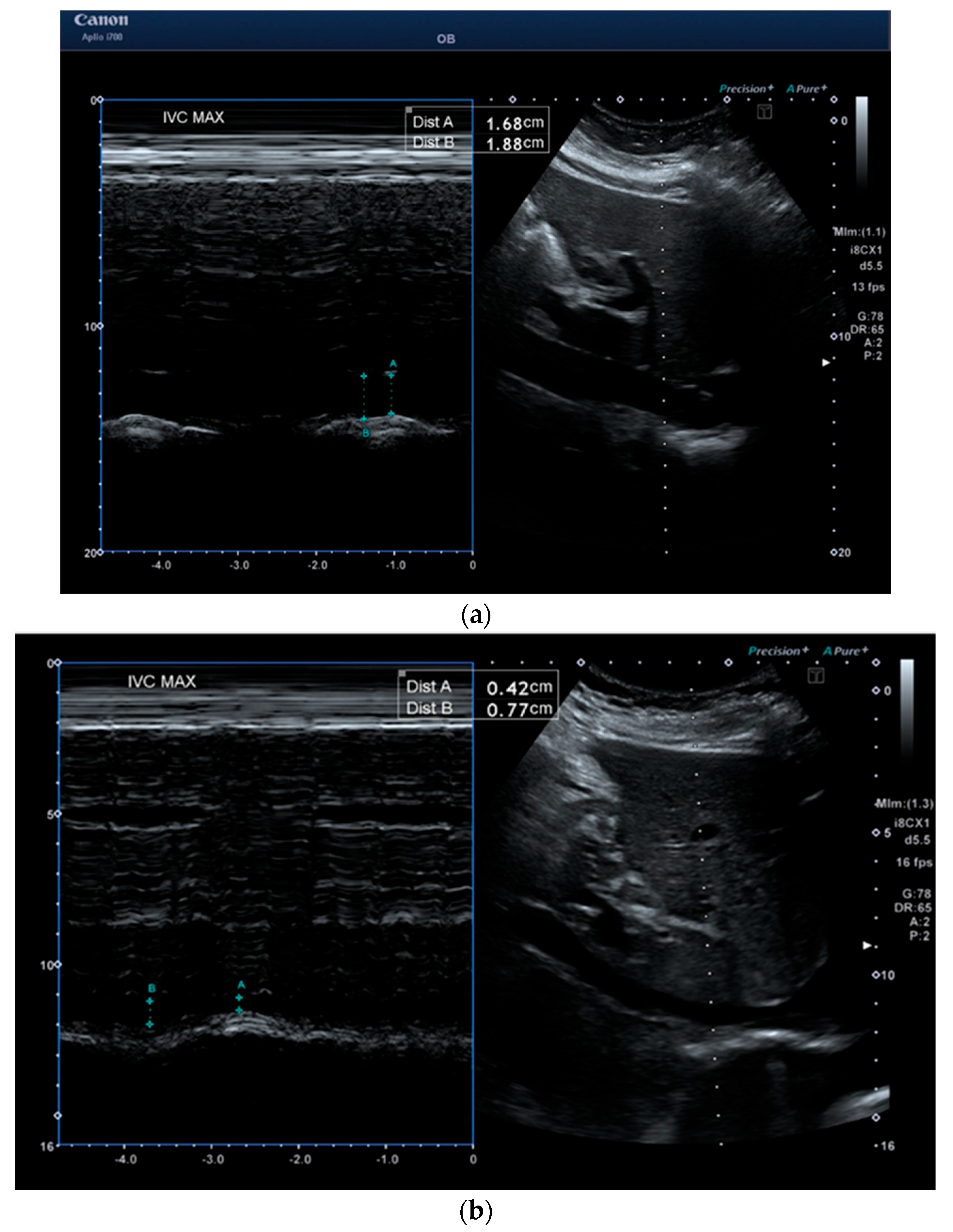
2.2. Measurements
2.3. Statistical Analysis
3. Results
4. Discussion
4.1. Advantages of Ultrasound over Traditional Hemodynamic Monitoring
4.2. Insights into Tailored Treatment for Hypertensive Disorders of Pregnancy
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Erez, O.; Romero, R.; Jung, E.; Chaemsaithong, P.; Bosco, M.; Suksai, M.; Gallo, D.M.; Gotsch, F. Preeclampsia and eclampsia: The conceptual evolution of a syndrome. Am. J. Obstet. Gynecol. 2022, 226, S786–S803. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Roberts, J.M. Preeclampsia epidemiology(ies) and pathophysiology(ies). Best. Pract. Res. Clin. Obstet. Gynaecol. 2024, 94, 102480. [Google Scholar] [CrossRef] [PubMed]
- Westerberg, A.C.; Degnes, M.L.; Andresen, I.J.; Roland, M.C.P.; Michelsen, T.M. Angiogenic and vasoactive proteins in the maternal-fetal interface in healthy pregnancies and preeclampsia. Am. J. Obstet. Gynecol. 2024, 231, 550.e1–550.e22. [Google Scholar] [CrossRef]
- Sibai, B.M.; Mabie, W.C. Hemodynamics of preeclampsia. Clin Perinatol. 1991, 18, 727–747. [Google Scholar] [CrossRef]
- Salas, S.P.; Marshall, G.; Gutiérrez, B.L.; Rosso, P. Time course of maternal plasma volume and hormonal changes in women with preeclampsia or fetal growth restriction. Hypertension 2006, 47, 203–208. [Google Scholar] [CrossRef] [PubMed]
- Szabó, M.; Bozó, A.; Darvas, K.; Horváth, A.; Iványi, Z.D. Role of inferior vena cava collapsibility index in the prediction of hypotension associated with general anesthesia: An observational study. BMC Anesthesiol. 2019, 19, 139. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ni, T.T.; Zhou, Z.F.; He, B.; Zhou, Q.H. Inferior Vena Cava Collapsibility Index Can Predict Hypotension and Guide Fluid Management After Spinal Anesthesia. Front. Surg. 2022, 9, 831539. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Orso, D.; Paoli, I.; Piani, T.; Cilenti, F.L.; Cristiani, L.; Guglielmo, N. Accuracy of Ultrasonographic Measurements of Inferior Vena Cava to Determine Fluid Responsiveness: A Systematic Review and Meta-Analysis. J. Intensive Care Med. 2020, 35, 354–363. [Google Scholar] [CrossRef] [PubMed]
- Sethi, D.; Jadhav, V.L.; Garg, G. Role of Inferior Vena Cava Collapsibility Index in the Prediction of Hypotension Associated With Central Neuraxial Block: A Prospective Observational Study. J. Ultrasound Med. 2023, 42, 1977–1985. [Google Scholar] [CrossRef] [PubMed]
- Easter, S.R.; Hameed, A.B.; Shamshirsaz, A.; Fox, K.; Zelop, C.M. Point of care maternal ultrasound in obstetrics. Am. J. Obstet. Gynecol. 2023, 228, 509.e1–509.e13. [Google Scholar] [CrossRef] [PubMed]
- Martins, J.G.; Waller, J.; Horgan, R.; Kawakita, T.; Kanaan, C.; Abuhamad, A.; Saade, G. Point-of-Care Ultrasound in Critical Care Obstetrics: A Scoping Review of the Current Evidence. J. Ultrasound Med. 2024, 43, 951–965. [Google Scholar] [CrossRef] [PubMed]
- Tomsin, K.; Mesens, T.; Molenberghs, G.; Peeters, L.; Gyselaers, W. Characteristics of heart, arteries, and veins in low and high cardiac output preeclampsia. Eur. J. Obstet. Gynecol. Reprod. Biol. 2013, 169, 218–222. [Google Scholar] [CrossRef]
- Yagel, S.; Cohen, S.M.; Admati, I.; Skarbianskis, N.; Solt, I.; Zeisel, A.; Beharier, O.; Goldman-Wohl, D. Expert review: Preeclampsia Type I and Type II. Am. J. Obstet. Gynecol. MFM 2023, 5, 101203. [Google Scholar] [CrossRef] [PubMed]
- Roberts, J.M.; Rich-Edwards, J.W.; McElrath, T.F.; Garmire, L.; Myatt, L.; Collaboration, G.P. Subtypes of preeclampsia: Recognition and determining clinical usefulness. Hypertension 2021, 77, 1430–1441. [Google Scholar] [CrossRef]
- Stott, D.; Bolten, M.; Paraschiv, D.; Papastefanou, I.; Chambers, J.B.; Kametas, N.A. Longitudinal hemodynamics in acute phase of treatment with labetalol in hypertensive pregnant women to predict need for vasodilatory therapy. Ultrasound Obstet. Gynecol. 2017, 49, 85–94. [Google Scholar] [CrossRef] [PubMed]
- Stott, D.; Papastefanou, I.; Paraschiv, D.; Clark, K.; Kametas, N. Serial hemodynamic monitoring to guide treatment of maternal hypertension leads to reduction in severe hypertension. Ultrasound Obstet. Gynecol. 2017, 49, 95–103. [Google Scholar] [CrossRef] [PubMed]
- Valensise, H.; Vasapollo, B.; Gagliardi, G.; Novelli, G.P. Early and late preeclampsia: Two different maternal hemodynamic states in the latent phase of the disease. Hypertension 2008, 52, 873–880. [Google Scholar] [CrossRef]
- Foo, F.L.; Mahendru, A.A.; Masini, G.; Fraser, A.; Cacciatore, S.; MacIntyre, D.A.; McEniery, C.M.; Wilkinson, I.B.; Bennett, P.R.; Lees, C.C. Association Between Prepregnancy Cardiovascular Function and Subsequent Preeclampsia or Fetal Growth Restriction. Hypertension 2018, 72, 442–450. [Google Scholar] [CrossRef] [PubMed]
- Tay, J.; Foo, L.; Masini, G.; Bennett, P.R.; McEniery, C.M.; Wilkinson, I.B.; Lees, C.C. Early and late preeclampsia are characterized by high cardiac output, but in the presence of fetal growth restriction, cardiac output is low: Insights from a prospective study. Am. J. Obstet. Gynecol. 2018, 218, 517.e1–517.e12. [Google Scholar] [CrossRef]
- Perry, H.; Binder, J.; Gutierrez, J.; Thilaganathan, B.; Khalil, A. Maternal haemodynamic function differs in pre-eclampsia when it is associated with a small-for-gestational-age newborn: A prospective cohort study. BJOG Int. J. Obstet. Gynaecol. 2021, 128, 167–175. [Google Scholar] [CrossRef]
- Ornaghi, S.; Caricati, A.; Di Martino, D.D.; Mossa, M.; Di Nicola, S.; Invernizzi, F.; Zullino, S.; Clemenza, S.; Barbati, V.; Tinè, G. Non-invasive maternal hemodynamic assessment to classify high-risk pregnancies complicated by fetal growth restriction. Front. Clin. Diabetes Healthc. 2022, 3, 851971. [Google Scholar] [CrossRef]
- Di Martino, D.D.; Stampalija, T.; Zullino, S.; Fusè, F.; Garbin, M.; Parasiliti, M.; Sterpi, V.; Farina, A.; Ferrazzi, E. Maternal hemodynamic profile during pregnancy and in the post-partum in hypertensive disorders of pregnancy and fetal growth restriction. Am. J. Obstet. Gynecol. MFM 2023, 5, 100841. [Google Scholar] [CrossRef]
- Mullan, S.J.; Vricella, L.K.; Edwards, A.M.; Powel, J.E.; Ong, S.K.; Li, X.; Tomlinson, T.M. Pulse pressure as a predictor of response to treatment for severe hypertension in pregnancy. Am. J. Obstet. Gynecol. MFM 2021, 3, 100455. [Google Scholar] [CrossRef] [PubMed]
- Maykin, M.M.; Mercer, E.; Saiki, K.M.; Kaneshiro, B.; Miller, C.B.; Tsai, P.S. Furosemide to lower antenatal severe hypertension: A randomized placebo-controlled trial. Am. J. Obstet. Gynecol. MFM 2024, 6, 101348. [Google Scholar] [CrossRef] [PubMed]
- Sampson, R.; Davis, S.; Wong, R.; Baranco, N.; Silverman, R.K. Pulse Pressure as a Hemodynamic Parameter in Preeclampsia with Severe Features Accompanied by Fetal Growth Restriction. J. Clin. Med. 2024, 13, 4318. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rafferty, T.D.; Berkowitz, R.L. Hemodynamics in patients with severe toxemia during labor and delivery. Am. J. Obstet. Gynecol. 1980, 138, 263–270. [Google Scholar] [CrossRef] [PubMed]
- Phelan, J.P.; Yurth, D.A. Severe preeclampsia. I. Peripartum hemodynamic observations. Am. J. Obstet. Gynecol. 1982, 144, 17–22. [Google Scholar] [CrossRef] [PubMed]
- Benedetti, T.J.; Kates, R.; Williams, V. Hemodynamic observations in severe preeclampsia complicated by pulmonary edema. Am. J. Obstet. Gynecol. 1985, 152, 330–334. [Google Scholar] [CrossRef] [PubMed]
- Sibai, B.M. Maternal and uteroplacental hemodynamics for the classification and prediction of preeclampsia. Hypertension 2008, 52, 805–806. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.J.; Cohn, J.H.; Ranasinghe, J.S. Cardiac output assessed by invasive and minimally invasive techniques. Anesthesiol. Res. Pract. 2011, 2011, 475151. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sorensen, M.B.; Bille-Brahe, N.E.; Engell, H.C. Cardiac output measurement by thermal dilution: Reproducibility and comparison with the dye-dilution technique. Ann. Surg. 1976, 183, 67–72. [Google Scholar] [CrossRef] [PubMed]
- Fegler, G. The reliability of the thermodilution method for determination of the cardiac output and the blood flow in central veins. Q. J. Exp. Physiol. Cogn. Med. Sci. 1957, 42, 254–266. [Google Scholar] [CrossRef]
- Vinayagam, D.; Patey, O.; Thilaganathan, B.; Khalil, A. Cardiac output assessment in pregnancy: Comparison of two automated monitors with echocardiography. Ultrasound Obstet. Gynecol. 2017, 49, 32–38. [Google Scholar] [CrossRef] [PubMed]
- Lavie, A.; Ram, M.; Lev, S.; Blecher, Y.; Amikam, U.; Shulman, Y.; Avnon, T.; Weiner, E.; Many, A. Maternal cardiovascular hemodynamics in normotensive versus preeclamptic pregnancies: A prospective longitudinal study using a noninvasive cardiac system (NICaS™). BMC Pregnancy Childbirth 2018, 18, 229. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hunter, S.; Robson, S.C. Adaptation of the maternal heart in pregnancy. Br. Heart J. 1992, 68, 540–543. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ouzounian, J.G.; Elkayam, U. Physiologic changes during normal pregnancy and delivery. Cardiol. Clin. 2012, 30, 317–329. [Google Scholar] [CrossRef] [PubMed]
- Kuhn, J.C.; Falk, R.S.; Langesæter, E. Haemodynamic changes during labour: Continuous minimally invasive monitoring in 20 healthy parturients. Int. J. Obstet. Anesth 2017, 31, 74–83. [Google Scholar] [CrossRef] [PubMed]
- Schrier, R.W. Pathogenesis of sodium and water retention in high-output and low-output cardiac failure, nephrotic syndrome, cirrhosis, and pregnancy (2). N. Engl. J. Med. 1988, 319, 1127–1134. [Google Scholar] [CrossRef]
- Opichka, M.A.; Rappelt, M.W.; Gutterman, D.D.; Grobe, J.L.; McIntosh, J.J. Vascular Dysfunction in Preeclampsia. Cells 2021, 10, 3055. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gevaerd Martins, J.; Saad, A.; Saade, G.; Pacheco, L.D. The role of point-of-care ultrasound to monitor response of fluid replacement therapy in pregnancy. Am. J. Obstet. Gynecol. 2024, 231, 563–573. [Google Scholar] [CrossRef] [PubMed]
- Melchiorre, K.; Sharma, R.; Thilaganathan, B. Cardiovascular implications in preeclampsia: An overview. Circulation 2014, 130, 703–714. [Google Scholar] [CrossRef] [PubMed]
- Gandhi, S.; Sun, D.; Park, A.L.; Hladunewich, M.; Silversides, C.K.; Ray, J.G. The Pulmonary Edema Preeclampsia Evaluation (PEPE) Study. J. Obstet. Gynaecol. Can. 2014, 36, 1065–1070. [Google Scholar] [CrossRef]
- Qasem, F.; Hegazy, A.F.; Fuller, J.G.; Lavi, R.; Singh, S.I. Inferior vena cava assessment in term pregnant women using ultrasound: A comparison of the subcostal and right upper quadrant views. Anaesth. Intensive Care. 2021, 49, 389–394. [Google Scholar] [CrossRef] [PubMed]
- Gagné, M.P.; Richebé, P.; Loubert, C.; Drolet, P.; Gobert, Q.; Denault, A.; Zaphiratos, V. Ultrasound evaluation of inferior vena cava compression in tilted and supine term parturients. Can. J. Anaesth. 2021, 68, 1507–1513. (In English) [Google Scholar] [CrossRef] [PubMed]
- Chong, Y.; Yu, Y.; Zhao, Y.; Zhang, Y. Value of inferior vena cava diameter and inferior vena cava collapse index in the evaluation of peripartum volume: A prospective cohort study. Eur. J. Obstet. Gynecol. Reprod. Biol. 2023, 285, 69–73. [Google Scholar] [CrossRef] [PubMed]
- Di Pasquo, E.; Giannubilo, S.R.; Valentini, B.; Salvi, S.; Rullo, R.; Fruci, S.; Filippi, E.; Ornaghi, S.; Zullino, S.; Rossi, F.; et al. The “Preeclampsia and Hypertension Target Treatment” study: A multicenter prospective study to evaluate the effectiveness of the antihypertensive therapy based on maternal hemodynamic findings. Am. J. Obstet. Gynecol. MFM 2024, 6, 101368. [Google Scholar] [CrossRef] [PubMed]
- Davis, A.M.; Blanchard, C.T.; Subramaniam, A.; Sinkey, R.G.; Tita, A.T.; Battarbee, A.N. Physiologic Treatment of Severe Hypertension in Pregnancy and Postpartum. Obstet. Gynecol. 2024, 143, 277–280. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Convertino, V.A.; Cooke, W.H.; Holcomb, J.B. Arterial pulse pressure and its association with reduced stroke volume during progressive central hypovolemia. J. Trauma 2006, 61, 629–634. [Google Scholar] [CrossRef] [PubMed]
- Duley, L.; Meher, S.; Jones, L. Drugs for treatment of very high blood pressure during pregnancy. Cochrane Database Syst Rev. 2013, 2013, CD001449. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- American College of Obstetricians and Gynecologists. Gestational Hypertension and Preeclampsia: ACOG Practice Bulletin, Number 222. Obstet. Gynecol. 2020, 135, e237–e260. [Google Scholar] [CrossRef] [PubMed]

| Gestational Age Matched Controls (n = 12) | PECS (n = 12) | |
|---|---|---|
| Age (years) | 30.8 (5.6) * | 28.3 (6.6) * |
| Parity | ||
| Nulliparous | 5 (41.7) | 8(66.7) |
| Multiparous | 7 (58.3) | 4 (33.3) |
| BMI (kg/m2) | 34.3 (7.4) | 37.8 (10.5) |
| Race/Ethnicity | ||
| White, non-Hispanic | 3 (60.0) | 2 (40.0) |
| Not white, non-Hispanic | 9 (47.4) | 10 (52.6) |
| Pre-pregnancy diabetes | 0 | 1 (8.3) |
| Gestational diabetes | 1 (8.3) | 0 |
| Chronic hypertension | 6 (50.0) | 5 (41.7) |
| Controls | PECS | ||
|---|---|---|---|
| IVC inhalation (cm) | 0.6 (0.2) | 1.5 (0.4) | p < 0.001 |
| IVC exhalation (cm) | 1.1 (0.3) | 1.9 (0.3) | p < 0.001 |
| IVC-CI (%) | 48.3 (13.6) | 20.1 (14.5) | p < 0.001 |
| Pulse pressure (mmHg) | 40.8 (4.1) | 61.4 (10.4) | p < 0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sampson, R.; Rojas Mendez, P.; Kaul, V. Hemodynamic Comparison of Inferior Vena Cava Collapsibility Index in Patients with Preeclampsia vs. Controls: A Pilot Study. Reprod. Med. 2025, 6, 35. https://doi.org/10.3390/reprodmed6040035
Sampson R, Rojas Mendez P, Kaul V. Hemodynamic Comparison of Inferior Vena Cava Collapsibility Index in Patients with Preeclampsia vs. Controls: A Pilot Study. Reproductive Medicine. 2025; 6(4):35. https://doi.org/10.3390/reprodmed6040035
Chicago/Turabian StyleSampson, Rachael, Patricia Rojas Mendez, and Viren Kaul. 2025. "Hemodynamic Comparison of Inferior Vena Cava Collapsibility Index in Patients with Preeclampsia vs. Controls: A Pilot Study" Reproductive Medicine 6, no. 4: 35. https://doi.org/10.3390/reprodmed6040035
APA StyleSampson, R., Rojas Mendez, P., & Kaul, V. (2025). Hemodynamic Comparison of Inferior Vena Cava Collapsibility Index in Patients with Preeclampsia vs. Controls: A Pilot Study. Reproductive Medicine, 6(4), 35. https://doi.org/10.3390/reprodmed6040035






